ABSTRACT
Introduction
High-dose melphalan (HD-Mel) has been successfully employed in autografting patients with multiple myeloma. An advantage of this regimen is that the total dose of Mel can be delivered in a single day, being particularly useful when non-frozen hematopoietic stem cells are employed in the autograft.
Material and Methods
All consecutive patients with R/R lymphomas, both HL and NHL studied and treated at two different centers were prospectively included in a study of ASCT employing a single dose of HD-Mel (200 mg/m2). A group of R/R HL or NHL autografted employing BEAM-like preparative regimens was constructed matched by diagnosis and age. The primary endpoint of the study was overall survival (OS), the secondary endpoint was event-free survival (EFS).
Results
Twenty-five R/R HL/NHL patients were prospectively accrued in the study. There were 8 (32%) females, 13 (52%) patients had at least 1 adverse effect: 7 (28%) developed mucositis, 5 (20%) neutropenic fever, and 6 (24%) grade IV nausea. In the HD-Mel group, median overall survival (OS) was not achieved and OS at 36 months was 71%, the transplant-related mortality being 0%. In the control group, median OS was not achieved and the 36-month OS was 76%, results not statistically significant (p 0.5). The EFS was also similar in both groups (p 0.5).
Conclusion
HD-Mel alone is non-inferior to a BEAM-like regimen as a preparative regimen for autografting patients with R/R HL and NHL. The regimen is adequate to graft persons with non-frozen stem cells.
Introduction
Lymphoma incidence has been rising since the 1980s leading to the development of different therapeutic strategies. Although many patients achieve long-term remission after standard combination chemotherapy, between 30% and 70% of non-Hodgkin’s lymphoma (NHL) patients will relapse [Citation1], this outcome being different for patients with Hodgkin’s lymphoma (HL), up to 80% of whom can be cured by means of chemotherapy with or without radiation [Citation1]. Autologous hematopoietic stem cell transplantation (ASCT) has become the cornerstone of treatment for relapsed and refractory (R/R) lymphomas [Citation2]. Treatment-related toxicities prevent many patients from gaining the benefits of this procedure, and suboptimal results remain a serious concern. Reduction in treatment toxicities, while achieving good transplantation outcomes, should allow more patients to undergo this procedure with a potential for long-term remission or cure. The first series of lymphoma patients given ASCT was reported in 1978, using the BACT regimen (carmustine [bis-chloroethylnitrosourea (BCNU)]-cytarabine-cyclophosphamide-thioguanine) [Citation3]. Many variants were derived from the same chemotherapeutic backbone, among which was the BEAM (BCNU-etoposide-cytarabine-melphalan), first reported in 1986 [Citation4]. The BEAM regimen has strong conceptual points favoring its widespread application: it uses readily available well-known drugs and is highly effective in relapsed and refractory HL and NHL. However, later on, carmustine was replaced by fotemustine in some centers (FEAM) [Citation5], thiotepa in others (TEAM) [Citation6], and thiotepa/cyclophosphamide in others [Citation6,Citation7], while other combinations of drugs have also been employed in conditioning such as busulfan, melphalan, etoposide [Citation8] and others. All these preparative regimens render relatively similar long-term results and most of them employ melphalan in their combinations [Citation4–8]. High-dose melphalan (HD-Mel, 200 mg/m2) alone has been successfully employed in autografting patients with multiple myeloma (MM) [Citation9]. Since the total dose of Mel can be delivered in a single day, this preparative regimen is particularly useful when non-frozen hematopoietic stem cells are employed in the transplant, being the prototype of the ‘short’ conditioning regimen to conduct ASCT [Citation10,Citation11]. We present here the results of autografting lymphoma patients (both HL and NHL) with HD-Mel as conditioning, showing that it is not inferior to other BEAM-like combinations as conditioning regimens.
Material and methods
Patients
All consecutive patients with refractory or relapsed (R/R) lymphoma (both HL and NHL) were grafted with a single dose of HD-Mel (200 mg/m2) in the Centro de Hematología y Medicina Interna de Puebla (Puebla, México) and the Jehovah witness patients grafted at the Instituto de Cancerología Las Américas (Medellin, Colombia) were prospectively included in the study. A group of R/R lymphoma patients, both HL and NHL, autografted employing BEAM-like preparative regimens was constructed by matching diagnosis, stage, age and sex, recruited from the Hospital Universitario de Nuevo León (Monterrey, México). The primary endpoint of the study was overall survival (OS), the secondary endpoint was event-free survival (EFS). The protocol of the study was approved by the Institutional Review Board of all centers. All patients signed a consent to participate in the study according to the Helsinki declaration.
