ABSTRACT
Life-threatening bleeding (LTB) is one of the major complications of hemophilia with a high risk of mortality.
Objective
The aim of this study was to assess incidence, risk factors, treatment and outcome of LTB in Senegalese people with hemophilia (PWH).
Methods
We analyzed the characteristics of LTB in a cohort of 274 PWH after 10 years of follow-up.
Results
We included 274 patients (241 HA and 33 HB). The mean age was 16.45 years and the median age was 13 years. The mean annual bleeding rate (ABR) was 1.65 (2.83 for severe form, 1.54 for moderate form, and 1.22 for mild form). A replacement therapy with clotting factor concentrates (CFC) was administered to 217 patients (79.2%); 56 patients (20.4%) received low-dose prophylaxis (LDP). Prevalence of inhibitors was 4.7% (13/274). All patients were HIV and HCV antibody negative. We observed 31 cases of LTB in 22 patients with an incidence of 8.03%. Central nervous system (CNS) bleeds were most frequent (6.2%) and accounted for 54.8% of severe bleeding. The delay between the first signs and the emergency visit was 78.9 hours. Inhibitors were positive in one patient among those who presented LTB. These bleeding were treated with CFC in 16 patients, surgical drainage (1 patient) and electrocoagulation during gastroscopy (1 patient). Eleven patients had complete remission and two had sequelae. We reported 0.32 death per 100 person-years. CNS bleeds were the main cause (77.7%). Four patients were secondarily on LDP. We observed a significant correlation between treatment (after 2 hours) and mortality.
Conclusion
LTB is a serious and lethal complication in PWH in absence of early management. A good awareness of patients and their family would further reduce this incidence, especially in resources-limited countries.
Introduction
Hemophilia is an X-linked congenital bleeding disorder with a prevalence of 17.1/100000 males and a prevalence at birth of 24.6/100000 males [Citation1]. It is caused by a deficiency of coagulation factor VIII (FVIII) or IX (FIX), named hemophilia A or hemophilia B [Citation2].
Over the past 75 years, the life expectancy and the quality of life of people living with hemophilia have improved dramatically, and their life is approaching that of non-hemophiliacs, especially in high-income countries. However, the high cost of hemophilia treatment limits the access to treatments for people living in low- and middle-income countries [Citation3]. Actually, 70% of PWH do not have access to replacement therapy in the worldwide [Citation4]. Their life expectancy is reduced and the consequences of joint and other bleeding lead to severe functional incapacity and inability to execute their daily activities.
PWH commonly suffer from joint and muscle bleeding. However, life-threatening bleeding (LTB) is a major complication of the disease with a risk of early death. About 10% of those with severe hemophilia have intracranial bleeding, and 30% of them die [Citation2,Citation5]. Central nervous system (CNS) bleeds are the most serious bleeding event that occur in patients with hemophilia. Its estimated mortality rate is approximately 20%, representing the largest number of deaths from bleeding. There is an up to 8% risk that a person with the disease will develop intracranial bleeding during his lifetime [Citation6,Citation7].
These LTB have become very rare in developed countries [Citation8] but continue to be a burden in countries where access to treatment is limited. Therefore, they can be an indicator of the quality of care in countries and their monitoring can help to assess the situation and the progress made in the management of hemophilia in different settings. A good understanding of their circumstances of occurrence would also enable the identification of the different factors to be considered to implement improvement strategies.
This observational cohort study aimed to describe all LTB that occurred in patients with hemophilia in Senegal over the past 10 years and to identify their risk factors, treatment and outcome.
Methodology
This was an observational study with the aim to describe the characteristics of LTB that occurred in a cohort of 274 PWH followed in Dakar Hemophilia Treatment Center (HTC) during the last 10 years (2012–2021). All male patients with mild, moderate and severe hemophilia A and B were included, and hemophilia was categorized as severe (<0.01 IU/mL), moderate (0.01–0.05 IU/mL), or mild (>0.05–0.30 IU/mL). Patients were followed regularly with at least two visits per year or in case of bleeding emergency. For severe events, patients had to go to the HTC where they received replacement therapy. These patients were treated either by on-demand treatment, continuous low-dose prophylaxis (25 IU/kg/week for HA and 30 IU/kg/ week for HB), or intermittent prophylaxis.
