ABSTRACT
Objectives:
Chemotherapy, including bendamustine, usually causes lymphocytopaenia and hypogammaglobulinaemia as side effects in patients with haematological malignancies. Therefore, the possibility has been considered that these immunological adverse events induced by bendamustine may lead to infectious diseases. However, lymphocytopaenia and/or hypogammaglobulinaemia have not yet been shown to have a statistically significant association with infection in cancer patients who receive bendamustine.
Methods:
We retrospectively studied 27 patients with relapsed or refractory indolent follicular lymphoma who were treated with bendamustine and rituximab (BR). In order to elucidate relationships between immune-related laboratory parameters (i.e. peripheral blood leukocyte, neutrophil, lymphocyte and immunoglobulin G [IgG]) and infectious events, receiver operating characteristic (ROC) curve and multivariate logistic regression analyses were performed.
Results:
Infectious diseases occurred in 11 patients (11/27, 41%), including 3 (3/27, 11%) with severe diseases. The area under the ROC curve (AUC) showed that the lowest IgG level during and after BR discriminated infectious events (cut-off value, 603 mg/dL) with 81.8% sensitivity and 68.8% specificity (AUC, 0.76; 95% CI, 0.52-0.90). Furthermore, a multivariate regression analysis revealed that the minimal serum IgG value during and after BR therapy was the only variable that was significantly associated with infection (odds ratio, 8.29; 95% CI, 1.19-57.62; p value, 0.03).
Conclusion:
Serum IgG ≤603 mg/dL during and after BR therapy was independently associated with an increased risk of infection. The monitoring of serum IgG during chemotherapy may help to predict the development of infection in blood cancer patients undergoing chemotherapy with bendamustine in combination with rituximab.
Introduction
Bendamustine, a unique cytotoxic chemotherapeutic drug with similar structures to both alkylating agents and purine analogues [Citation1,Citation2], was first developed in the former German Democratic Republic in 1963 and has increasingly been used worldwide for the treatment of patients with lymphoid malignancies [Citation3,Citation4]. Recent randomized clinical trials have demonstrated that chemotherapy including bendamustine is superior to standard treatment for patients who are newly diagnosed with indolent non-Hodgkin lymphoma (NHL) [Citation5,Citation6] and those with relapsed or refractory indolent NHL [Citation7]. Hence, bendamustine has become the first choice of treatment for indolent B-cell lymphomas [Citation4].
While beneficial to patient survival, chemotherapy with bendamustine generally causes lymphocytopaenia and/or hypogammaglobulinaemia as side effects during treatment, leading to impaired cellular and/or humoral immunity in some patients [Citation8]. In addition, patients treated with bendamustine-containing chemotherapy sometimes suffer from infection [Citation4,Citation8]. According to reports on the clinical trials of bendamustine in patients with lymphoid tumours, 6-68% of subjects developed infectious adverse events [Citation5,Citation7,Citation9–15]. A recent retrospective study of a large cohort of indolent NHL patients reported that bendamustine was significantly associated with an increased risk of both common and opportunistic infections [Citation16]. This report also found a larger number of previous treatments to be an independent risk factor for infection [Citation16], which may imply that relapsed or refractory NHL patients receiving bendamustine are susceptible to infection. Based on these previously reported findings on adverse events, the possibility has been considered that lymphocytopaenia and/or hypogammaglobulinaemia induced by bendamustine lead to the development of infectious diseases [Citation17–20]. However, to our knowledge, no direct link between lymphocytopaenia and/or hypogammaglobulinaemia and infection has been demonstrated in cancer patients undergoing chemotherapy with bendamustine.
In the present cross-sectional study, we examined the accuracy of immunological laboratory parameters in the diagnosis of infectious events in lymphoma patients treated with bendamustine plus rituximab using receiver operating characteristic (ROC) curve. Furthermore, a multivariate logistic regression analysis was conducted in order to investigate whether or not a clinical laboratory parameter that was found to have an optimal cut-off value for discriminating infectious diseases by an ROC analysis could be independently associated with the development of infection.
Materials and methods
Patients
Twenty-seven consecutive patients diagnosed with relapsed or refractory follicular lymphoma (grade 1, 2 or 3A), who were treated in the Department of Hematology, NHO Kyushu Cancer Center from 2011 to 2017, were enrolled in this study. The disease subtype of each tumour was determined according to the WHO classification [Citation21]. Patients received 1–6 cycles of bendamustine in combination with rituximab (BR) [Citation7]. None of the patients had previously been treated with bendamustine. Ethical approval was obtained from the IRB of NHO Kyushu Cancer Center. Written informed consent for the study was obtained from each patient.
