ABSTRACT
Objectives
The aim of this retrospective study was to evaluate the safety and efficacy of SEAM regimen followed by auto-SCT in lymphoma.
Patients and methods
We retrospectively reviewed the records of patients with lymphoma who underwent auto-SCT with SEAM conditioning regimen from January 2010 to June 2018 at our centre. In total, 97 patients were analysed.
Results
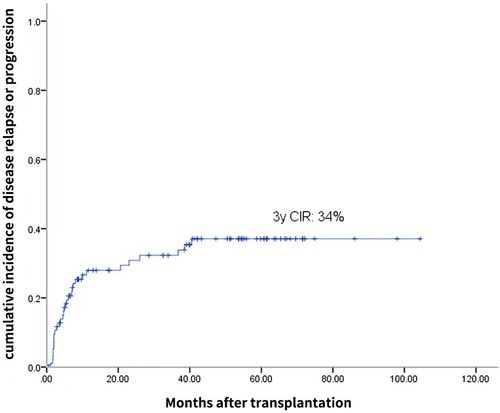
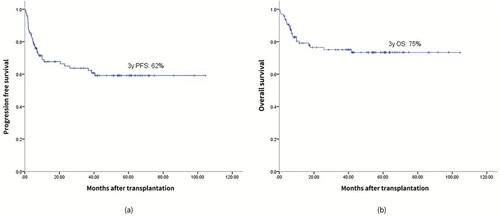
The median time to neutrophil engraftment and platelet engraftment was 9.5 days (range, 7–15 days) and 12 days (range, 7–25 days), respectively. Grade 3–4 nausea/vomiting, mucositis and diarrhoea were observed in 21.6%, 36.1%, and 11.3% of patients, respectively. Treatment-related mortality at 100 days occurred in 2 patients (2.1%). After a median follow-up time of 53.9 months, the 3-year incidence of disease relapse or progression was 34%. The estimated progression-free survival and overall survival at 3 years were 62% and 75%, respectively. Compared with previous studies using BEAM as the conditioning regimen, this study shows that the SEAM regimen has a comparable efficacy and safety profile.
Conclusions
The SEAM regimen is feasible and might be an ideal alternative to BEAM regimen for lymphoma auto-SCT.
Introduction
High-dose chemotherapy (HDC) followed by autologous stem cell transplantation (auto-SCT) is still the standard treatment for malignant lymphoma patients who are resistant to the initial treatment or relapse after the initial treatment [Citation1–3]. The conditioning regimens play a key role in auto-SCT. The conditioning regimens for auto-SCT in lymphoma can be divided into the following two different types: total body irradiation (TBI)-based regimens and chemotherapy-only regimens. Patients treated with TBI-based conditioning regimens reportedly have increased non-relapsed mortality (NRM) due to a higher incidence of second malignancies than patients treated with chemotherapy-based conditioning regimens [Citation4,Citation5]. Therefore, many centres have shifted from TBI-based regimens to chemotherapy-only regimens. In addition, the carmustine, etoposide, cytarabine, and melphalan (BEAM) regimen has been the most widely used chemotherapy-only conditioning regimen before auto-SCT in patients with Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) and is considered the gold standard due to its limited morbidity and toxicities and high efficacy [Citation6–8]. Unfortunately, carmustine (BCNU), one of the components of BEAM, is consistently in short supply. Consequently, BEAM or other BCNU-containing regimens are not available for all physicians; thus, identifying an alternative conditioning regimen is critical and urgent. Semustine (Me-CCNU), which is another oral nitrosourea agent, has been chosen as a potential substitute. Furthermore, it has been reported that conditioning regimens that include Me-CCNU are generally very effective and well tolerated in acute leukemia and multiple myeloma patients [Citation9,Citation10]. Hence, we used a modified regimen involving Me-CCNU, etoposide, cytarabine, and melphalan (SEAM) as a conditioning regimen for lymphoma auto-SCT in which BCNU was replaced with Me-CCNU. This study aimed to evaluate the safety and efficacy of the SEAM regimen followed by auto-SCT for malignant lymphoma patients.
