ABSTRACT
Background
Acute myeloid leukemia (AML) with t(8;21) is generally associated with a favorable clinical course. Loss of sex chromosome (LOS) are frequently observed in t (8;21) AML, but the prognostic value of LOS remains uncertain.
Methods
A total of 73 patients with AML with t(8;21) were studied and divided into t(8;21) with LOS group (n = 36) and t(8;21) alone group (n = 37). The patients with t(8;21) AML with ACAs other than LOS were excluded. The clinical characteristics of these two groups were compared, and the prognostic value of LOS was evaluated based on disease-free survival (DFS) and overall survival (OS).
Results
The clinical characteristics (except for gender) were found to have no significant difference between these two groups, and the male patients tended to account for a larger proportion in the former group (P = .001). The OS of the t(8;21) AML with LOS group was significantly longer than that of the t(8;21) AML alone group (P = .005). While not obvious, the patients with LOS seemed to have longer DFS (P = .061). The multivariable analysis also showed LOS to be an independent favorable prognostic factor of t(8;21) AML (P = .022).
Conclusions
Our results suggested that LOS could be associated with a favorable prognosis in t(8;21) AML patients without other ACAs, and for this subtype of AML, longer DFS and a satisfactory and stable survival can be achieved with high-dose cytarabine (HDAC) consolidation treatment.
Introduction
The t(8;21)(q22;q22) is one of the common translocations identified in de novo acute myeloid leukemia (AML), occurring in nearly 40% of cases of FAB-M2 AML and 8% to 20% of all cases of AML [Citation1,Citation2]. There is considerable clinical and biological heterogeneity within these patients. AML with t(8;21) is recognized as a unique entity with high complete remission (CR) rates after chemotherapy and favorable prognosis [Citation3]. However, some patients still show poor prognosis, and the relapse incidence of this group reaches up to 30–40% [Citation4,Citation5].
The translocation (8;21) involves the acute myeloid leukemia 1 (AML1) (also call runt-related transcription factor 1(RUNX1)) gene on chromosome 21 and the corepressor eight-twenty-one (ETO) (MTG8, RUNX1T1) gene on chromosome 8 [Citation6], which is more frequently associated with additional cytogenetic abnormalities (ACAs). The most common cytogenetic abnormalities associated with t(8;21) include the loss of sex chromosome (LOS), deletions of the long arm of chromosome 9 (del9q) and complex abnormalities [Citation7]. The LOS is the most common ACA, occurring in t(8;21) AML in a relatively high percentage of cases (32%−66%) [Citation8–10]. Although some studies have reported the clinical significance of the loss of sex chromosome (LOS) in t(8;21) AML, its prognostic value is still a matter of debate. Some studies have suggested that the prognosis of t(8;21) AML with LOS was poor [Citation11,Citation12], while several reports have concluded that deletion of the sex chromosome was a favorable prognostic factor [Citation13,Citation14]. However, few studies have considered the possible negative impact of del (9q) or complex karyotype on prognosis, which has been reported in previous reports [Citation7,Citation15], which may result in conflicting data regarding the prognostic value.
Therefore, we perform a retrospective study to evaluate the prognostic value of LOS in t(8;21) AML and explore whether there are other variables related to the outcome of t(8;21) AML. To avoid the potential effect of additional cytogenetic aberration other than LOS, we excluded this group in our study and consequently focused on the t(8;21) with LOS group and the t(8;21) alone group.
Patients and methods
Patients and data collection
From May 2011 to June 2019, the clinical data of 117 patients with t(8;21) AML who initially presented to the First Affiliated Hospital, Zhejiang University, were collected in this retrospective study. All the cases were definitively diagnosed by bone marrow morphology, cytogenetics, immunophenotype and molecular biology examination, with completion of at least 1 cycle of induction chemotherapy and effective evaluation. Diagnosis was based on the criteria of the 2018 World Health Organization (WHO) classification.