Data collection
The following patient characteristics were collected before BR therapy: age, sex, Eastern Cooperative Oncology Group performance status [Citation22,Citation23], underlying diseases, subtype [Citation21], Ann Arbor stage [Citation24,Citation25], clinical laboratory test results, Follicular Lymphoma International Prognostic Index (FLIPI) [Citation26], the number of previous lines of treatment and the number of prior chemotherapy regimens including rituximab (). Data regarding chemotherapy, such as the number of BR therapy cycles administered to each patient, the dose of bendamustine in each cycle and the date of the commencement of each cycle, were also obtained. The medical record of each infected patient was reviewed to confirm information, particularly information on infection, including the disease name, the causative microorganism, the severity graded according to the Common Terminology Criteria for Adverse Event (CTCAE) version 5.0 [Citation27] and the date on which the infectious disease occurred. Blood tests of laboratory parameters that represent the immune system function, namely the peripheral blood leukocyte count, neutrophil count, lymphocyte count and immunoglobulin G (IgG) level, were conducted every four weeks when chemotherapy was administered or follow-up examinations were performed. In addition, the examination was repeated at the physician’s discretion, depending on the patient’s bone marrow function, the occurrence of infection or other adverse events. Regarding the statistical analyses mentioned below, we used laboratory data on these parameters and clinical information on infectious events, both of which were collected from the initiation of BR therapy, either until 6 months after the last BR cycle or until the commencement of the next chemotherapy regimen if the treatment was started within 6 months after the completion or discontinuation of BR therapy. Based on the hypothesis that the lowest level of each immunological laboratory parameter during and after BR therapy may potentially reflect the impairment of the immune function induced by chemotherapy, we focused especially on the minimal immune-related laboratory values after the commencement of the treatment.
Table 1. Patient characteristics.
Statistical analyses
Clinical characteristics were compared between patients with and without infectious events by the chi-squared test for categorical variables and the Wilcoxon rank sum test for continuous variables. An ROC analysis was performed to judge the ability of immunological parameters to discriminate infectious events; a parameter was defined as an effective discriminator when the lower limit of the 95% confidence interval (CI) of the area under the ROC curve (AUC) was >0.5 [Citation28]. The maximum Youden index was applied to the ROC curve in order to determine the optimal cut-off value for each parameter for discriminating events [Citation29,Citation30]. The odds ratios (ORs) of clinicopathological and immunological variables for infection were examined by a logistic regression analysis. All immune-related variables were converted to binary variables using cut-off values that were identified by the ROC analysis, and then were applied to the analysis. When a variable proved to have a significant OR in the univariate analysis, the variable was included in a multivariate logistic regression analysis to identify factors independently associated with infection. Two-sided p values of <0.05 were considered to indicate statistical significance. The analyses were performed using JMP version 13 and SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Immune-related laboratory parameters and infection in lymphoma patients receiving BR therapy
The clinicopathological characteristics of the patients (male, n = 9; female, n = 18; median age at treatment, 68 years [range, 53-86]) are shown in . The majority of patients had systemic lymphoma (stage III, 42%; stage IV, 42%). FLIPI was judged to be high risk in 52% (14/27) of patients. The median number of previous chemotherapy lines and prior rituximab-based chemotherapeutic regimens were two and one, respectively.
Patients received a median of 4 cycles (range, 1-6) of BR therapy [Citation7] (). The relative dose intensity (RDI) [Citation31,Citation32] of bendamustine ranged from 0.40–1.00, with a median of 0.90 (). All patients received prophylactic treatment with sulfamethoxazole/trimethoprim and acyclovir throughout chemotherapy. Nine of twenty-seven (33%) patients were treated with granulocyte colony-stimulating factor (G-CSF) for neutropaenia. Intravenous immunoglobulin (IVIG) was administered to three patients with hypogammaglobulinaemia (3/27, 11%) as immunoglobulin replacement therapy during and/or after BR (). Of the three patients, two received IVIG at regular intervals due to extremely severe hypogammaglobulinaemia (patient code FL03) and a history of disseminated herpes zoster (patient code FL15) ().
