ABSTRACT
Objectives: Allogeneic hematopoietic stem cell transplantation (allo-HCT) is the only curative treatment for myelodysplastic syndromes (MDS), although predicting post-transplant outcomes remains inconclusive. This study evaluated patients who underwent allo-HCT for MDS to identify prognostic factors and develop a clinical risk model.
Methods: We evaluated 55 patients between June 2000 and March 2015 to identify prognostic factors and develop a model for three-year overall survival (OS) and event-free survival (EFS). Cox regression analysis was performed on four factors: age ≥55 years; Hematopoietic Cell Transplant-Comorbidity Index >2; intermediate or worse cytogenetic status based on revised International Prognostic Scoring System; and unrelated donor status associated with poor OS in the univariate analysis. A clinical risk model was constructed using the sum of the regression coefficients and evaluated using receiver operating characteristic analysis and five-fold cross-validation.
Results: Patient median age was 51 (range: 30–67) years. Median follow-up was 45.8 (range: 1.27–193) months; the three-year OS and EFS rates were 61.8% and 56.4%, respectively. The areas under the curves (AUCs) for OS and EFS were 0.738 and 0.778, respectively, and the average AUC for 50 times five-fold cross-validation were 0.711 and 0.723 for three-year OS and EFS, respectively.
Conclusion: A four-clinical-risk-factor model that could effectively predict post-transplantation outcomes and help decision-making in MDS treatment was developed.
Introduction
Myelodysplastic syndromes (MDS) are a group of hematologic disorders that are characterized by ineffective hematopoiesis and progression to acute myeloid leukemia (AML) [Citation1]. Allogeneic hematopoietic stem cell transplantation (allo-HCT) is the only curative treatment for MDS, especially for high-risk patients [Citation2–4]. However, a significant proportion of patients succumb to transplant-related deaths and relapse after transplantation, which is affected by various factors including the heterogeneity of the disease, the patient’s characteristics, and post-transplantation complications [Citation5–7]. We have, therefore, continued to refine supportive care and adapted the transplantation protocol to improve outcomes and expand its application to patients who otherwise could not undergo allo-HCT [Citation8]. Nevertheless, it remains difficult to accurately predict post-transplantation outcomes and to make clinical decisions as to which patients with MDS should undergo allo-HCT.
The International Prognostic Scoring System (IPSS) [Citation9] is widely used to predict the prognosis of patients with MDS, as well as their risk of progression to leukemia. The Revised International Prognostic Scoring System (IPSS-R) [Citation10] was subsequently developed and has also become widely used. Both systems are used to determine whether allo-HCT should be performed and to predict the outcomes after allo-HCT in MDS patients. However, these systems rely on patient characteristics at the time of diagnosis. Furthermore, clinicians have sometimes experienced difficulty predicting post-transplantation outcomes using the IPSS or IPSS-R, especially for patients who have experienced disease progression or received chemotherapy after diagnosis. A previous study has suggested that an assessment with the IPSS before allo-HCT could be beneficial for stratifying the risk of post-transplantation outcomes [Citation11]. However, the reported rate of non-relapse mortality (NRM) is still 26% [Citation8], and the outcomes may also be associated with parameters that are not included in the IPSS, such as age, general condition, and complications. The Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), [Citation7] a scoring system that relies on complications, is frequently used to predict the risk of post-transplantation NRM. However, the HCT-CI does not include patient age or disease status and is not sufficient on its own to predict post-transplantation outcomes. Therefore, developing a novel system to comprehensively predict post-transplantation outcomes of MDS patients is clinically warranted. Several studies have proposed new systems comprising patient background, blast level, karyotype, and recent somatic mutation [Citation12–15]. This retrospective study aimed to analyze the characteristics of Japanese patients with MDS who underwent allo-HCT to identify clinical risk factors based on baseline and pre-HCT characteristics.
Materials and methods
Patients and data
This retrospective study included ≥18-year-old patients with MDS who underwent their first allo-HCT in centers administered by Yokohama City University Hospital Department of Hematology/Rheumatology/Infectious Diseases, Yokohama City University Medical Center Department of Hematology, and Kanagawa Cancer Center Department of Hematology, belonging to Yokohama Cooperative Study Group for Hematology (YACHT) between January 2000 and March 2015. MDS diagnosis and categorization was based on the 2008 criteria from the World Health Organization (WHO) [Citation1]. Patients were excluded if they had ≥20% blasts in the bone marrow (BM) or peripheral blood (PB) at any time and if they had chronic myelomonocytic leukemia or an excess of blasts in transformation (RAEB-t) based on the French-American-British (FAB) classification [Citation1]. We also excluded patients undergoing BM and PB stem cell transplantation from donors with ≥2/6 mismatched human leukocyte antigens (HLAs).
