ABSTRACT
Background
FAM230B serves as an oncogenic lncRNA in both gastric cancer and papillary thyroid cancer, while its role in acute myeloid leukemia (AML) is unclear. We predicted that FAM230B could be a target of miR-140, a well-characterized tumor suppressor, and analyzed their interaction in AML.
Methods
Differential expressions of FAM230B and miR-140 in bone marrow mononuclear cells (BMMNCs) were determined by RT-qPCR. Correlations were analyzed by Pearson’s correlation coefficient. Subcellular FAM230B location was determined using cellular fractionation assay. The direct interaction between FAM230B and miR-140 was confirmed by RNA pull-down assay. The roles of FAM230B and miR-140 in cell proliferation were explored using BrdU assay.
Results
High FAM230B expression level and low miR-140 expression level were observed in AML. FAM230B and miR-140 were inversely correlated and directly interacted with each other. FAM230B could be detected in both cytoplasm and nuclear samples. MiR-140 overexpression downregulated FAM230B expression and suppressed the enhancing effects of FAM230B overexpression on cell proliferation.
Conclusion
MiR-140 may target FAM230B to suppress cell proliferation in AML.
Background
As a type of malignancy originating from bone marrow, Acute myeloid leukemia (AML) is characterized by the arrested hematopoietic precursors as early developmental stages [Citation1]. AML has been reported to be closely correlated with several risk factors, such as drug exposures, environmental exposures, familial syndromes, and antecedent hematologic disorders. However, most de novo AML patients show no identifiable risk factors [Citation2,Citation3], suggesting the complex molecular mechanism of this disease [Citation4,Citation5]. AML patients are usually treated with combined treatment of chemotherapy and radiotherapy, and bone marrow transplant is also performed in some cases [Citation6,Citation7]. However, even with active treatment, the overall survival of AML patients is still poor. It is estimated that only fewer than 25% of AML patients can survive 5 years after diagnosis [Citation8]. Therefore, more therapeutic approaches are needed.
Targeted therapies are emerging novel anti-cancer approaches that can be applied to suppress cancer development by affecting related gene expression [Citation9,Citation10]. Certain genes, such as HIF-1α and CD64, have been shown to be potential targets for AML targeted therapy [Citation10,Citation11]. However, AML targeted therapy is still under research, and more molecular targets are needed to improve its safety and efficiency. Without protein-coding capacity, lncRNAs and miRNAs affect related protein synthesis in cancers [Citation12,Citation13]. Specifically, lncRNAs may interact with other non-coding RNAs, DNAs, and proteins to affect chromatin stability and behaviors of cancer cells [Citation12]. Therefore, lncRNAs can be targeted to treat cancers. FAM230B is an oncogenic lncRNA in both gastric cancer and papillary thyroid cancer [Citation14,Citation15]. FAM230B promotes the progression of papillary thyroid cancer and gastric cancer by regulating miR-378a-3p/WNT5A axis and miR-27a-5p/TOP2A axis, respectively. However, its role in AML is unclear. We predicted that FAM230B is a target of miR-140. It is known that miR-140 can target many oncogenes, such as YES1, to suppress the oncogenic behaviors of cancer cells [Citation16]. Therefore, we speculated that FAM230B could interact with miR-140 to regulate cancers and analyzed their interaction in AML.
Materials and methods
AML patients and bone marrow mononuclear cells (BMMNCs)
Bone marrow samples were donated by a total of 60 AML patients and 60 controls at Hainan Cancer Hospital from January 2018 to January 2021 (Ethics Committee of Hainan Cancer Hospital approved this study). The 60 controls were with suspected non-malignant diseases, such as myelofibrosis and thrombocytopenic purpura. These conditions were excluded after analysis. About 2–5 ml of bone marrow was collected through bone marrow puncture, and mononuclear cells (BMMNCs) were isolated using lymphocyte separation medium (Sigma-Aldrich). All BMMNCs were stored in liquid nitrogen prior to the subsequent assays. All patients and controls signed informed consent. Patients’ clinical data were presented in .
Table 1. Clinical data of 60 AML patients and 60 controls included in this study.
AML cells and transfections
Two AML cell lines, KG-1 (ECACC, UK) and THP-1 (ATCC, USA), were used in this study and cultured in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% FBS at 37°C in an incubator with 5% CO2 and 95% humidity. To overexpress FAM230B and/or miR-140, KG-1 and THP-1 cells were transfected with FAM230B expression vector (pcDNA3.1 vector) and/or miR-140 mimic (RiboBio) using Lipofectamine 2000 (Invitrogen), with empty vector or miRNA mimic as the negative controls (NCs). Transfected cells were incubated in fresh media for 48 h prior to the subsequent analyses.