Figure 1. Serum immunoglobulin G and infectious diseases during the clinical course in three patients treated with immunoglobulin replacement therapy. Results representative of the events are shown as follows: red bar, immunoglobulin G (IgG) level; blue bar, a period of an infectious disease; grey box, one cycle of bendamustine and rituximab (BR) therapy; black arrow, one dose of intravenous immunoglobulin (IVIG). The patient code (FL03, FL15 and FL20) is indicated on the left side.
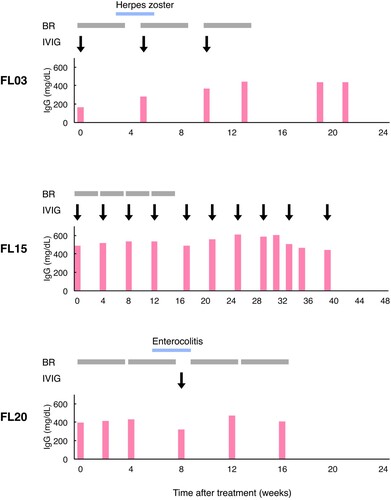
Before the commencement of BR therapy, the median baseline measurements of four laboratory parameters related to the immune system function, that is the leukocyte count, neutrophil count, lymphocyte count and IgG level, were 4640/μL (range, 1020-8950), 2730/μL (range, 1118-6623), 898/μL (range, 315-2437) and 808 mg/dL (range, 168-2137), respectively. In contrast, the median minimal leukocyte, neutrophil, lymphocyte and IgG values during and after BR were 1850/μL (range, 490-3910), 751/μL (range, 55-2424), 134/μL (range, 23-346) and 603 mg/dL (range, 203-1598), respectively. Grade 4 neutropaenia occurred in 37% (10/27) of the patients. The lowest IgG levels of the patients receiving IVIG ranged from 280 to 490 mg/dL ().
Infectious events were observed in 41% (11/27) of the patients (). Bacterial and viral infections occurred in 7 (26%) and 3 (11%) patients, respectively. One patient (4%) developed pulmonary aspergillosis, a fungal infection. Three (11%) patients had grade ≥3 infectious events, all of which were bacterial infections (). Of the 3 patients treated with immunoglobulin replacement therapy, 2 suffered infectious diseases (). Specifically, one patient (patient code FL03) developed herpes zoster during the first cycle of BR therapy despite commencing the regular administration of IVIG for pre-existing extremely severe hypogammaglobulinaemia (). The other patient (patient code FL20) suffered from enterocolitis after undergoing the second cycle of BR and was treated with oral medicines, G-CSF and IVIG (). Nine patients developed infections during BR therapy, whereas two developed infections after the completion of BR therapy (). No significant differences in clinicopathological features were noted between patients with and without infection (). Patients who developed infectious diseases tended to have diabetes mellitus as an underlying disease (p = 0.05) and higher soluble interleukin-2 receptor levels (p = 0.07) ().
Table 2. Infection occurring in relapsed or refractory follicular lymphoma patients treated with chemotherapy with bendamustine.
Table 3. Comparison of clinicopathological characteristics between infected and uninfected lymphoma patients.
We examined the incidence of the minimal immune-related laboratory values and infectious events in the clinical course (Supplementary Figure 1). With respect to lymphocytes, the minimal values were most frequently detected at 4 weeks after the commencement of BR therapy (10/27 patients, 37%); the incidence then continued to gradually decline until 28 weeks after treatment. In contrast to the lymphocyte count, the minimal IgG levels were widely distributed in the 32 weeks after the initiation of BR, except for one event occurring in the 60th week of treatment. Seven infectious events occurred within eight weeks after the initiation of BR, and thereafter the cumulative number of infections gradually increased up to 11 by the 48th week.