Data were collected from the registry of HCT cases (the Transplant Registry Unified Management Program in the Japanese Date Center for Hematopoietic Cell Transplantation). Additional patients’ data were collected from their institutional medical records. The laboratory data before HCT were measured just before initiation of conditioning chemotherapy; ferritin, Wilms’ tumor (WT)−1 [Citation16], and β2-microglobrin [Citation17] were measured within one month of transplantation. The disease risk index (DRI) [Citation18] was not evaluated because of the difficulty of its precise assessment for pre-transplant disease status due to clinical characteristics of MDS. The retrospective protocol was approved by the institutional ethics review boards. According to previous reports, cytogenetics were categorized based on the IPSS-R classification [Citation10], and myeloablative or reduced-intensity conditioning regimens were defined [Citation19].
Statistical analysis
The primary outcome was defined as three-year overall survival (OS), and the secondary outcomes were defined as three-year event-free survival (EFS) and NRM. The OS intervals were defined as the time from stem cell infusion to death, and the EFS interval was defined as the time from stem cell infusion to either death or relapse or progression. NRM was defined as death from any cause except MDS. The OS and EFS outcomes were summarized using the Kaplan–Meier method.
The potential prognostic factors were considered significant with values of p < 0.10 (using the log-lank test), to increase the sensitivity of analysis. The potential prognostic factors were evaluated as risk factors using multivariable Cox regression analysis on all patients. As a relatively small number of cases was expected to be included in this study, we considered that it would be difficult to derive clinically meaningful results using general multivariate analysis with statistically significant covariates because of low statistical power. Therefore, these factors were also evaluated by receiver operating characteristic (ROC) analysis for the three-year OS and EFS; a risk model was constructed by summing the regression coefficients of each factor obtained by Cox regression analysis and was evaluated and validated by the area under the curve (AUC) of the ROC curve. To examine over-fitting, the factors were also examined by five-fold cross-validation; all were assigned randomly: four-fifths to a training set and one-fifth to a validation set. The coefficient regression of each factor was found on each training set, and the risk model was constructed by summing the coefficient regressions of applicable risk factors using multivariable Cox regression analysis. The risk model was evaluated using ROC analyses. The five-fold cross-validation was repeated 50 times to calculate the average AUC. This analysis was performed by IBM SPSS software version 22 (IBM Corp., Armonk, NY).
Results
Patient characteristics
Seventy-two patients who underwent allo-HCT for MDS were identified from the database. Seventeen patients were excluded due to the presence of >20% of blasts in the BM/PB, a diagnosis of RAEB-t based on the FAB classification (eight cases) or chronic myelomonocytic leukemia (six cases), allogenic bone marrow transplant (allo-BMT) from an HLA-haplo-identical donor (one case), a second transplantation (one case), and other reasons (one case). The characteristics of the 55 patients who progressed to the analysis are shown in . The median age was 51 (range: 30–67) years, and the median follow-up was 45.8 (range: 1.27–193 months) months. Six (10.9%) patients had secondary MDS. Based on the WHO 2008 classification, the disease stages at diagnosis were refractory cytopenia with unilineage dysplasia (RCUD) in six cases (10.9%), refractory cytopenia with multilineage dysplasia (RCMD) in 21 cases (38.1%), refractory anemia with excess blasts (RAEB)−1 in 15 cases (27.2%), and RAEB-2 in 13 cases (23.6%). The disease stages before HCT were RCUD in five cases (9.1%), RCMD in 15 cases (27.3%), RAEB-1 in 17 cases (30.9%), and RAEB-2 in 18 cases (32.7%). The median time between diagnosis and transplantation was seven months (range: 1–178 months), and the diseases during this interval were progressive, stable, and improved in 16 (29.1%), 34 (61.8%), and five cases (9.1%), respectively. The values for WT-1 and β2-microglobulin were only measured in eight and 12 patients, respectively. shows the OS and EFS of all patients, with one-year, two-year, and three-year OS rates of 74.5%, 61.8%, and 61.8%, respectively, as well as one-year, two-year, and three-year EFS rates of 63.6%, 58.2%, and 56.4%, respectively. The three-year incidence rates of relapse and NRM were 22.3% (95% confidence interval [CI] 9.0–33.7%) and 22.2% (95% CI 9.6–33.0%), respectively. No patient underwent planned maintenance treatment with hypomethylating agents or DLI after allo-HCT.