Autologous transplants
The peripheral blood stem cell (PBSC) mobilization schedule began at least 30 days after the last dose of chemotherapy. Subcutaneous G-CSF (10 µg/kg/day/five days) was given to mobilize the stem cells, starting day −5. Using either a peripheral vein or a Mahurkar-type subclavian catheter, the apheresis procedures were performed on days −3 to −1, using a COBE® Spectra (Terumo BCT, Lakewood, CO), Spectra Optia® (Terumo BCT, Lakewood, CO) or a Fenwal Amicus® (Fenwal, Lake Zurich, IL), and the Spin-Nebraska protocol [Citation9,Citation10]. The apheresis objective was to reach at least 1 × 106 viable CD34+ cells/kg.
Conditioning and autografting
Intravenous melphalan, 200 mg/m2 in a single I.V. dose was used on day −1 in the HD-Mel only group. In the group of patients autografted with BEAM-like regimens, the following combinations were employed: I.V. etoposide 300 mg/m2 and cyclophosphamide 50 mg/kg administered on days −3 to −1; alternative therapies were i.v. Mel combined with cyclophosphamide 100 mg/m2 and 600 mg/kg, respectively, infused on days −2 and −1, or i.v. etoposide and Mel 300 and 100 mg/m2, respectively, on days −2 and −1 (see ). Ondansetron (8 mg i.v. every 12 h after chemotherapy), ciprofloxacin (250 mg. bid) and fluconazole (200 mg bid) were used in all patients; they were used until granulocytes were greater than 0.5 × 109/L. G-CSF was given to all patients from day −5 until three consecutive days with >0.5 × 109/L granulocytes. In persons in whom prevention of transfusions (Jehovah witness) was important, additional support was given with 2000 IU erythropoietin every day and eltrombopag 50 mg per day. All patients had daily laboratory workup and clinical studies. All patients had complete blood testing every 24–48 h until day +30, after that, studies were done each 15 days until day +90. Complete blood chemistry samples were collected every 15 days until day +30, with monthly recollection afterwards.
Table 1. Summary of the conditioning regimens employed in the autografts.
Apheresis product preservation, studies and infusion
The products of the apheresis and 1 ml aliquots were kept in ACD-A (Baxter Healthcare, Deerfield, IL, USA) at 4°C, in 300 ml transfer packs (Baxter Healthcare) composed of gas impermeable, polyvinyl chloride plastic film for up to 72 h. Enumeration of the total white mononuclear cells (MNC) and CD34 positive cells was done by flow-cytometry in an EPICS Elite ESP apparatus (Coulter Electronics, Hialeah, FL, USA), using for the latter subpopulation the anti-CD34 monoclonal antibody HPCA-2 (Becton Dickinson, San José, CA, USA), gating in propidium iodide-excluding CD45(+) MNC population according to forward and 90° angle light scattering. Additional viability studies of the MNC used propidium iodide exclusion and anti-cell antibodies on a flow-cytometer. No purging procedures were performed. The apheresis products obtained on days −3 to −1 were reinfused to the patients on days 0 to +2, respectively, after keeping them in the conventional blood bank refrigerator [Citation9,Citation10]. Flow-cytometry was employed to evaluate CD34+ cell viability. Upon recollection, the products were maintained at 1–4°C in a standard blood bank refrigerator; the autograft was kept in these conditions until reinfusion on day 0.
Statistical analysis
Descriptive quantitative data are presented in mean and ranges in all cases and qualitative in absolute frequency and percentages (%). Mann-Whitney U and chi-square tests were employed to compare differences in baseline features between comparison groups. Survival analysis under the Kaplan–Meier method was conducted to assess overall survival (OS) and event-free survival (EFS). Univariate and multivariate Cox-regression models were employed to assess factors related to OS and EFS. Covariates for multivariate analyses employed age, diagnosis, sex and years of diagnosis and transplantation. Hazard ratios (HR) and their 95% confidence intervals were calculated and reported, as well as two-sided p-values for every statistical test. Statistical significance was set to an alpha level of 0.05. All the analyses were conducted in Stata MP version 16.0.
Results
Patients
Twenty-five patients were prospectively grafted employing HD-Mel as conditioning, 8 females and 17 males. The median age was 41 years (range 26.5–48). There were 12 NHL and 13 HL: 17 relapsed and 8 refractory. See .
Table 2. Salient features of the HD-Mel and BEAM-like groups.
Control group
A group of 25 patients with R/R NHL or HL autografted with BEAM-like conditioning was selected from the database of the Hospital Universitario de Nuevo León, to build a group of patients paired by diagnosis, stage, age and gender. shows the salient features of both groups of R/R HL or NHL patients autografted with HD-Mel alone or BEAM-like regimens.
PBSC mobilization and apheresis
All patients conditioned with HD-Mel received a full dose (200 mg/m2); the conditioning regimen was administered on an outpatient basis in 19/25 patients (76%). No patients that were treated with BEAM-like regimen underwent chemomobilization.