Patients and methods
Patient selection
We retrospectively reviewed the records of patients with lymphoma who underwent auto-SCT with SEAM conditioning from January 2010 to June 2018 at our centre. The inclusion criteria were as follows: (i) patients aged 15–65 years; (ii) patients with adequate cardiac, hepatic, and renal function prior to transplantation; (iii) patients with an Eastern Cooperative Oncology Group (ECOG) performance status of <2; and (iv) patients from whom we obtained an adequate number of CD34-positive stem cells. The number of CD34-positive progenitor cells collected was required to be greater than 2 ×106/kg. Our study was approved by the Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. The patients provided written informed consent prior to participating in the study.
Conditioning regimen and supportive care
The SEAM regimen consisted of 250 mg/m2 Me-CCNU p.o. on day −8 (i.e. the 8th day before the stem cell infusion), 100 mg/m2 etoposide every 12 h i.v. on days −7 to −4 (total dose 800 mg/m2), 200 mg/m2 cytarabine every 12 h i.v. on days −7 to −4 (total dose 1600 mg/m2) and 140 mg/m2 melphalan i.v. on day −3. Granulocyte colony-stimulating factor (G-CSF) (5 µg/kg/d) was given from day 1 until the absolute neutrophil count (ANC) was >1×109/L on three consecutive days. Irradiated blood products were administered to maintain a hemoglobin level >60 g/L and platelet count (PLTc) >20×109/L. Prophylactic treatments against bacterial, fungal, viral, and pneumocystis infections were administered.
Response evaluation and definitions
Following auto-SCT, a response evaluation via positron emission tomography (PET)/computed tomography (CT) was performed at 3, 6, 12, and 24 months. The response to treatment was evaluated according to the standardized response criteria developed by the Revised International Working group for malignant lymphoma [Citation11]. The toxicity assessment was performed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Neutrophil and platelet engraftment were defined as the first day of three consecutive days with an ANC ≥0.5×109/L with no subsequent decline and a PLTc ≥20×109/L on three consecutive days without transfusion, respectively. Overall survival (OS) was calculated as the time from transplantation to death from any cause, with surviving patients censored at the last follow-up. Progression free survival (PFS) was calculated as the time from transplantation to disease relapse, progressive disease, or death from any cause, with surviving patients without disease progression censored at the last follow-up. Treatment-related mortality (TRM) was defined as death from any cause other than disease relapse or progression occurring within the first 100 days after auto-SCT.
Statistical analysis
The primary end points studied included the OS and PFS. The secondary end point was the incidence of disease relapse or progression (CIR). The probabilities of OS and PFS were calculated using the Kaplan-Meier method. The estimates of the CIR were calculated using cumulative incidence curves. The variables that showed potential prognostic significance in the univariate analyses (p < 0.1) were included in the multivariate model. A Cox proportional hazards regression model was employed to evaluate the relative risk of the subjects. All statistical analyses were performed using Statistical Package for Social Science (SPSS 22.0) software.
Results
Patient characteristics
In total, 97 patients (57 males and 40 females) were included in the study. The characteristics of the transplanted patients are summarized in . The median age of the patients was 42.0 years (range, 15-65). Refractory or relapsed lymphoma was present in 81.4% of the patients. Seventy-three (75.3%) patients with advanced disease (stage III or IV) were included in the study. Eight patients (8.2%) were diagnosed with bone marrow involvement.
Table 1. Patient characteristics.