Figure 1. Kaplan–Meier plots of (a) OS and (b) EFS in 55 patients. The 1-year, 2-year, and 3-year OS rates were 74.5%, 61.8%, and 61.8%, respectively. The 1-year, 2-year, and 3-year EFS rates were 63.6%, 58.2%, and 56.4%, respectively.
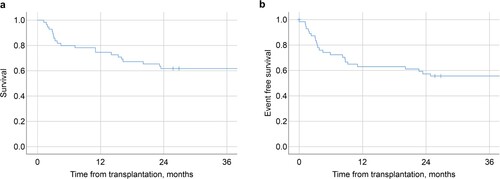
Table 1. Characteristics of the 55 patients.
Predictors of OS
The univariate analyses () revealed that poor OS and EFS rates were associated with ages ≥55 years (p = 0.005, 0.010), an HCT-CI >2 (p = 0.004, <0.001), intermediate or worse cytogenetic status (poor or very poor) before allo-HCT based on IPSS-R classification (p = 0.019, 0.024), an absolute neutrophil count (ANC) of <800/µL before allo-HCT (p = 0.022, 0.009), an elevated C-reactive protein (CRP) level before allo-HCT (p = 0.016, 0.011), and unrelated donor status (BM or PB stem cell transplantation from an unrelated donor and cord blood transplantation) (p = 0.084, 0.096). IPSS-R before HCT was not associated with OS (p = 0.159), but only with EFS (p = 0.004). The OS and EFS outcomes were not significantly associated with hemoglobin level, platelet count, blasts in the BM or PB, lactate dehydrogenase level, or intensity of conditioning therapy (). Eastern Cooperative Oncology Group performance status (ECOG PS) was excluded from this analysis because there was a prominent imbalance in the number of cases between ECOG PS ≤1 (53 cases) and ≥2 (two cases). Regarding the parameters at diagnosis, BM blasts ≥5% (p = 0.125), PB blasts ≥3% (p = 0.964), and intermediate or worse cytogenetic status (p = 0.436) were not significant factors for OS by univariate analysis.
Table 2. Clinical variables for the 55 patients and p-values by univariate analysis for OS and EFS.
As six factors were disproportionate considering the number of cases in this study and because they included various elements as risk factors, four factors were chosen to be subjected to Cox multivariate analysis: age; HCT-CI; cytogenetic status before allo-HCT; and unrelated donor status. These are known factors to have an impact on MDS patients who underwent allo-HCT, rather than ANC or CRP level. Since age and HCT-CI [Citation7] are fundamental factors and have an obvious significant impact on prognosis, it was imperative to incorporate them into the multivariate analysis. Cytogenetic status was also necessary, as it is an essential feature of the disease itself and also has a significant impact on prognosis [Citation10]. Transplantation conditions, including basic information in examining the prognosis of transplantation and the status of the unrelated donor, were selected. shows that in the Cox multivariate analyses, age and HCT-CI were independent predictors of three-year OS. Cytogenetic status and unrelated donor status were not significant factors. For three-year EFS, only HCT-CI was an independent predictor. Age, cytogenetic status, and unrelated donor status were not significant factors.
Table 3. Multivariate analysis, regression coefficient, and scoring system for 3-year OS and EFS.
Model development and validation
A clinical risk model for three-year OS was calculated using multivariable Cox regression models by summing the regression coefficients of the applicable factor in the four factors as follows: age (0 or 1.042) + HCT-CI (0 or 1.660) + cytogenetic status before allo-HCT (0 or 0.670) + unrelated donor status (0 or 0.965). A risk model for three-year EFS was also calculated as follows: age (0 or 0.778) + HCT-CI (0 or 1.822) + cytogenetic status before allo-HCT (0 or 0.632) + unrelated donor status (0 or 0.864). We tested the ability of the model to identify the three-year OS and EFS using ROC analysis. Three patients were not included in the calculation because of missing values.