Serum IgG has an independent association with infection in patients treated with chemotherapy including bendamustine
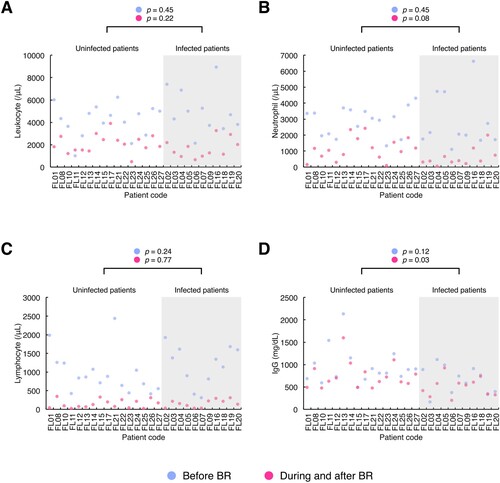
The clinical laboratory values of the immunological parameters (i.e. peripheral blood leukocyte count, neutrophil count, lymphocyte count and IgG level) were compared between the infected and uninfected patient groups using dot plots (). No significant differences in the median leukocyte or lymphocyte counts were found between the patient groups ((A, C)). Patients who developed infection tended to have lower neutrophil counts during and after BR therapy (infected patient group: median, 388 (range, 55-2008); uninfected patient group: median, 1109 (range, 88-2424); p = 0.08) ((B)). The serum IgG level before the initiation of BR therapy in patients with and without infection did not differ to a statistically significant extent, whereas the median minimal IgG value measured during and after BR was significantly lower in the infected patient group (infected patient group: median, 540 (range, 203-922); uninfected patient group: median, 663 (range, 473-1598); p = 0.03) ((D)).
Figure 2. Laboratory parameters reflecting the immune system function in patients with and without infection before, during and after chemotherapy with the combination of bendamustine and rituximab. The results of routine laboratory tests of immune-related parameters, i.e. peripheral blood leukocyte count, neutrophil count, lymphocyte count and immunoglobulin G (IgG) value, were collected from the initiation of bendamustine and rituximab (BR) either until 6 months after the completion or discontinuation of BR or until the commencement of the subsequent chemotherapy regimen if the treatment started within 6 months after the end of BR therapy. Patients were divided into two groups, i.e. infected and uninfected patient groups (grey and white areas in the graphs, respectively). The dot plots of each parameter ((A), leukocyte count; (B), neutrophil count; (C), lymphocyte count; (D), IgG level) were drawn with the baseline measurements before BR and the minimal measurements during and after BR therapy per patient (blue dot, the baseline measurement; red dot, the minimal measurement). The X-axis shows the patient code, while the y-axis shows the value of each laboratory parameter. Differences between the patient groups were analysed using Wilcoxon rank sum test and indicated above the graphs. Before BR, the baseline value before the commencement of BR therapy; During and after BR, the minimal value obtained from the initiation of BR therapy until either 6 months after the last cycle of BR or the commencement of the next chemotherapy regimen if the treatment was started within 6 months after the completion or discontinuation of BR therapy; IgG, immunoglobulin G.
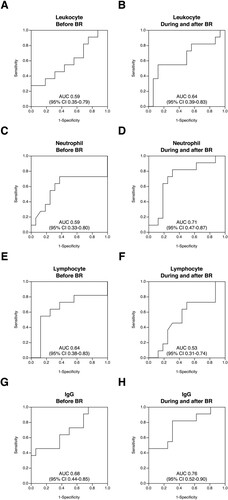
We performed an ROC analysis to assess the performance of the four immune-related laboratory parameters in discriminating infectious events. The ROC curves for the diagnostic accuracy of each parameter are shown in . The ROC curve analysis revealed that the minimal IgG value during and after BR therapy effectively discriminated infectious events (cut-off value, 603 mg/dL; sensitivity, 81.8%; specificity, 68.8%; AUC, 0.76 [95% CI, 0.52-0.90]) ((H)). No other parameters showed the lower limit of the 95% CI of AUC over 0.50 ((A-G)).
Figure 3. Receiver operating characteristic curves of immunological laboratory parameters for infection before, during and after BR therapy. The receiver operating characteristic (ROC) analysis was performed to assess the diagnostic accuracy of the leukocyte count, neutrophil count, lymphocyte count and immunoglobulin G (IgG) level for the occurrence of infection, with both the baseline and minimal values of each parameter. When the lower limit of the 95% confidence interval of the area under the ROC curve was >0.5, the parameter was considered to be effective for discriminating infectious events. The curves of each parameter before ((A), leukocyte count; (C), neutrophil count; (E), lymphocyte count; (G), IgG level), during and after ((B), leukocyte count; (D), neutrophil count; (F), lymphocyte count; (H), IgG level) BR therapy are shown. AUC, area under the curve; Before BR, before the commencement of BR therapy; CI, confidence interval; During and after BR, from the initiation of BR therapy until either 6 months after the last cycle of BR or the commencement of the subsequent chemotherapy regimen if the treatment was started within 6 months after the completion or discontinuation of BR therapy; IgG, immunoglobulin G.