Hematopoietic engraftment and toxicity
The engraftment results are summarized in . The median dose of CD34-positive progenitor cells transplanted was 4.67×106/kg (range, 2.0-28.86×106/kg). All patients were evaluated for hematopoietic engraftment, except for two patients, who died at +8 days and +28 days. The median time of neutrophil engraftment was 9.5 days (range, 7–15 days), and the median time of platelet engraftment was 12 days (range, 7–25 days). The non-hematological regimen-related toxicities are summarized in . The common toxicities (>50% in all cases and all grades) included nausea/vomiting, mucositis, diarrhoea and febrile neutropenia. The most common grade 3–4 non-hematological toxicity was mucositis (36.1%). Of these patients, eight patients had mild gastrointestinal bleeding. Febrile neutropenia occurred in 51 patients (52.6%). Of these patients, seven patients had positive blood cultures, and 22 patients developed pulmonary infection. Hepatic and renal impairment occurred in 27 patients (grade I n = 15, grade II n = 10, and grade III n = 2) and 9 patients (grade I n = 8 and grade II n = 1), respectively. One patient with a prior history of seizure suffered another seizure during transplantation. Two patients experienced cardiac toxicity (one patient with frequent atrial premature beat and paroxysmal atrial tachycardia and one patient with frequent ventricular premature beat) and recovered after antiarrhythmic treatment. No cases of idiopathic pulmonary syndrome (IPS) or veno-occlusive disease (VOD) were observed throughout the follow-up period. TRM occurred in 2 patients (2.1%) at 100 days. Both patients died of respiratory failure and septic shock caused by severe pulmonary infection (on days +8 and +28). One patient was admitted to the intensive care unit (ICU) due to severe pulmonary infection at 7 months after the transplantation, and the blood culture was positive for Pseudomonas aeruginosa; the anti-infective treatment was poor, and he died of septic shock. Three months after the transplantation, one patient's white blood cell was still low at approximately 2.0-3.0×109, and the patient required G-CSF intermittently for a year. Two years after the transplantation, one patient developed pain in the right lower abdomen and underwent capsule endoscopy, which revealed an ileocecal ulceration. He was cured and discharged from the hospital after treatment in the gastroenterology department. Although no patient had a secondary tumour, one patient was found to have a meningioma (benign) more than a year after the transplantation. The meningioma was surgically removed in the brain surgery department of our hospital, and the patient is still alive.
Table 2. Hematopoietic engraftment after auto-SCT conditioned with SEMA.
Table 3. Toxicity associated with SEMA conditioning regimen and response to auto-SCT.
Response to transplantation and survival analysis
Ninety-five patients were evaluated to determine their treatment response at three months after hematopoietic stem cell transplantation (). Of these patients, 66 patients (69.5%) achieved complete remission, 14 patients (14.7%) achieved partial remission, and 15 patients (15.8%) showed no response or progressive disease. After a median follow-up time of 53.9 months, 31 patients (32.0%) showed relapse or progression over a median duration of 4.87 months (range, 1.1-40.53) after the transplantation. Of these 31, 4 patients received allogeneic transplantation, 9 patients received salvage chemotherapy, 2 patients received a second auto-SCT, 12 patients received radiation therapy, 3 patients received radiation therapy combined with salvage chemotherapy, and 1 patient received chimeric antigen receptor T cell (CAR-T) therapy after relapse. At the end of the study, 12 patients out of the 31 who relapsed or progressed were still alive either in remission (n = 9) or with evidence of disease (n = 3). In addition, the 3-year CIR was 34% (), and the estimated PFS and OS at 3 years were 62% and 75%, respectively ().
Prognostic factors
The results of the univariate and multivariate analyses are shown in . The univariate analysis revealed that serum LDH >250 U/L before auto-SCT, relapsed or refractory disease and no-remission disease status at auto-SCT were significant negative prognostic factors of both PFS and OS. In the multivariate analysis, serum LDH >250 U/L before auto-SCT and no-remission disease status at auto-SCT were identified as independent factors with a negative influence on PFS (HR: 2.386; 95% CI: 1.233-4.616; p = 0.010; HR: 4.609; 95% CI: 2.208-9.628; p < 0.001) and OS (HR: 3.254; 95% CI: 1.399-7.569; p = 0.006; HR: 0.048; 95% CI: 2.588-6.649; p < 0.001).