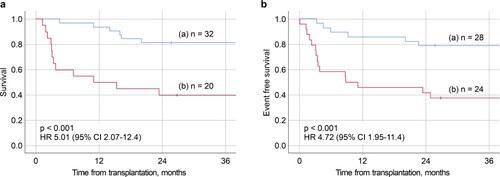
The ROC curves are shown in , which provided AUCs of 0.738 for three-year OS and 0.778 for three-year EFS. To examine the model’s over-fitting, we also performed five-fold cross-validation, in which all patients were randomly assigned to a training set (n = 43) and a validation set (n = 12); we evaluated the ROC curves and the AUCs for three-year OS and EFS 50 times. The average AUC of 50 times was 0.711 for three-year OS and 0.723 for three-year EFS. To further examine the risk model, one group with lower than or equal values to the cutoff value (1.635) (n = 32) and one group with higher values than the cut off (n = 20), were compared by the Kaplan–Meier method (a). The group with values lower than or equal to the cutoff value was associated with better survival (p < 0.001; Hazard ratio [HR] 5.01, 95% CI 2.07–12.4). The three-year OS rates were 81.2% (95% CI 62.9–91.1%) for the lower group and 40.0% (95% CI 19.3–60.0) for the higher group. In terms of three-year-EFS, the group with values lower than or equal values to the cutoff value (1.420) (n = 28) was associated with better EFS compared with the group with higher values than the cutoff value (n = 24) (p < 0.001; HR 4.72, 95% CI 1.95–11.4) (b). The three-year EFS rate were 78.6% (95% CI 58.4–89.8%) for the lower group, and 37.5% (95% CI 19.0–56.0%) for the higher group. As the detailed score is difficult to apply clinically, we created a new risk model for three-year OS by converting the regression coefficients for each factor into integer scores so that the sum of the integer scores for each case perfectly matched the two groups classified by the sum of the regression coefficients; two points for age, three points for HCT-CI, and one point for cytogenetic status and unrelated donor status; a score ≥ three points was defined as the high-risk group, and the Kaplan–Meier curve of the new risk model was also consistent with that presented in a. For three-year EFS, we used the same method; age and cytogenetic status were scored as one point, HCT-CI as three points, and unrelated donor status as two points; a score ≥ three points was defined as the high-risk group, which also exactly matched b. When the risk model was applied to 42 patients having undergone transplantation between 2000 and March 2015, the low-risk group was associated with better survival (p = 0.007; HR 3.63, 95%CI 1.42-9.27), and the same occurred for EFS (p = 0.027; HR 2.93, CI 1.13-7.57).
Figure 2. ROC curves of the risk model for three-year (a) OS and (b) EFS in all patients. The area under the curve were (a) 0.738 and (b) 0.778 for OS and EFS, respectively. The cutoff values for OS and EFS were (a) 1.635 and (b) 1.420, respectively.
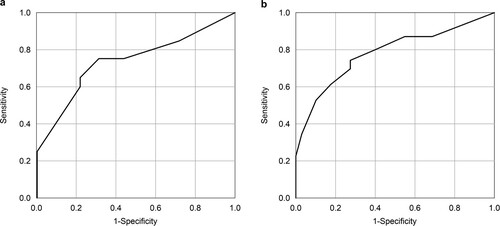
Figure 3. (a) Kaplan–Meier plots of the group (a) lower and (b) higher than the cutoff value point in the risk model for three-year OS. The three-year OS rates were 81.2% for the lower group and 40.0% for the higher group. (b) Kaplan–Meier plots of the group (a) lower and (b) higher than the cutoff value point in the risk model for three-year EFS. The three-year EFS rates were 78.6% for the lower group and 37.5% for the higher group.