We next performed a logistic regression analysis in order to investigate whether or not the lowest IgG level during and after BR therapy that was identified by the ROC analysis was an independent predictor of infection (). A univariate analysis of the factors potentially associated with infectious events revealed that when the cases were dichotomized using a cut-off value of IgG 603, there was a significant association between the minimal serum IgG value and infection (OR, 9.90; 95% CI, 1.54-63.67; p = 0.02) (). Preexisting diabetes tended to be associated with infection in the univariate analysis (p = 0.08). Other variables, e.g. the number of prior chemotherapeutic regimens, the number of previous treatments including rituximab and the RDI of bendamustine, showed no significant association with infection (). Furthermore, we conducted a multivariate regression analysis that included variables which tended to show an association with infection (p <0.10) based on the findings of a univariate analysis. A multivariate analysis revealed that the lowest IgG level of ≤603 mg/dL during and after BR was the only variable to show a significant association with the development of infection (OR, 8.29; 95% CI, 1.19-57.62; p = 0.03) (). These results thus showed that the minimal IgG level during and after BR therapy was independently associated with an increased risk of infection, which may suggest the possibility that the minimal IgG value after commencing treatment helps to predict the development of infection in blood cancer patients who are treated with chemotherapy including bendamustine.
Table 4. Associations between clinicopathological and immunological variables and infection in follicular lymphoma patients treated with bendamustine and rituximab.
Discussion
In this single centre study of relapsed or refractory indolent follicular lymphoma patients treated with BR therapy, 41% of patients developed infection, with 11% developing severe, grade ≥3 infectious disease. The median minimal IgG value during and after BR was significantly lower in patients suffering from infection. Furthermore, using an ROC curve analysis, we identified the cut-off value of the lowest serum IgG level of ≤603 mg/dL that discriminated infectious disease. This value was found to show an independent association with infection according to a multivariate logistic regression analysis.
Thus far, decreases in immune-related laboratory parameters during and/or after chemotherapy with bendamustine have been reported in the literature, and findings from some previous studies were similar to our results. Regarding serum IgG, which is a routine laboratory parameter that reflects the humoral immune system, a few previous retrospective studies appear to have presented relatively similar results to our data. For example, Ito et al. reported that the median serum IgG level at the end of BR therapy was 581 mg/dL in relapsed or refractory NHL patients [Citation18]. Gafter-Gvili and colleagues reported that the serum IgG level was <500 mg/dL in 40% of patients at the end of follow-up after BR therapy [Citation33]. Our results in relation to neutropaenia, which is one of the most widely acknowledged side effects of chemotherapy with bendamustine [Citation34], were also somewhat in line with previous studies [Citation20,Citation33]. On the other hand, the median lowest lymphocyte count in the present study (134/μL) was lower in comparison to previous reports. Specifically, one study showed that the median lowest lymphocyte count was 365/μL [Citation19], and another reported that grade 3 or 4 lymphocytopaenia developed in 51% of patients [Citation33]. However, some research groups have described infectious diseases that are related to a decreased lymphocyte count (i.e. cellular immunodeficiency), such as herpes zoster and cytomegalovirus colitis [Citation19,Citation35,Citation36], some of which also occurred in this study (). Despite these similarities between previous reports and the present study, we revealed—for the first time—a statistically significant association between a routinely measured laboratory parameter, serum IgG, and infection in cancer patients treated with chemotherapy that included bendamustine.
The present study seems to have had several advantages in clarifying the connection between impaired immunity and the occurrence of infection. Firstly, we collected and analysed immunological laboratory parameters that were examined at arbitrary points during and after BR therapy. In contrast, the majority of previous studies, in which decreased serum IgG levels and/or a decreased lymphocyte count were not reported to show statistically significant associations with infection, measured the parameters at uniform time points [Citation5,Citation7,Citation17,Citation18,Citation33,Citation35–39]. When the approach used in these previous studies is applied to measurement, values that are appropriate for inclusion in the analysis are occasionally missed; thus, such results can reduce the diagnostic accuracy of the laboratory values with respect to the patient’s actual immune function. In some of our patients, extra laboratory measurements, in addition to regular testing, were performed at the physician’s discretion just before or just after the onset of an infectious disease. Thus, it is likely that this testing approach contributed to obtaining laboratory values that more appropriately reflected the immune function. Next, the optimal cut-off value of the minimal IgG during and after BR therapy for discriminating the occurrence of infection was determined by an ROC analysis ((H)), and this value was shown to be independently associated with an increased risk of infection in a logistic regression analysis (). These results suggest the possibility that using the lowest level of each immune-related laboratory parameter in the clinical course was beneficial for evaluating optimal cut-off values for predicting infection, in contrast with previous studies [Citation18,Citation33]. In other words, the minimal laboratory values measured during and after BR therapy may indeed have reflected the impaired immune function, as we expected (see Materials and methods). Finally, an independent association between the lowest serum IgG and infectious events was identified despite the relatively small number of patients in the present study. Excluding heterogeneity in the lymphoma subtypes, disease status and treatment regimen may have helped to overcome the paucity of subjects and events in our study.