To avoid the potential effects of additional cytogenetic aberrations other than LOS, this group of t(8;21) AML was excluded from our study. Ultimately, 73 cases were included in our study and were divided into a t(8;21) with LOS group (n = 36) and a t(8;21) alone group (n = 37). The patients were followed up by medical records consultation or telephone contact. The deadline of follow-up was March 31, 2020. This study was conducted in compliance with the institutional policy regarding the protection of patients’ private information and was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Cytogenetic and molecular analysis
The bone marrow samples were collected from all patients with AML at the time of diagnosis. In accordance with the routine chromosome preparation and technical operation of R-band display, cytogenetic aberration descriptions were provided according to the 2013 International System for Human Cytogenetic Nomenclature by microscopic analysis [Citation16]. Molecular screening for the AML1-ETO gene, Nucleophosmin 1(NPM1) mutation, Fms-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) mutation and c-KIT mutation was completed by the Hematology Institute in our hospital as previously described [Citation17].
Treatment
For younger (<60 years) patients and older (≥60 years) fit patients with AML, the regimens of the first cycle of induction chemotherapy were IA (idarubicin 8–10 mg/m2 per day on days 1–3, cytarabine 100 mg/m2 per day on days 1–7) or HAA (homoharringtonine 2 mg/m2 per day on days 1–7, cytarabine 100 mg/m2 per day on days 1–7, aclarubicin 20 mg per day on days 1–7). Patients who failed to achieve a first complete remission (CR) received a second cycle of induction chemotherapy or changed to a second-line regimen. High-dose cytarabine (1–3 g/m2 every 12 h for 3 days) was used for post-remission consolidation chemotherapy. For older patients with AML who were unfit for intensive therapy, a low-intensity regimen such as CAG (cytarabine 20 mg every 12 h on days 1–14, aclarubicin 10–14 mg/m2/day on days 1–4; granulocyte-stimulating factor 150 µg/m2 every 12 h on days 0–14), decitabine or decitabine combined with CAG was given as induction therapy. The follow-up treatment was determined by individual clinicians’ choice and patients’ decision. Hematopoietic stem cell transplantation (HSCT) was performed in patients who experienced bone marrow relapse, those with c-KIT mutations, and those with molecular relapse defined by real-time quantitative PCR or flow cytometry.
Definitions of treatment response and end points
Definitions of treatment response were employed as previously recommended [Citation18].
Complete remission (CR) was defined as <5% bone marrow blasts in the setting of peripheral blood cell count recovery (neutrophils >1,000/µL; platelets >100,000/µL) after single chemotherapy induction or after second induction if completed within 30 days of first induction. Overall survival (OS) time was defined as the time from the clear diagnosis of the patient's disease until death (including death of any cause) or missing or the end of follow-up. Disease-free survival (DFS) refers to the interval from the date of CR to the date of leukemia relapse, death (death from any cause) or follow-up deadline.
Statistical analysis
Continuous variables were expressed as medians (ranges) and were compared using the independent sample t-test or Mann–Whitney non-parametric test. Count data were compared using chi-square test or Fisher’s exact test. Survival analysis used the Kaplan-Meier method, and the differences between groups were compared using the log-rank test. The criterion for statistical significance was P < .05. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model for OS and DFS. Differences between the comparative test results were considered significant if the two-sided P-value was <.05. All statistical analyses were performed using SPSS 19.0.
Results
Patient characteristics
Among the 77 cases of AML patients, there were 36 cases in the t(8;21) with LOS group (27 males and 9 females) and 37 cases in the t(8;21) alone group (14 males and 23 females). There were proportionately more male patients in the t(8;21) with LOS group (P = .001) when compared with the t(8;21) alone group. There were no significant differences in the other variables between the two groups (P > .05). The molecular mutations, including c-KIT, FLT3-ITD and NPM1 also had no obvious differences between the t(8;21) with LOS group and the t(8;21) alone group. See for details.
Table 1. The clinical characteristics of patients in t(8;21) with LOS group (n = 36) and t(8;21) alone group (n = 37).