Discussion
The present study was a multicenter study investigating prognostic factors that could predict OS and develop a clinical risk model in MDS patients undergoing allo-HCT. Based on the results, we identified the following clinical risk factors: age ≥55 years, HCT-CI >2, intermediate or worse cytogenetic status before allo-HCT based on the IPSS-R classification, ANC <800/µL before allo-HCT, elevated CRP before allo-HCT, and unrelated donor status. In this context, the patients’ age and HCT-CI reflect their background status, which is known to influence outcomes. In contrast, cytogenetic status, ANC, and CRP reflect the patients’ disease risk and status, while unrelated donor status reflects transplantation condition. Thus, these clinical risk factors serve as comprehensive indicators in predicting outcomes in this population. In the multivariable analysis, only two factors, HCT-CI and age, were significant predictors of three-year OS, and only one factor, HCT-CI, was associated with the poor outcome of three-year EFS. However, the prognosis after transplantation for MDS cannot be predicted with these factors. The ROC curves of the Cox regression model, including the four factors other than ANC and CRP, had an average AUC of 0.711 for three-year OS and 0.723 for three-year EFS, and the results indicated the four factors had the ability to estimate the outcome, although they are not independent predictors. It is clear that age and HCT-CI are strong predictors of OS [Citation7], and cytogenetic status is included in the IPSS-R criteria [Citation10], which is a strong predictor. Donor type is generally an important factor in transplantation. Therefore, it is easy to consider these four factors as prognostic predictors. In multivariate analysis, HCT-CI was an independent predictor for three-year OS and EFS. In our study, seven out of nine (77.8%) cases with high HCT-CI had poor cytogenetic status (especially four cases with a very poor status). Moreover, reduced-intensity conditioning was performed in two out of nine cases (22.2%) with high HCT-CI, which was a slightly higher percentage compared to that of patients with low HCT-CI who underwent this procedure (nine out of 46 cases; 19.7%). These facts may have caused HCT-CI to become an independent factor of EFS as well as OS. Conversely, blast in BM or PB, platelet, and Hb, which are included in IPSS-R criteria [Citation10], did not become predictors in this study. Some patients who had many blasts in BM or PB had good cytogenetics status and outcome. This fact indicated that non-remission transplantation, which should have poor outcomes, was possibly expected to lead to long-term survival if the four factors are good categories. Additionally, in cases where the total regression coefficient of applicable risk factors is less than the cutoff value point (1.635), a three-year OS rate of more than 80% is possible, which strongly supports the decision of transplantation. Conversely, transplantation may be high risk if the total regression coefficient is higher than the cutoff value point. The same is true for three-year EFS.
Previous studies have identified various risk factors and new prognostic systems. Della Porta et al. proposed a ‘MDS transplantation risk index calculation’ that classified patients into four risk categories based on age, IPSS-R, HCT-CI, and refractoriness to induction chemotherapy [Citation15]. Shaffer et al. developed a novel scoring system that included age, Karnofsky performance status, cytogenetics status, blasts in PB before HCT, and platelet count before HCT [Citation20], which is similar to our focus on various aspect factors, and on parameters and disease status before HCT. These studies also suggest that post-transplantation outcomes are not solely determined by disease risk or status, and patients’ backgrounds could also affect outcome. We emphasize that the good features of this study is the possibility of predicting prognosis with only four simple factors before HCT, without the need for parameters based on detailed blood test results.
The present study evaluated laboratory data and cytogenetic status before allo-HCT, not at diagnosis. In this context, pre-HCT laboratory data should provide a better prognostic value than data from the diagnosis, as previous reports have suggested that the pre-HCT IPSS can be used to stratify the risk of post-transplantation outcomes [Citation11]. BM blasts, PB blasts, and cytogenetic status at diagnosis were not significant factors for OS in this study. Moreover, classification of disease status based on the WHO 2008 status of 16 patients (29.1%) experienced progression or improvement. Cytogenetic status at the IPSS-R category also changed in 16 patients (29.1%) (improved in six and progressed in ten patients), whereas it stayed the same in 36 patients and was missing in three patients. This indicates that the change in disease and cytogenetic status may confound prognostic predictions based on the date available only at diagnosis, and the pre-HCT parameters may be more prognostic than parameters from at the time of diagnosis. Moreover, many cases with unchanged IPSS-R or cytogenetic status had not been treated before allo-HCT, especially before the launch of azacitidine, and the disease status of future cases may further change between the time of diagnosis and HCT.
This study has several limitations. First, it was a small cohort including only 55 patients from three facilities. We excluded the two factors of ANC and CRP, which were suggested as possible prognostic factors by univariate analysis. There were no significant differences in OS for two risk factors, cytogenetic status and unrelated donor status, in the multivariate analyses. Some parameters, such as ECOG PS, had a bias and were difficult to evaluate. Thus, it is possible that a larger cohort could detect independent effects of other factors, such as blasts in the BM or PB, and finer classification of cytogenetic status, which might provide more accurate clinical risk factors or scoring systems. Second, this study was retrospective, and there was possibly selection bias which impacted the results. Some prospective studies are currently ongoing to analyze outcomes or reveal prognostic factors, and the results are pending. Third, although we selected the clinical cutoff values or values from the IPSS-R classification for each parameter, additional studies are needed to refine these cutoff values to better reflect the disease risk and status of MDS. Fourth, the current study did not examine additional treatment for relapse after allo-HCT, which may have modified the prognosis of the high-risk group.