RNA isolations and RT-qPCRs
Total RNAs were isolated from BMMNCs and both KG-1 and THP-1 cells and incubated with DNase I for 2 h to remove genomic DNAs. The concentration and integrity of all RNA samples were determined with Bioanalyzer. All samples had a RIN value higher than 9, indicating high RNA integrity.
cDNA samples were prepared with 3000 ng total RNA as template and subjected to qPCRs to determine the expression of FAM230B and miR-140 with 18S rRNA and U6 as internal controls, respectively. Ct values of target genes were normalized to corresponding internal controls using the 2−ΔΔCt method.
RNA pull-down assay
FAM230B and NC RNAs were prepared through in vitro transcriptions and labeled with desthiobiotinylation at 3’end using Pierce RNA 3’End Desthiobiotinylation Kit (Thermo Fisher Scientific). The labeled RNAs were named Bio-FAM230B and Bio-NC, respectively. The two labeled RNAs were transfected into KG-1 and THP-1 cells. At 48 h of post-transfection, cells were lysed and incubated with Streptavidin-Dyna beads (Invitrogen) to pull down RNA complex. After that, RNAs were extracted and subjected to qRT-PCRs to determine miR-140 levels.
Cellular fractionation assay
Nuclear and cytoplasmic fractions of KG-1 and THP-1 cells were separated with PARIS kit (Thermo Fisher Scientific) through centrifugation. Total RNAs were separated from these two fractions and subjected to RT-PCRs to determine FAM230B level. PCR products were separated on 1% agarose gels, stained using ethidium bromide, photographed with MyECL imager, and analyzed.
BrdU proliferation assay
BrdU incorporation was analyzed to reflect cell proliferation. Briefly, transfected cells were cultured in fresh media for 48 h and incubated with BrdU (10 µM, BD Pharmingen) for another 2 h. After that, cells were fixed for 1 h and incubated with peroxidase-coupled anti-BrdU-antibody (Sigma–Aldrich) for 1 h at RT. After PBS washing, cells were incubated with peroxidase substrate (tetramethylbenzidine) for 30 min, and OD values at 450 nm were determined.
Statistical analysis
Comparisons between two and multiple independent groups were performed with paired t test and ANOVA Tukey’s test, respectively. Correlations were done with Pearson’s correlation coefficient. P < 0.05 was statistically significant.
Results
Fam230B and miR-140 are differentially expressed in AML
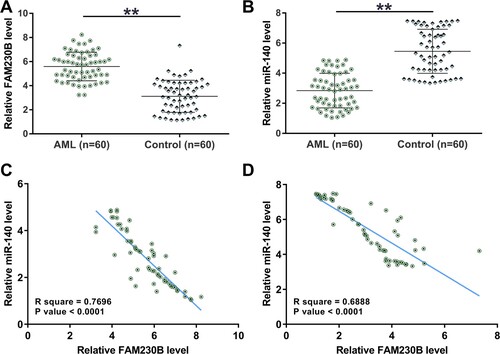
Total RNAs were isolated from BMMNCs from both AML patients and controls and subjected RT-PCRs to analyze differential expression of FAM230B and miR-140. It is worth noting that no significant differences in age and gender were observed between AML patients and controls. However, white blood cell count (WBC), hemoglobin (HB)level, and platelet (PLT) count were significantly higher in AML patients than in controls (p < 0.05 or 0.0001), reflecting the disease condition of AML (). Modified MRC risk stratification and ELN risk stratification [Citation17–19] of AML patients were also presented (). Compared to the controls, significantly higher FAM230B levels (A, p < 0.01) and lower miR-140 levels (B, p < 0.01) were observed in AML patients. Correlation analysis revealed that FAM230B and miR-140 were inversely and significantly correlated across both AML (C) and control (D) samples.
Figure 1. Differential expression of FAM230B and miR-140 in AML.
Note: The differential expression of FAM230B (A) and miR-140 (B) in BMMNCs from both AML patients and controls were analyzed through RNA isolations, followed by RT-PCRs. Correlations between FAM230B and miR-140 across both AML (C) and Control (D) samples were analyzed using Pearson’s correlation coefficient. **p < 0.01.