Analysis of LOS on the impact of the efficacy and prognosis of AML patients
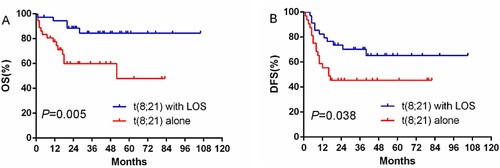
Among the 73 patients, 54 (74.0%) patients achieved CR after the first cycle of induction therapy. After the second repeated induction therapy, 13 patients achieved a CR. In total, 35 cases (97.2%) in the t(8;21) with LOS group and 32 cases (88.9%) in the t(8;21) alone group achieved overall CR. A total of 67 (91.8%) patients who achieved a CR were enrolled in the DFS analysis. Among 24 patients who received HSCT, 21 cases received allo-HSCT, and 3 cases received auto- HSCT. Of 21 patients who received allo-HSCT, 3 cases had c-KIT mutations, 13 cases relapsed, including molecular relapse, and 4 cases urged for HSCT for personal reasons. Sixteen cases received HSCT after the first cycle of induction therapy (8 [22.9%] of the t(8;21)with LOS group and 8 [25%] of the t(8;21) alone group; P = .863). The OS of the t(8;21) with LOS group was significantly longer than that of the t(8;21) alone group, and the median OS times were 37 and 17.5 months, respectively, with statistical significance (P = .005). However, the DFS of the two groups was not statistically significant (P = .263).
In the t(8;21) with LOS group, 15 patients were treated with high-dose cytarabine (HDAC) after first remission, with the outcome that only one of them died, and 8 patients received HSCT, with the outcome that one of them died due to graft-versus-host disease (GVHD). In the t(8;21) alone group, 15 patients were treated with HDAC as consolidation therapy; three of them died, and two cases who received HSCT died due to multiple organ failure.
In our research, 30 t(8;21) AML patients received HDAC therapy during consolidation. In the subgroup of patients with HDAC therapy, the DFS of the t(8;21) with LOS group was longer than that of the other group (P = .041) through Kaplan-Meier method, while the OS times of these two groups were not significantly different (P = .181). For the patients receiving HSCT after CR, both the OS (P = .362) and DFS (P = .362) of these two groups were not significantly different. See and and for details.
Figure 2. Curves comparison of OS and DFS for t(8;21) with LOS group and t(8;21) alone group with HDAC consolation therapy.
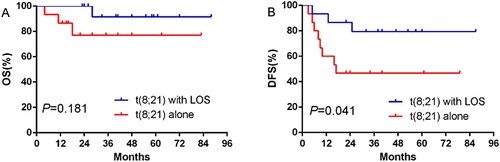
Table 2. Treatments and outcomes of t(8;21) with LOS group (n = 36) and t(8;21) alone group (n = 37).
Univariate and multivariable Cox regression analysis of t(8;21) AML patients’ clinical parameters
To determine independent prognostic predictors for multivariable logistic regression, significant variables were identified by univariate analysis (P < .1). The cut-offs for continuous variables were based on a previous report [Citation13]. In the analysis of DFS, five variables were identified by univariate analysis, and blasts ≥50% and FLT3-ITD mutation were confirmed as independent prognostic predictors for t(8;21) AML patients. In the analysis of OS, univariate analysis indicated that FLT3 mutations were all significantly associated with poor overall survival (P = .005). Conversely, t(8;21) with LOS AML patients tended to have better outcomes (P = .029). Ultimately, LOS and FLT3-ITD mutation were confirmed as independent prognosis predictors of t(8;21) AML by multivariable logistic regression analysis (LOS, HR 0.258, 95% confidence interval 0.077–0.871, P = .029; FLT3-ITD, HR 5.677, 95% confidence interval 1.681–19.168, P = .005). LOS was a favorable factor for overall survival, while FLT3-ITD mutation was significantly associated with poor progression-free survival. See and for details.
Table 3. Univariate and multivariable cox regression analysis of t (8;21) AML patients’ clinical parameters in DFS.
Table 4. Univariate and multivariable cox regression analysis of t (8;21) AML patients’ clinical parameters in OS.
Discussion
The t(8;21) AML is firmly established as a distinct biological and clinically relevant AML subtype with a favorable prognosis, while a portion of these patients still relapse after achieving CR and show poor prognosis. LOS is the most common ACA in t(8;21) AML, but its prognostic significance remains controversial. In this retrospective cohort study, LOS was identified as a favorable prognostic factor in t(8;21) AML patients without other ACAs.