Table 4. Univariate and multivariate analysis of factors potentially associated with survivals.
Discussion
BEAM is considered the gold standard conditioning regimen for auto-SCT in lymphoma. However, a shortage of old drugs is a worldwide problem, and BCNU is one of the drugs in short supply. Therefore, alternative regimens are being developed and used by many institutions[Citation12–16]. In this study, Me-CCNU was used as a substitute for BCNU to provide a modified SEAM conditioning regimen. BCNU and Me-CCNU are both nitrosourea derivatives that interfere with the synthesis and function of DNA, RNA, and proteins via alkylation and carbamoylation. The two medicines have a qualitatively similar spectrum of antitumor activities, clinical toxicities, kinetic effects on the hemopoietic system, metabolic disposition, and degradation characteristics; in addition, these agents appear to act through similar degradation products [Citation17].
The results of our study show that the SEAM regimen including Me-CCNU instead of BCNU was well tolerated in lymphoma patients. The non-hematological regimen-related toxicities were not as severe as those observed in previous studies using BEAM as the conditioning regimen [Citation13,Citation18,Citation19]. As expected, the common early toxicities in our study included nausea/vomiting, mucositis and diarrhoea; these complications rapidly disappeared after hematopoietic engraftment. Nitrosoureas do not usually cause mucositis when used in monotherapy[Citation20–22]; however, nitrosoureas may cause severe mucositis (grade 3-4) if used in combination, with a reported occurrence of 30.5% to 68% in the BEAM regimen[Citation12,Citation16,Citation23,Citation24]. Grade 3–4 mucositis was observed in 36.1% of the patients in our study. Intestinal mucositis may result in severe diarrhoea. Grade 3–4 diarrhoea has been reported at an occurrence rate of 6% to 47% with the BEAM regimen[Citation12,Citation16,Citation23,Citation25,Citation26]. Compared with the BEAM regimen, the SEAM regimen had a similar incidence of grade 3–4 diarrhoea (11%). A known concern related to BCNU is the development of pulmonary toxicity, especially in regimens with higher BCNU doses [Citation27,Citation28], when BCNU is combined with cyclophosphamide [Citation29] or when patients receive radiation therapy prior to auto-SCT [Citation28]. Cardiac toxicity was observed in 2 patients (2.2%). In 2004, a study reported that 11% of patients subjected to the BEAM conditioning regimen developed cardiac toxicity [Citation25]. In 2008, Zaucha et al. [Citation26] reported that 12% patients subjected to the BEAM conditioning regimen developed greater than or equal to two grades cardiac toxicity. The incidence of cardiac toxicity in our study is lower than that reported in the above literature. IPS, which is a diffuse and severe lung injury without evidence of infection, is a serious complication of hematopoietic stem cell transplantation with high morbidity and mortality [Citation30–33]. Chen et al. [Citation27] reported that the incidence of IPS following the BEAM conditioning regimen (n = 1730) was 3% (2%−4%) within 1 year. The BCNU dose was approximately 300 mg/m2 (median 293 mg/m2, range 227-347 mg/m2). In the study conducted by Weaver et al. [Citation28], IPS was not observed in the patients who received 300 mg/m2 BCNU but occurred in 23% of the patients who received 600 mg/m2 BCNU. In our study, no cases of IPS were observed in these patients who received 250 mg/m2 Me-CCNU. The cumulative incidence of TRM at 100 days was 2.1%, which is similar to that previously reported [Citation34].