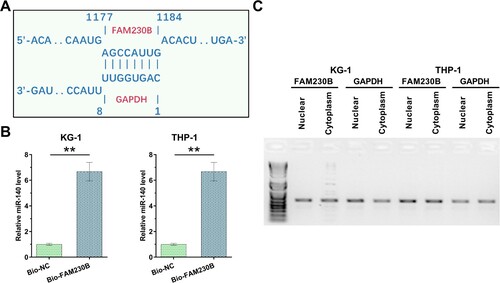
Direct interaction between FAM230B and miR-140 and subcellular FAM230B location in KG-1 and THP-1 cells
The potential interaction between FAM230B and miR-140 was predicted using IntaRNA 2.0. It was observed that FAM230B and miR-140 might form multiple base pairings (A). RNA pull-down assay was carried out to confirm the direct interaction between FAM230B and miR-140. Compared to Bio-NC pull-down samples, Bio-FAM230B pull-down samples showed significantly higher miR-140 level, suggesting the direct interaction between them (B, p < 0.01). Cellular fractionation assay was performed to determine the subcellular FAM230B location in KG-1 and THP-1 cells. It was observed that FAM230B could be detected in both nuclear and cytoplasm fractions (C), suggesting that FAM230B in cytoplasm fraction may directly interact with miR-140.
Figure 2. Direct interaction between FAM230B and miR-140 and subcellular FAM230B localization in KG-1 and THP-1 cells.
Note: IntaRNA 2.0 was used to predict the potential interaction between FAM230B and miR-140 (A). RNA pull-down assay was carried out to study the direct interaction between FAM230B and miR-140 (B). Cellular fractionation assay was performed to determine the subcellular localization of FAM230B in KG-1 and THP-1 cells (C). **p < 0.01.
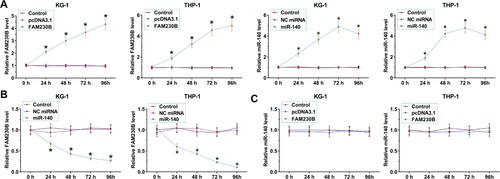
MiR-140 regulates FAM230B expression in AML cells
Overexpression of FAM230B or miR-140 in KG-1 and THP-1 cells were performed by transfection of their expression vector and confirmed every 24 h until 96 h using RT-qPCR (A, p < 0.05). Interestingly, miR-140 overexpression significantly downregulated FAM230B level (B, p < 0.05), while FAM230B overexpression did not significantly affect miR-140 expression (C), suggesting that. FAM230B may be targeted by miR-140 in AML cells.
Figure 3. The role of miR-140 in FAM230B expression.
Note: FAM230B or miR-140 were overexpressed in KG-1 and THP-1 cells, and FAM230B and miR-140 overexpression was confirmed every 24 h until 96 h (A). The role of FAM230B expression in miR-140 expression (B) and the role of miR-140 expression in FAM230B expression (C) in AML cells were analyzed with RT-qPCRs. *p < 0.05.
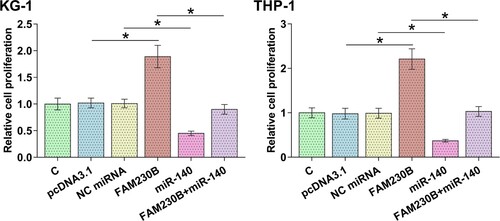
The role of miR-140 and FAM230B in AML cell proliferation
BrdU assays were performed to study the role of miR-140 and FAM230B in both KG-1 and THP-1 cell proliferation (). FAM230B overexpression increased the proliferation of both cell lines (p < 0.05) while miR-140 overexpression decreased proliferation of both cell lines (p < 0.05). In addition, miR-140 overexpression suppressed the enhancing effects of FAM230B overexpression on cell proliferation (p < 0.05).
Discussion
This study explored the role of miR-140 and FAM230B in AML and studied their interactions. The results showed that miR-140 and FAM230B expression was altered in AML. In addition, miR-140 might target FAM230B in AML cells to suppress AML cell proliferation.
The oncogenic roles of FAM230B have only been reported in papillary thyroid cancer and gastric cancer [Citation14,Citation15]. In papillary thyroid cancer, FAM230B is overexpressed and sponges miR-378a-3p to upregulate WNT5A, thereby accelerating tumor metastasis [Citation14]. In gastric cancer, FAM230B overexpression increases TOP2A expression by sponging miR-27a-5p to promote both tumor growth and metastasis [Citation15]. However, the role of FAM230B in other cancers, such as AML, is unclear. This study showed that FAM230B is overexpressed in AML. Moreover, FAM230B overexpression increased proliferation of two AML cell lines. Therefore, FAM230B may play an oncogenic role in AML by increasing cell proliferation. However, the downstream targets of FAM230B in AML cells are unknown. It is possible that FAM230B may also regulate TOP2A and WNT5A through miRNA sponging to regulate AML cell behaviors. Future studies are needed to test this possibility.