LOS is more frequently observed in t(8;21) AML than other subtypes of AML. There were several studies exploring the effect of LOS on the pathogenesis of t(8;21) AML. Kuchenbauer F et al found that AML1-ETO alone is not enough to induce tumorigenesis [Citation19]. In their murine models, overexpression of AML1-ETO without additional genetic and molecular mutations did not show a leukemic transformation [Citation20]. In another study, Matsuura et al discovered that the gene encoding the GM-CSF receptor α subunit (CSF2RA) on the human sex chromosome plays an unexpected tumor-suppressor role. The occurrence of LOS certainly accompanied by the loss of the tumor suppressive effect of GM-CSF [Citation8]. Therefore, loss of the CSF2RA may be a critical mutation explaining the high incidence of LOS in t(8;21)(q22;q22). The studies above both explained the synergistic effect of LOS and AML1-ETO on pathogenesis of AML.
Despite the fact that the prognostic significance of LOS in AML has been explored by many studies, the prognostic impact of LOS in AML has remained controversial. Our study found that LOS might be a favorable prognostic indicator in t(8;21) AML patients without other ACAs. Krauth, M. T. et al suggested that LOS (either -X or -Y) was associated with a significantly better outcome, which is consistent with our results [Citation14]. In contrast to our study, Wei Zhou et al showed an adverse prognostic impact of loss of the Y chromosome in younger adult male patients with t(8;21) AML on high-dose cytarabine consolidation therapy [Citation12]. This discrepancy may vary slightly due to the use of different therapies or different age distributions. On the other hand, the conflicting data in previous reports may be induced by the impact of other ACAs in t(8;21) AML. Some studies suggested that secondary cytogenetic aberrations are associated with a subset of t(8;21) AML patients with poor survival, such as the deletion of the long arm of chromosome 9 (del 9q), which was thought to be an unfavorable prognostic factor [Citation15]. The result suggested that the ACAs had a potential prognostic value in t(8;21) AML.
In the t(8;21) with LOS group, 15 patients were treated with high-dose cytarabine (HDAC) after first remission, with the outcome that only one of them ultimately died. For the patients who received HDAC after first remission, the t(8;21) patients with LOS had significantly longer DFS compared with the t(8;21) alone patients. Accordingly, our research may put forward a hypothesis that the t(8;21) AML patients with LOS are always associated with favorable outcome and that this subtype may achieve longer DFS with chemotherapy consolidation therapy. Chen, G. also found that allo-HSCT may not be necessary for patients with -X; chemotherapy could be an appropriate consolidation regimen for this group [Citation2]. In the NCCN guidelines, HSCT is only recommended for t(8;21) AML with high risk of relapse [Citation4]. HSCT was not suitable for t(8;21) AML with LOS without poor prognostic factors and sometimes could even cause a second recurrence and chronic graft-versus-host disease. However, for the t(8;21) AML patients with LOS who relapsed or occurred with other unfavorable molecular abnormalities such as C-Kit or FLT3 mutation, HCST is still a possibly better choice to achieve long-term survival. The t(8;21) AML is characterized by clinical and biological heterogeneity, which determines the need for more detailed prognostic stratification.
The study has limitations due to its retrospective nature; therefore, heterogeneities of induction and consolidation therapies exist in our study. A few patients in our study did not receive gene mutation tests; as a result, there was no prognostic impact of c-KIT mutations in patients with t(8;21). Due to the removal of patients with t(8;21) AML with other ACAs, the number of patients in the two groups was limited. Therefore, further researches with larger sample size or clinical trials need to be carried out.
Conclusions
Our study suggests that LOS is an independent prognostic factor related to better outcome with longer OS in t(8;21) AML without other ACAs, while FLT3-ITD mutation was associated with a poor prognosis. Our results indicated that the patients with LOS have relatively better outcome among the t(8;21) subgroup.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Peterson LF, Boyapati A, Ahn EY, et al. Acute myeloid leukemia with the 8q22;21q22 translocation: secondary mutational events and alternative t(8;21) transcripts. Blood. 2007;110(3):799–805. doi:10.1182/blood-2006-11-019265.