All bleeding episodes were mentioned in the medical records and/or in the patient’s notebook, which the physician could consult at each visit. All these data were also recorded in the World Federation of Hemophilia’s World Bleeding Disorder Register (WBDR) since 2019 [Citation9].
LTB was defined as a severe bleed that requires immediate treatment and medical attention due to the high risk of rapid death. Diagnosis of such bleeding was suspected based on clinical symptoms (neurological, abdominal … .), confirmed by medical imaging evaluation if required. Data of these severe bleeding were extracted from patient diary and medical records.
For LTB, clotting factor concentrate (CFC) VIII or IX replacement therapy protocols were established with a target factor level of 100% during all the high risk of death period, and dose regression was made accordingly.
The incidence, risk factors, location of bleeds, management strategies, and outcome were evaluated. Risk factors for morbidity and mortality of LTB were assessed.
Results
The cohort was constituted by 241 individuals with hemophilia A (87.9%) and 33 with hemophilia B (12.1%). The mean age of patients was 16.45 years and the median age was 13 years. A majority of patients resided in Dakar (capital of Senegal). A family history of hemophilia was found in 151 patients. Consanguinity was observed in 9.5% of patients. The mean annual bleeding rate (ABR) was 1.65 (2.83 for severe form, 1.54 for moderate, and 1.22 for mild form).
Replacement therapy with CFC was administered to 217 patients (79.2%); 56 patients (20.4%) received low dose prophylactic treatment with 32 intermittent and 24 continuous prophylaxis. The prevalence of inhibitors was 4.7% (13/274). All patients were HIV and HCV antibody negative ().
Table 1. General characteristics of study population.
Over a period of 10 years, we observed 31 cases of severe bleeding in 22 individuals, i.e. incidence of 8.03%. These 22 patients were distributed as follows: 17 severe forms (77.3%), 5 moderate forms (22.7%), and no mild form, and their mean age was 15.6 years (4 months–37 years). The prevalence of LTB was 11.8% (19/161) among patients on demand treatment versus 5.3% (3/56) among patients under low-dose prophylaxis treatment.
CNS bleeds were most frequent and were estimated at 6.2% and accounted for 54.8% of all LTB in our cohort. The delay between the first signs and the emergency room visit was 78.9 hours. All patients were negative for HIV and HCV serology, and only one patient was positive for inhibitor screening with low titer.
The correlation between the occurrence of LTB and type and severity of hemophilia, the bleeding circumstances, and the bleeding sites are represented in . Ten episodes of recurrent bleeds were observed in five patients. Of these, three patients had hematemesis of great abundance in ulcer sites and two other intra-abdominal bleeding.
Table 2. Type of hemophilia, bleeding circumstances and bleeding sites for patients with life-threatening bleeding.
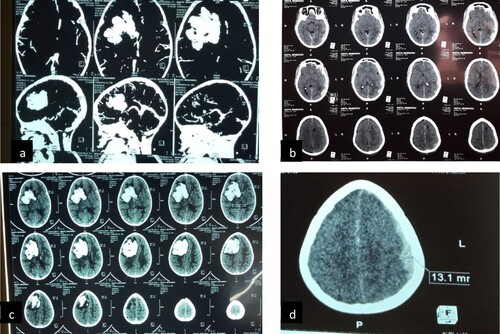
We did not note any recurrences of intracranial bleeding. Imagery was performed in 10 patients. They were digestive endoscopy in three patients, cerebral scan in three patients (), cerebral ultrasound in one patient, spinal scan in one patient, abdominal scan in one patient, and muscular ultrasound in one patient.
Figure 1. Cerebral hematoma. (a and c) subacute hemorrhagic stroke with falcorial engagement in an 11-month-old infant; (b) left hemispherical multiple acute extradural hematoma associated with hyperdensities of the subarachnoid spaces; (d) left hemispheric acute subdural hematoma.
Strategies management and outcomes are presented in . Sixteen of the 22 patients received emergency CFC and six were not treated. Three patients with CNS bleeds received physiotherapy with an average duration of 7 months (4–12). .