In our study, the median time to neutrophil engraftment and platelet engraftment was 9.5 days (range, 7–15 days) and 12 days (range, 7–25 days), respectively. The probability of PFS and OS at 3 years among the patients conditioned with SEAM was 62% and 75%, respectively. The hematopoietic engraftment and survival efficacy were similar to those in previous studies using BEAM as the conditioning regimen. Studies using BEAM as the conditioning regimen have demonstrated that (1) the median time to neutrophil engraftment and platelet engraftment were 10–12 days and 12–18.5 days, respectively; (2) three- and five-year PFS rates of 51% to 75% and 47% to 66.7%, respectively; and (3) three- and five-year OS rates of 64% to 86% and 47% to 77.8%, respectively [Citation13,Citation16,Citation19,Citation23,Citation24,Citation27,Citation35]. In February 2015, Chen et al. [Citation27] published the results of a study involving 4917 patients in the Center for International Blood and Marrow Transplant Research (CIBMTR). Among these patients, 1730 patients were subjected to the BEAM conditioning regimen. The 3-year PFS of NHL and HL were 61% and 51%, respectively. The 3-year OS of NHL and HL were 64% and 79%, respectively. Kothari et al. [Citation35] showed similar results in patients who received BEAM or LEAM. The median time required for neutrophil engraftment and platelet engraftment were 11 and 12 days with BEAM and 11 and 12 days with LEAM, respectively. The 3-year PFS and 3-year OS were 75% and 86% with BEAM and 69% and 86% with LEAM, respectively. Compared with other BCNU alternatives, the BeEAM regimen showed similar hematopoietic engraftment and non-hematological regimen-related toxicities [Citation14]. Sharma et al. [Citation12] reported that the 2-year event free survival (EFS) and 2-year OS of patients who received LEAM were 41.1% and 62.7%, respectively, which are slightly worse than those in our study. Our previous data [Citation36] showed that in lymphoma patients who underwent auto-SCT with a modified BuCy (mBuCy) conditioning regimen, neutrophil engraftment was somewhat faster compared with that with SEAM. The mBuCy group had less mucositis and diarrhoea than the SEAM group, which may be because melphalan and nitrosoureas were not used. The mBuCy group showed PFS and OS similar to those in the SEAM group.
According to previous reports, the prognostic factors for poor survival include a poor disease status at auto-SCT, high International Prognostic Index (IPI) (or age-adjusted IPI), extranodal involvement and high LDH levels [Citation37–42]. Similarly, our results demonstrated that serum LDH >250 U/L before auto-SCT, relapsed or refractory disease and a no-remission disease status at auto-SCT were negative prognostic factors that impacted PFS and OS in the univariate analysis and that serum LDH >250 U/L before auto-SCT and a no-remission disease status at auto-SCT were the only factors that impacted PFS and OS in the multivariate analysis. Thus, the overall clinical efficacy can be improved by reducing the tumour load and increasing the remission rates before auto-SCT.
The limitations of our study include the retrospective design, lack of a BEAM control group, and small sample size. Although toxicities were evaluated with CTCAE, this was performed retrospectively using medical records. Therefore, it is possible that the documentation of side effects and toxicity differed due to different physicians, which may result in a reporting bias. A prospective randomized trial with an adequate number of patients should be conducted to determine whether one of these regimens is superior to the other regimens. Moreover, thus far, no direct comparison has been conducted in the same cohort. To the best of our knowledge, a single exception is the prospective trial comparing the BeEAM (bendamustine, etoposide, cytarabine, melphalan) regimen with the BEAM regimen (NCT02278796). This trial completed recruitment, but the results are still unavailable.
In conclusion, the incidence and severity of the non-hematological regimen-related toxicities of the SEAM conditioning regimen in this study were almost identical to those of the BEAM conditioning regimen as reported in previous studies. The SEAM conditioning regimen followed by auto-SCT was well tolerated and had an efficacy and safety profile comparable to that of BEAM. Meanwhile, Me-CCNU can be conveniently administered and is readily available. Therefore, the SEAM conditioning regimen is feasible in terms of efficacy, safety and sources of drugs and might be an ideal alternative to the BEAM conditioning regimen for auto-SCT in lymphoma.
Disclosure of interest
The authors have no conflicts of interest to report.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-hodgkin's lymphoma. N Engl J Med. 1987;316(24):1493–1498.