- Chen G, Zhou W, Gong D, et al. Loss of X chromosome predicts favorable prognosis in female patients with t(8;21) acute myeloid leukemia. Leuk Lymphoma. 2020;61(5):1168–1177. doi:10.1080/10428194.2019.1709836.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196.
- Duployez N, Marceau-Renaut A, Boissel N, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood. 2016;127(20):2451–2459. doi:10.1182/blood-2015-12-688705.
- Khoury H, Dalal BI, Nevill TJ, et al. Acute myelogenous leukemia with t(8;21)–identification of a specific immunophenotype. Leuk Lymphoma. 2003;44(10):1713–1718. doi:10.1080/1042819031000116698.
- Appelbaum FR, Kopecky KJ, Tallman MS, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135(2):165–173. doi:10.1111/j.1365-2141.2006.06276.x.
- Matsuura S, Yan M, Lo MC, et al. Negative effects of GM-CSF signaling in a murine model of t(8;21)-induced leukemia. Blood. 2012;119(13):3155–3163. doi:10.1182/blood-2011-04-350694.
- Liu XP, Xue YP, Liu SH, et al. [An analysis of cytogenetic characteristics and prognosis of 189 t (8; 21) acute myeloid leukemia patients]. Zhonghua nei ke za zhi. 2006;45(11):918–921.
- Parihar M, Kumar JA, Sitaram U, et al. Cytogenetic analysis of acute myeloid leukemia with t(8;21) from a tertiary care center in India with correlation between clinicopathologic characteristics and molecular analysis. Leuk Lymphoma. 2012;53(1):103–109. doi:10.3109/10428194.2011.603447.
- Zhu CY, Yang H, Niu JH, et al. [Clinical analysis of acute myeloid leukemia with t(8;21) (q22;q22) and loss of Y chromosome]. Zhongguo shi yan xue ye xue za zhi. 2014;22(4):950–956. doi:10.7534/j.issn.1009-2137.2014.04.013.
- Zhou W, Chen G, Gong D, et al. Loss of the Y chromosome predicts a high relapse risk in younger adult male patients with t(8;21) acute myeloid leukemia on high-dose cytarabine consolidation therapy: a retrospective multicenter study. Leuk Lymphoma. 2020;61(4):820–830. doi:10.1080/10428194.2019.1683734.
- Jung HA, Maeng CH, Park S, et al. Prognostic factor analysis in core-binding factor-positive acute myeloid leukemia. Anticancer Res. 2014;34(2):1037–1045.
- Krauth MT, Eder C, Alpermann T, et al. High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia. 2014;28(7):1449–1458. doi:10.1038/leu.2014.4.
- Schoch C, Haase D, Haferlach T, et al. Fifty-one patients with acute myeloid leukemia and translocation t(8;21)(q22;q22): an additional deletion in 9q is an adverse prognostic factor. Leukemia. 1996;10(8):1288–1295.
- Shaffer L, Mcgowanjoran J, Schmid M, et al. (2013). ISCN 2013: An International System for Human Cytogenetic Nomenclature.
- Wang L, Xu WL, Meng HT, et al. FLT3 and NPM1 mutations in Chinese patients with acute myeloid leukemia and normal cytogenetics. Journal of Zhejiang University Science B. 2010;11(10):762–770. doi:10.1631/jzus.B1000052.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2003;21(24):4642–4649. doi:10.1200/jco.2003.04.036.
- Kuchenbauer F, Schnittger S, Look T, et al. Identification of additional cytogenetic and molecular genetic abnormalities in acute myeloid leukaemia with t(8;21)/AML1-ETO. Br J Haematol. 2006;134(6):616–619. doi:10.1111/j.1365-2141.2006.06229.x.
- Kuchenbauer F, Feuring-Buske M, Buske C. AML1-ETO needs a partner: new insights into the pathogenesis of t(8;21) leukemia. Cell Cycle (Georgetown, Tex). 2005;4(12):1716–1718. doi:10.4161/cc.4.12.2256.