In contrast to serum IgG, neutropaenia was not identified as a significant discriminator of infection in the ROC curve analysis, even though severe neutropaenia was found in some of our patients ((B), (D)). This result may be partly due to our patients having developed infectious diseases that were mainly related to impaired humoral immunity, which are reflected by decreased IgG levels. Additionally, G-CSF was administered to approximately 30% of patients during BR therapy in this study (see Results). The prophylactic use of G-CSF plays an important role in preventing or weakening infections, e.g. febrile neutropaenia and bacteraemia, which are usually caused by neutropaenia due to chemotherapy [Citation40–44]. These biological effects of G-CSF may have modified the association between the lowest neutrophil count and infection in our present study.
Infection does occur in patients undergoing chemotherapy with bendamustine [Citation4,Citation8,Citation16]. Thus, our results seem to shed light on the utility of monitoring serum IgG levels during chemotherapy with bendamustine because such monitoring is likely to contribute to predicting the optimal timing of prophylaxis or treatment of infection. On the other hand, the management of infection in lymphoma patients treated with bendamustine has been controversial. In a recent study on risk factors for infectious complications in patients receiving bendamustine and anti-CD20 monoclonal antibody therapy, the authors indicated that the relatively low incidence of infection was attributed to the prophylactic use of antimicrobial drugs or G-CSF [Citation20]. In addition, a review of phase II and III clinical trials for bendamustine in indolent NHL patients mentioned that infectious events were manageable without much difficulty [Citation34]. By contrast, some groups have shown considerable infection rates and various infection types in patients treated with bendamustine, and thus discussed the importance of establishing prophylaxis against infection [Citation16,Citation33,Citation45]. In the present study, despite the prophylactic administration of antimicrobial agents and/or G-CSF as mentioned above, 41% (11/27) of patients suffered from infectious diseases, which was a similar result to infection rates reported in some previous randomized trials [Citation9,Citation12,Citation15]. Even more unfortunately, once a patient developed infection, the physician usually delayed treatment or reduced the drug dose. It should be noted that these modifications in treatment weaken the dose intensity of chemotherapy, and sometimes ultimately affect patient survival [Citation46–48]. Thus, given the association between serum IgG and infection in this study, careful observation through the monitoring of serum IgG levels during and after treatment will help to predict the occurrence of infectious diseases, control infection and finally lead to improved survival in patients with lymphoid malignancies who receive bendamustine.
On the other hand, there has been no standard and/or optimal treatment for hypogammaglobulinaemia due to chemotherapy and/or disease itself, i.e. secondary immunodeficiency, in patients with haematological malignancies [Citation49]. The prophylactic and therapeutic effects of immunoglobulin replacement therapy in relation to infection could not be discussed in the present study because of the cross-sectional design and the paucity of events (). Further studies therefore should be warranted to elucidate whether or not immunoglobulin replacement therapy, such as IVIG, is effective for treating infection and whether it therefore contributes to better outcomes in lymphoma patients treated with bendamustine.