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. The Lancet. 2002;359(9323):2065–2071.
- Gribben JG, Linch DC, Singer CR, et al. Successful treatment of refractory Hodgkin's disease by high-dose combination chemotherapy and autologous bone marrow transplantation. Blood. 1989;73(1):340–344.
- Darrington DL, Vose JM, Anderson JR, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994;12(12):2527–2534.
- Montoto S, Canals C, Rohatiner AZ, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007;21(11):2324–2331.
- Mills W, Chopra R, McMillan A, et al. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995;13(3):588–595.
- Caballero MD, Rubio V, Rifon J, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant 1997;20(6):451–458.
- Mounier N, Gisselbrecht C. Conditioning regimens before transplantation in patients with aggressive non-Hodgkin's lymphoma.
- Ann Oncol. 1998;9(Suppl 1):S15–S21.
- Gu B, Zhang X, Chen G, et al. Efficacy of haploidentical hematopoietic stem cell transplantation compared to HLA-matched transplantation for primary refractory acute myeloid leukemia. Ann Hematol 2018.
- Zhang XH, Huang XJ, Liu KY, et al. Modified conditioning regimen busulfan-cyclophosphamide followed by allogeneic stem cell transplantation in patients with multiple myeloma. Chin Med J. 2007;120(6):463–468.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.
- Sharma A, Kayal S, Iqbal S, et al. Comparison of BEAM vs. LEAM regimen in autologous transplant for lymphoma at AIIMS. SpringerPlus. 2013;2:489.
- Kim JE, Lee DH, Yoo C, et al. BEAM or BuCyE high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma patients: a single center comparative analysis of efficacy and toxicity. Leuk Res 2011;35(2):183–187.
- Visani G, Malerba L, Stefani PM, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118(12):3419–3425.
- Stuart MJ, Chao NS, Horning SJ, et al. Efficacy and toxicity of a CCNU-containing high-dose chemotherapy regimen followed by autologous hematopoietic cell transplantation in relapsed or refractory Hodgkin's disease. Biol Blood Marrow Transplant. 2001;7(10):552–560.
- Olivieri J, Mosna F, Pelosini M, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: An observational study on 1038 patients from fondazione italiana linfomi. Biol Blood Marrow Transplant. 2018;24(9):1814–1822.
- Wheeler GP. Studies related to the mechanisms of action of cytotoxic alkylating agents: a review. Cancer Res 1962;22:651–688.
- Jo JC, Kang BW, Jang G, et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol 2008;87(1):43–48.
- Shi Y, Liu P, Zhou S, et al. Comparison of CBV, BEAM and BEAC high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation in non-hodgkin lymphoma: Efficacy and toxicity. Asia Pac J Clin Oncol. 2017;13(5):e423–e429.
- Khayat D, Lokiec F, Bizzari JP, et al. Phase I clinical study of the new amino acid-linked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res 1987;47(24 Pt 1):6782–6785.
- Young RC, Walker MD, Canellos GP, et al. Initial clinical trials with methyl-CCNU 1-(2-chlorethyl)-3-(4-methyl cyclohexyl)-1-nitrosourea (MeCCNU). Cancer. 1973;31(5):1164–1169.
- Bouffet E, Khelfaoui F, Philip I, et al. High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol 1997;39(4):376–379.
- Khattry N, Gupta A, Jain R, et al. LACE versus BEAM conditioning in relapsed and refractory lymphoma transplant: retrospective multicenter analysis of toxicity and efficacy. Int J Hematol 2016;103(3):292–298.
- Marchesi F, Capria S, Giannarelli D, et al. BEAM vs FEAM high-dose chemotherapy: retrospective study in lymphoma patients undergoing autologous stem cell transplant. Bone Marrow Transplant 2018;53(8):1051–1054.
- Wang EH, Chen YA, Corringham S, et al. High-dose CEB vs BEAM with autologous stem cell transplant in lymphoma. Bone Marrow Transplant 2004;34(7):581–587.