Table 3. Management strategies and outcomes for life-threatening bleeding.
The mortality rate of LTB in the cohort was 0.32 deaths per 100 person-years (9 deaths/22). The causes of death were mainly cerebral hemorrhage, intra-abdominal hemorrhage, and septic shock in the context of compressive hemorrhage. CNS bleeds represented 77.7% of the total cause of death (). Among the survivors, 11 patients were in complete remission and two patients had sequelae (a ptosis of the right eye for one patient and a steppage for the other patient). For secondary prevention, four patients were put on low-dose prophylaxis for an average of 4.7 months (3–6) at a rate of 15 U/Kg twice a week, and six patients were on prophylaxis with Emicizumab.
Table 4. Characteristics of deceased patients.
The time from diagnosis to death averaged 23.7 hours (1 hour–5 days). We observed a significant correlation between late CFCs treatment (after 2 hours) and mortality (p = .014).
Discussion
We identified 31 episodes of LTB in a cohort of 274 patients over 10 years of follow-up, occurring mainly on patients with severe form and under on demand treatment.
This prevalence is significantly lower in rich countries where around 10–15% of patients with severe hemophilia tend to have rare/mild types of bleeding [Citation8]. Unlike, in resource-limited countries, particularly in Africa, that represents less than 3% of patients identified as having hemophilia, and only 2% of those use CFCs for treatment, LTB remains a real challenge with a still high incidence [Citation10].
Prophylaxis is now the gold standard of Hemophilia treatment and the use of extended half-life products especially in the context of low-dose prophylaxis, has contributed to a reduction in the number of LTB events, mortality, and improvement in patients’ quality of life [Citation10].
In developed countries, with the era of prophylaxis, LTB is no longer a frequent threat compared to the new challenges and risks for these patients, such as the recrudescence of other non-communicable diseases related to the increase of their life expectancy [Citation11].
CNS hemorrhage is one of the most common causes of mortality among hemophiliacs patients and its incidence in this study was 6.2%. This incidence of CNS bleeds is in a range of what was reported in the literature ranging from 2% to 7.8%, and occurring mainly in countries where access to diagnosis and treatment is more limited [Citation6].
Moderate trauma is the most frequent cause of CNS bleeds in our cohort. The possibility of CNS bleeds should not be excluded neither on the basis of the absence of head trauma history nor on the absence of signs. The literature suggests that all hemophiliacs patients with minor or moderate head trauma should receive early replacement therapy, in order to minimize the risk of bleed, and an urgent CT scan is needed only in the presence of symptoms [Citation2,Citation12,Citation13]. Persistent and prolonged headache, even without neurological symptoms or signs, should be investigated because it could be related to CNS bleed [Citation2].
Ten recurrent episodes were observed in 5 PWH, 80% of them within one year after the initial episode. This can be explained by the fact that two of them had ulcer disease and one presented low titer inhibitors.
The two youngest patients in our cohort had less than 2 years, presented CNS bleeds and were moderate hemophilia. In the pediatric population, CNS bleeds are more frequently observed in children aged 2 years or under with severe hemophilia and who are not yet on prophylaxis [Citation14]. The protective role of early treatment with coagulation factor concentrate has been recently reported [Citation15] and confirmed the recommendations from international guidelines on hemophilia management [Citation2].
Concerning mortality, this study shows a rate of 0.32 deaths per 100 person-years, and CNS bleeds were the main cause of death (77%) of patients who presented LTB. Six out of nine patients who died were unable to receive factor supplementation in an emergency situation.
Finally, the most important aspect of the treatment of LTB in PWH patients is the prompt replacement of coagulation factor, which raises the plasma level to about 100% and especially a multidisciplinary team approach [Citation16–20].
However, the etiological diagnosis and management of severe bleeding is a challenge for African countries [Citation21]. The difficulty resides in the fact that patients living in the countryside have very limited access to sufficient clotting factors and specialized medical care [Citation10]. That is why it is very important to have an increased awareness among stakeholders (governments, physicians, patients), about the need of available and affordable CFC in African countries.