The present study was associated with some limitations. First, the small number of subjects limited the number of events, thus possibly preventing a sufficient evaluation of the association between immune-related laboratory parameters and specific subtypes of infection. For example, fungal infection, which is considered to mainly develop due to cellular immunodeficiency [Citation50–52], occurred in only one patient (). Thus, the possible association between decreased lymphocytes, which certainly reflect impaired cellular immunity, and infectious events, especially fungal diseases, appears to have been insufficiently analysed in this study. Second, the involvement of CD4-positive lymphocytes in infection could not be assessed because the CD4+ T cell count was not examined as a routine clinical test in our patients. CD4-positive T cells generally play a crucial role in cellular immunity [Citation53–55]. The capability of bendamustine to deplete lymphocytes, including CD4+ T cells, is well recognized [Citation35,Citation56,Citation57], suggesting susceptibility to infections in patients who are treated with bendamustine [Citation58]. According to the literature [Citation19,Citation56], the total lymphocyte count and CD4-positive T lymphocyte count appear to change in parallel during and after chemotherapy using bendamustine. In the present study, the total lymphocyte count was therefore substituted for the CD4+ T cell count in analyses, as data on the number of CD4-positive lymphocytes were unavailable. Indeed, the routine testing of lymphocyte subsets, as indicated by real-world data [Citation59], is not frequently conducted in the monitoring of the immune system status during treatment with bendamustine, depending on the clinician’s discretion. Further studies are therefore needed to clarify the optimal cut-off values of the specific subsets of lymphocytes for discriminating infection. Third, all patients in this study were treated with the combination of bendamustine and rituximab. Both agents have been reported to have the potential to affect humoral and/or cellular immunity [Citation18,Citation33,Citation45,Citation56,Citation60,Citation61]. In addition to bendamustine, rituximab may have led to modification of the humoral and/or cellular immunity in our patients. Finally, our findings, which were determined by a cross-sectional analysis, do not necessarily show causality between laboratory parameters and infection [Citation62,Citation63].
In conclusion, the lowest IgG level was independently associated with the development of infection in relapsed or refractory indolent follicular lymphoma patients who received BR therapy. The application of the lowest levels of immune-related laboratory parameters during and after treatment in the ROC analysis may have helped to show our findings. The monitoring of serum IgG throughout chemotherapy should be performed in order to predict infection in patients with haematological malignancies receiving chemotherapy with bendamustine. Needless to say, we should verify our hypothesis in a larger cohort in order to confirm and generalize the results of our study. These efforts will lead to truly personalized approaches for the more effective treatment of patients with blood cancer.
Ethical approval
Ethical approval for this study was obtained from the institutional review board of NHO Kyushu Cancer Center. All procedures performed in studies involving the patients were in accordance with the ethical standards of the governmental guidelines and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each individual participant.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplemental Material
Download PDF (607.5 KB)Acknowledgements
We are grateful to K. Yamaguchi for her helpful support in data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
AH collected all the clinical data, conducted all the statistical analyses and wrote the paper. TT, KH, TN, HU, HO, EN, YT and IC treated patients and commented on the results from a clinical point of view. KT commented on the results from a laboratory point of view. YS managed the study. KM conceived of and designed the study and wrote the paper.
Additional information
Funding
References
- Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. J Clin Oncol. 2009;27:1492–1501.
- Darwish M, Bond M, Hellriegel E, et al. Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites. Cancer Chemother Pharmacol. 2015;75:1143–1154.
- Kalaycio M. Bendamustine: a new look at an old drug. Cancer. 2009;115:473–479.
- Cheson BD, Brugger W, Damaj G, et al. Optimal use of bendamustine in hematologic disorders: treatment recommendations from an international consensus panel - an update. Leuk Lymphoma. 2016;57:766–782.
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210.
- Flinn IW, van der Jagt R, Kahl B, et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37:984–991.
- Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016;17:57–66.
- Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016;57:512–519.
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952.
- Herold M, Schulze A, Niederwieser D, et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin's lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19). J Cancer Res Clin Oncol. 2006;132:105–112.
- Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–4384.
- Niederle N, Megdenberg D, Balleisen L, et al. Bendamustine compared to fludarabine as second-line treatment in chronic lymphocytic leukemia. Ann Hematol. 2013;92:653–660.
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942.
- Michallet AS, Aktan M, Hiddemann W, et al. Rituximab plus bendamustine or chlorambucil for chronic lymphocytic leukemia: primary analysis of the randomized, open-label MABLE study. Haematologica. 2018;103:698–706.
- Pönisch W, Mitrou PS, Merkle K, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone–a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J Cancer Res Clin Oncol. 2006;132:205–212.
- Fung M, Jacobsen E, Freedman A, et al. Increased risk of infectious complications in older patients with indolent non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis. 2019;68:247–255.
- Muñoz R G, Izquierdo-Gil A, Muñoz A, et al. Lymphocyte recovery is impaired in patients with chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann Hematol. 2014;93:1879–1887.