- Zaucha R, Gooley T, Holmberg L, et al. High-dose chemotherapy with BEAM or busulphan/melphalan and thiotepa followed by hematopoietic cell transplantation in malignant lymphoma. Leuk Lymphoma. 2008;49(10):1899–1906.
- Chen YB, Lane AA, Logan B, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046–1053.
- Weaver CH, Appelbaum FR, Petersen FB, et al. High-dose cyclophosphamide, carmustine, and etoposide followed by autologous bone marrow transplantation in patients with lymphoid malignancies who have received dose-limiting radiation therapy. J Clin Oncol. 1993;11(7):1329–1335.
- Alessandrino EP, Bernasconi P, Colombo A, et al. Pulmonary toxicity following carmustine-based preparative regimens and autologous peripheral blood progenitor cell transplantation in hematological malignancies. Bone Marrow Transplant 2000;25(3):309–313.
- Clark JG, Hansen JA, Hertz MI, et al. NHLBI workshop summary. idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis 1993;147(6 Pt 1):1601–1606.
- Demirer T, Weaver CH, Buckner CD, et al. High-dose cyclophosphamide, carmustine, and etoposide followed by allogeneic bone marrow transplantation in patients with lymphoid malignancies who had received prior dose-limiting radiation therapy. J Clin Oncol. 1995;13(3):596–602.
- Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American thoracic society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183(9):1262–1279.
- Todd NW, Peters WP, Ost AH, et al. Pulmonary drug toxicity in patients with primary breast cancer treated with high-dose combination chemotherapy and autologous bone marrow transplantation. Am Rev Respir Dis. 1993;147(5):1264–1270.
- Robinson SP, Boumendil A, Finel H, et al. High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-hodgkin lymphoma: no differences in toxicity or outcome. A matched-control study of the EBMT-lymphoma working party. Bone Marrow Transplant 2018;53(12):1553–1559.
- Kothari J, Foley M, Peggs KS, et al. A retrospective comparison of toxicity and initial efficacy of two autologous stem cell transplant conditioning regimens for relapsed lymphoma: LEAM and BEAM. Bone Marrow Transplant 2016;51(10):1397–1399.
- Huang H, Zhang L, Jiang Y, et al. Modified BuCy is an alternative conditioning regimen for lymphoma patients undergoing autologous stem cell transplantation. Ann Hematol 2019;98(5):1259–1266.
- Ko OB, Jang G, Kim S, et al. Autologous stem cell transplantation for diffuse large B-cell lymphoma with residual extranodal involvement. Korean J Intern Med 2008;23(4):182–190.
- Sohn BS, Park I, Kim EK, et al. Comparison of clinical outcome after autologous stem cell transplantation between patients with peripheral T-cell lymphomas and diffuse large B-cell lymphoma. Bone Marrow Transplant 2009;44(5):287–293.
- Qian F, Fan W, Wei X, et al. Prognostic factors of lymphoma patients after autologous stem cell transplantation. Ann Transplant 2015;20:225–232.
- Yang DH, Kim WS, Kim SJ, et al. Prognostic factors and clinical outcomes of high-dose chemotherapy followed by autologous stem cell transplantation in patients with peripheral T cell lymphoma, unspecified: complete remission at transplantation and the prognostic index of peripheral T cell lymphoma are the major factors predictive of outcome. Biol Blood Marrow Transplant. 2009;15(1):118–125.
- Hosing C, Saliba RM, Okoroji GJ, et al. High-dose chemotherapy and autologous hematopoietic progenitor cell transplantation for non-hodgkin's lymphoma in patients >65 years of age. Ann Oncol. 2008;19(6):1166–1171.
- Pham RN, Gooley TA, Keeney GE, et al. The impact of histologic grade on the outcome of high-dose therapy and autologous stem cell transplantation for follicular lymphoma. Bone Marrow Transplant. 2007;40(11):1039–1044.