Conclusion
LTB is a serious complication in hemophilia if not managed early. It is a fatal hemorrhage with a high morbidity and mortality. CFC substitution is needed as soon as the diagnosis is suspected to lower the risk of morbidity and mortality. A good awareness of patients and their families would further reduce this incidence.
Acknowledgments
The authors would like to thank the WFH for humanitarian donations of Clotting factors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males. Ann Intern Med. 2019;171:540–546.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020 Aug;26(Suppl 6):1–158.
- Hassan S, Monahan RC, Mauser-Bunschoten EP, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001-2018. J Thromb Haemost. 2021 Mar;19(3):645–653.
- Mahlangu J, Cerquiera M, Srivastava A. Emerging therapies for haemophilia - global perspective. Haemophilia. 2018;24(Suppl. 6):15–21.
- Aronstam A, Rainsford SG, Painter MJ. Patterns of bleeding in adolescents with severe haemophilia A. Br Med J. 1979;1:469–470.
- Zanon E, Pasca S. Intracranial haemorrhage in children and adults with haemophilia A and B: a literature review of the last 20 years. Blood Transfus Trasfus Sangue. 2019;17:378–384.
- Cho JY, Lee WS, Park YS, et al. Clinical characteristics and prognostic factors in hemophiliacs with Intracranial Hemorrhage: A single-center, retrospective experience. Indian J Hematol Blood Transfus. 2016;32:488–493.
- Hay CRM, Nissen F, Pipe SW. Mortality in congenital hemophilia A - a systematic literature review. J Thromb Haemost. 2021 Jan;19(Suppl 1):6–20.
- Toure SA, Youttananukorn T, Seck M, et al. Improving data collection in hemophilia patients in Senegal. Haemophilia. 2021;27(Suppl. 2):18–181. DOI: 10.1111/hae.14236. ABS 129, p90, https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/hae.14236?download = true.
- Mbanya DN, Diop S, Ndoumba Mintya AN, et al. Hemophilia care in Africa: Status and challenges. Transfus Clin Biol. 2021 May;28(2):158–162.
- Skinner MW, Nugent D, Wilton P, et al. Achieving the Unimaginable: Health Equity in Haemophilia. Haemophilia. 2020;26(1):17–24. 2.
- Witmer CM, Manno CS, Butler RB, et al. The clinical management of hemophilia and head trauma: a survey of current clinical practice among pediatric hematology/oncology physicians. Pediatr Blood Cancer. 2009;53:406–410.
- Antunes SV, Vicari P, Cavalheiro S, et al. Intracranial haemorrhage among a population of haemophilic patients in Brazil. Haemophilia. 2003;9:573–577.
- Traivaree C, Blanchette V, Armstrong D, et al. Intracranial bleeding in haemophilia beyond the neonatal period–the role of CT imaging in suspected intracranial bleeding. Haemophilia. 2007;13:552–559.
- Andersson NG, Auerswald G, Barnes C, et al. Intracranial haemorrhage in children and adolescents with severe haemophilia A or B - the impact of prophylactic treatment. Br J Haematol. 2017;179:298–307.
- Stieltjes N, Calvez T, Demiguel V, et al. Intracranial haemorrhages in French haemophilia patients (1991-2001): clinical presentation, management and prognosis factors for death. Haemophilia. 2005;11:452–458.
- Chalmers EA, Alamelu J, Collins PW, et al. Intracranial haemorrhage in children with inherited bleeding disorders in the UK 2003-2015: A national cohort study. Haemophilia. 2018;24:641–647.
- Haque Q, Abuduaini Y, Li H, et al. Intracranial Hemorrhage in children with inherited bleeding disorders: A Single Center study in China. J Pediatr Hematol Oncol. 2019;41:207–209.
- Aras M, Oral S. Management of intracranial hemorrhage in hemophilia A patients. Childs Nerv Syst. 2020;36:2041–2046.
- Hegde A, Nair R, Upadhyaya S. Spontaneous intracerebral hemorrhage in hemophiliacs – A treatment dilemma. Int J Surg Case Rep. 2016;29:17–19.
- Diop S, Seck M, Sy-Bah D, et al. Implementing haemophilia care in Senegal, West Africa. Haemophilia. 2014 Jan;20(1):73–77.