Conclusion
We developed a clinical risk model for allo-HCT in MDS, which was based on age ≥55 years, HCT-CI >2, intermediate or worse cytogenetic status before allo-HCT based on IPSS-R classification, and unrelated donor status. Nevertheless, comprehensive prognostic scoring or clinical risk models are still needed to accurately incorporate disease risk, disease stage, and patient characteristics, facilitating better and more personalized treatments.
Statement of ethics
This study was performed in accordance with the Declaration of Helsinki and the Ethics Guidelines for Clinical Research published by the Ministry of Health, Labor, and Welfare of Japan. This retrospective study was approved by Ethical Committee for Medical and Biological Research Involving Human Subjects of Yokohama City University Hospital (B170400006). Approval for the protocol and written informed consent forms were obtained from the ethics committees at each institution. The written decision can be presented upon request.
Acknowledgments
We would like to thank the patients and clinical staff of each institution and the Yokohama Cooperative Study Group for Hematology (YACHT) for their cooperation in this study.
Data availability statement
The data supporting the results of this study are available from the corresponding author upon a reasonable request.
Disclosure statement
TT reports personal fees from Otsuka, Novartis, Pfizer, BMS, Daiichi Sankyo, and Astellas outside of the submitted work. The other authors declare no conflicts of interest.
References
- Vardiman JW. The 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia: rationale and important changes/JW Vardiman, J. Thiele, D. Arber. Blood. 2009;114:937–951.
- Malcovati L, Hellström-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–2964.
- de Witte T, Hagemeijer A, Sucio S, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European intergroup trial. Haematologica. 2010;95:1754–1761.
- Basquiera AL, Rivas MM, Remaggi G, et al. Allogeneic hematopoietic stem cell transplantation in adults with myelodysplastic syndrome: Experience of the argentinean group of bone marrow transplantation (GATMO). Hematology. 2015;21:162–169.
- Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2012;104:579–585.
- Platzbecker U, Schetelig J, Finke J, et al. Allogeneic hematopoietic cell transplantation in patients age 60-70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: comparison with patients lacking donors who received azacitidine. Biol Blood Marrow Transplant. 2012;18:1415–1421.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)– specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919.
- Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088.
- Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465.
- Lee JH, Lee JH, Lim SN, et al. Allogeneic hematopoietic cell transplantation for myelodysplastic syndrome: prognostic significance of pre-transplant IPSS score and comorbidity. Bone Marrow Transplant. 2010;45:450–457.
- Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376:536–547.
- Patel SS, Sekeres MA, Nazha A. Prognostic models in predicting outcomes in myelodysplastic syndromes after hypomethylating agent failure. Leuk Lymphoma. 2017;58:2532–2539.
- Germing U, Hildebrandt B, Pfeilstöcker M, et al. Refinement of the international prognostic scoring system (IPSS) by including LDH as an additional prognostic variable to improve risk assessment in patients with primary myelodysplastic syndromes (MDS). Leukemia. 2005;19:2223–2231.
- Della Porta MG, Alessandrino EP, Bacigalupo A, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS strati fi ed according to the revised IPSS-R. Blood. 2014;123:2333–2343.
- Casalegno-Garduño R, Schmitt A, Spitschak A, et al. Immune responses to WT1 in patients with AML or MDS after chemotherapy and allogeneic stem cell transplantation. Int J Cancer. 2016;138:1792–1801.
- Gatto S, Ball G, Onida F, et al. Contribution of β-2 microglobulin levels to the prognostic stratification of survival in patients with myelodysplastic syndrome (MDS). Blood. 2003;102:1622–1625.
- Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–913.
- Baron F, Ruggeri A, Beohou E, et al. RIC versus MAC UCBT in adults with AML: A report from Eurocord, the ALWP and the CTIWP of the EBMT. Oncotarget. 2016;7:43027–43038.
- Shaffer BC, Ahn KW, Hu Z-H, et al. Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 2016;34:1864–1871.