- Ito K, Okamoto M, Ando M, et al. Influence of rituximab plus bendamustine chemotherapy on the immune system in patients with refractory or relapsed follicular lymphoma and mantle cell lymphoma. Leuk Lymphoma. 2015;56:1123–1125.
- Saito H, Maruyama D, Maeshima AM, et al. Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B-cell and mantle cell lymphoma. Blood Cancer J. 2015;5:e362.
- Sarlo KM, Dixon BN, Ni A, et al. Incidence of infectious complications with the combination of bendamustine and an anti-CD20 monoclonal antibody. Leuk Lymphoma. 2020;61:364–369.
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). Lyon: IARC; 2017.
- Zubrod CG, Schneiderman M, Frei EI, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860–1861.
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636.
- Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265.
- U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; published on 27 November 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 2021 31 March.
- Krzanowski WJ, Hand DJ. ROC curves for continuous data. New York: Chapman & Hall/CRC; 2009.
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35.
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577.
- Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–1937.
- Loibl S, Skacel T, Nekljudova V, et al. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer. 2011;11:131.
- Gafter-Gvili A, Ribakovsky E, Mizrahi N, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma. 2016;57:63–69.
- Brugger W, Ghielmini M. Bendamustine in indolent non-Hodgkin's lymphoma: a practice guide for patient management. Oncologist. 2013;18:954–964.
- Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31:2103–2109.
- Hasegawa T, Aisa Y, Shimazaki K, et al. Cytomegalovirus reactivation with bendamustine in patients with low-grade B-cell lymphoma. Ann Hematol. 2015;94:515–517.
- Hoy SM. Bendamustine: a review of its use in the management of chronic lymphocytic leukaemia, rituximab-refractory indolent non-Hodgkin's lymphoma and multiple myeloma. Drugs. 2012;72:1929–1950.
- Mondello P, Steiner N, Willenbacher W, et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with indolent non-Hodgkin's lymphoma: evidence from a multicenter, retrospective study. Ann Hematol. 2016;95:1107–1114.
- Tsutsumi Y, Ito S, Ohigashi H, et al. Sustained CD4 and CD8 lymphopenia after rituximab maintenance therapy following bendamustine and rituximab combination therapy for lymphoma. Leuk Lymphoma. 2015;56:3216–3218.
- Aapro MS, Bohlius J, Cameron DA, et al. Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2010;2011(47):8–32.
- Bennett CL, Djulbegovic B, Norris LB, et al. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368:1131–1139.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212.
- Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25:3295–3304.
- National Comprehensive Cancer Network. Hematopoietic Growth Factors Version 2.2021. In NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). <https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf>. Accessed 2021 31 March.
- Hosoda T, Yokoyama A, Yoneda M, et al. Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54:1327–1328.
- Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108.
- Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77:221–240.
- Havrilesky LJ, Reiner M, Morrow PK, et al. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203–210.
- Na IK, Buckland M, Agostini C, et al. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur J Haematol. 2019;102:447–456.
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23.
- Antachopoulos C. Invasive fungal infections in congenital immunodeficiencies. Clin Microbiol Infect. 2010;16:1335–1342.
- Kumaresan PR, da Silva TA, Kontoyiannis DP. Methods of controlling invasive fungal infections using CD8(+) T cells. Front Immunol. 2018;8:1939.
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569.
- Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148.
- Deeks SG, Overbaugh J, Phillips A, et al. HIV infection. Nat Rev Dis Primers. 2015;1:15035.
- Schöffski P, Seeland G, Engel H, et al. Weekly administration of bendamustine: a phase I study in patients with advanced progressive solid tumours. Ann Oncol. 2000;11:729–734.
- Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36:2395–2404.
- Medicines and Healthcare Products Regulatory Agency. Drug Safety Update Volume 10 Issue 11, July 2017: 2. <https://www.gov.uk/drug-safety-update/bendamustine-levact-increased-mortality-observed-in-recent-clinical-studies-in-off-label-use-monitor-for-opportunistic-infections-hepatitis-b-reactivation>. Accessed 2022 28 February.
- Martínez-Calle N, Hartley S, Ahearne M, et al. Kinetics of T-cell subset reconstitution following treatment with bendamustine and rituximab for low-grade lymphoproliferative disease: a population-based analysis. Br J Haematol. 2019;184:957–968.
- Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13:106–111.
- Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423.
- Hiebert R, Nordin M. Methodological aspects of outcomes research. Eur Spine J. 2006;15(Suppl 1):S4–S16.
- Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020;158:S65–S71.