MiR-140 targets different oncogenes in different cancers to play tumor-suppressive roles [Citation16,Citation20]. For instance, miR-140 targets YES1 in gastric cancer to suppress cancer cell invasion, migration, and proliferation [Citation16]. In ovarian cancer, miR-140 targets PDGFRA to inhibit ovarian cancer cell growth [Citation20]. This study is the first to report miR-140 downregulation in AML and its inhibitory effect on cell proliferation. Although the function of FAM230B in cancer biology and its downstream targets have been reported [Citation14,Citation15], its upstream regulators in cancer biology are hardly known. Interestingly, we showed that FAM230B is located in both nuclear and cytoplasm fractions of AML cells and directly interacts with miR-140. In addition, miR-140 overexpression decreases FAM230B expression. It is well known that mature miRNAs, including miR-140, are mainly enriched in the cytoplasm. Therefore, FAM230B in the cytoplasm can be targeted by miR-140. Besides protein-coding genes, miR-140 can also target lncRNAs to play its tumor-suppressive role. Our study reported a novel function of miR-140 in cancer biology. Although we did not explore the interaction between FAM230B and miR-140 in normal cells, they are negatively correlated across both AML and control samples. Therefore, FAM230B may be targeted by miR-140, and the decreased miR-140 expression in AML results in increased FAM230B to promote AML. However, the mechanism that mediates miR-140 decrease in AML remains to be studied.
In conclusion, miR-140 is downregulated in AML and FAM230B is overexpressed in AML. In addition, miR-140 may target FAM230B in AML cells to suppress AML cell proliferation.
List of abbreviations
Acute myeloid leukemia (AML); bone marrow mononuclear cells (BMMNCs)
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184.
- Estey E. Acute myeloid leukemia: 2016 update on risk-stratification and management. Am J Hematol. 2016;91(8):824–846. doi:10.1002/ajh.24439.
- Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31(1):63–76. doi:10.1016/j.blre.2016.08.005.
- Lagunas-Rangel FA, Chávez-Valencia V, Gómez-Guijosa M, et al. Acute myeloid leukemia-genetic alterations and their clinical prognosis. Int J Hematol Oncol Stem Cell Res. 2017;11(4):328–339.
- Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2016;127(1):29–41. doi:10.1182/blood-2015-07-604496.
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. doi:10.1182/blood-2015-08-604520.
- Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767–774. doi:10.1182/blood-2014-08-551499.
- Cortes JE, Heidel FH, Fiedler W, et al. Survival outcomes and clinical benefit in patients with acute myeloid leukemia treated with glasdegib and low-dose cytarabine according to response to therapy. J Hematol Oncol. 2020;13(1):92. doi:10.1186/s13045-020-00929-8.
- Yu J, Jiang PYZ, Sun H, et al. Advances in targeted therapy for acute myeloid leukemia. Biomark Res. 2020;8:1–11. doi:10.1186/s40364-020-00196-2.
- Wang Y, Liu Y, Bailey C, et al. Therapeutic targeting of TP53-mutated acute myeloid leukemia by inhibiting HIF-1α with echinomycin. Oncogene. 2020;39(14):3015–3027. doi:10.1038/s41388-020-1201-z.
- Yong SB, Chung JY, Kim SS, et al. CD64-targeted HO-1 RNA interference enhances chemosensitivity in orthotopic model of acute myeloid leukemia and patient-derived bone marrow cells. Biomaterials. 2020;230:119651. doi:10.1016/j.biomaterials.2019.119651.
- Jiang MC, Ni JJ, Cui WY, et al. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9(7):1354–1366.
- Ishida M, Selaru FM. miRNA-Based therapeutic strategies. Curr Pathobiol Rep. 2013;1(1):63–70. doi:10.1007/s40139-012-0004-5.
- Zhou Q, Feng J, Yin S, et al. LncRNA FAM230B promotes the metastasis of papillary thyroid cancer by sponging the miR-378a-3p/WNT5A axis. Biochem Biophys Res Commun. 2021;546:83–89. doi:10.1016/j.bbrc.2021.01.109.
- Cui Y, Pu R, Ye J, et al. LncRNA FAM230B promotes gastric cancer growth and metastasis by regulating the miR-27a-5p/TOP2A axis. Dig Dis Sci. 2020;66(8):2637–2650. doi:10.1007/s10620-020-06581-z.
- Fang Z, Yin S, Sun R, et al. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16(1):139. doi:10.1186/s12943-017-0708-6.
- Metzeler KH, Herold T, Rothenberg-Thurley M, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128(5):686–698. doi:10.1182/blood-2016-01-693879.
- Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom medical research council trials. Blood. 2010;116(3):354–365. doi:10.1182/blood-2009-11-254441.
- Röllig C, Bornhäuser M, Thiede C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758–2765.doi:10.1200/jco.2010.32.8500.
- Lan H, Chen W, He G, et al. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed Pharmacother. 2015;75:117–122. doi:10.1016/j.biopha.2015.07.035.