Conditioning and autografting
All patients conditioned with HD-Mel received a full dose (200 mg/m2); the conditioning regimen was administered on an outpatient basis in 19/25 patients (76%).
Apheresis product studies
In patients given HD-Mel, the median number of transplanted CD34+ viable cells was 1.8 × 106/kg (range 1.0–7.4); in all cases, the viability of the CD34+ cells was above 85% prior to being reinfused to the patients. For the BEAM-like regimen, the median number of transplanted CD34+ viable cells was 4 × 106/kg (range 1.2–21.6); a significant difference was observed between both groups (p 0.004).
Engraftment and response
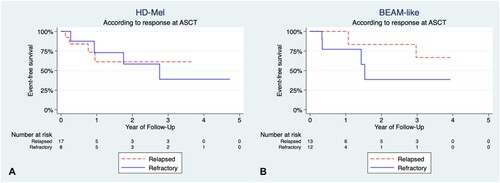
The time to achieve more than 0.5 × 109/L granulocytes had a median of 12 days (range 10–13) and 12 days (range 10–18) for HD-Mel and BEAM-like, respectively; no significant differences were found (p 0.7); whereas the time to recover more than 20 × 109/L platelets had a median of 14 days (range 10–25) and 12 days (range 9–20) for HD-Mel and BEAM-like, p 0.002. Median OS of the HD-Mel group was not achieved and 36-month OS was 71%, and Cox-regression models did not find any significant association (). Median EFS for the HD-Mel group was 33.8 months, whereas the 36-month EFS was 47%. Median OS for HL patients was not achieved, with a 36-month OS of 72%. No clinical factors were related to EFS on univariate and multivariate analyses (see ). Median OS for NHL patients was not achieved either, and the 36-month OS was 53%. The 36-month OS for refractory patients was 75%, and the 36-month OS for relapsed patients was 68%, the differences being not statistically significant ().
Figure 1. Overall survival (OS) comparison according to conditioning and main features. (A) OS of patients in the HD-Mel group according to response at ASCT (p 0.9). (B) OS patients in the BEAM-like group according to response at ASCT (p 0.4).
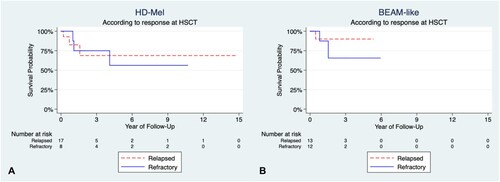
Table 3. Results of univariate and multivariate Cox-regression models for overall survival in patients transplanted with HD-Mel.
Table 4. Results of univariate and multivariate Cox-regression models for event-free survival in patients transplanted with HD-Mel.
Toxicity
3/25 (28%) patients had grade III/IV nausea or vomiting which was easily controlled with antiemetics. Five patients (16%) had neutropenic fever and 2 (40%) had to be admitted to the hospital for the delivery of intravenous antibiotics. 7/25 patients (28%) had mucositis. No patient died during the procedure, therefore, the transplant-related mortality for the HD-Mel group was 0%.
Comparison with the control group
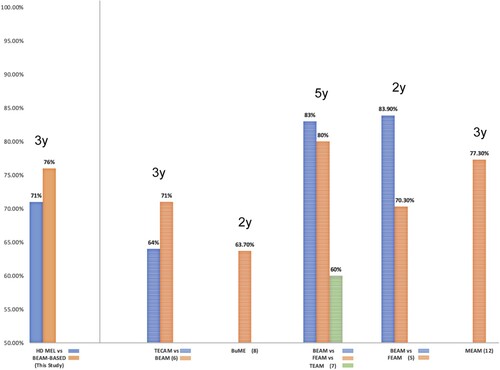
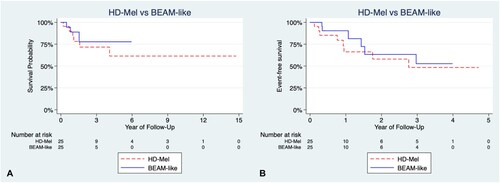
The salient features of the control group of R/R lymphoma patients grafted with other Mel-based conditioning regimens are shown in ; the differences in age, sex, type of lymphoma, stage, etc., are not statistically significant as shown. shows the OS of both groups of patients: those conditioned with HD-Mel and those conditioned with BEAM-like combinations; differences in both OS and EFS are not significant as depicted in . No differences were found when comparing with the clinical response at ASCT using HD-Mel or BEAM-like regimens (see ). refers to the three-year OS of patients with R/R lymphomas autografted with different conditioning regimens. The analysis shows that there are no statistically significant differences among the three-year OS of the patients.
Figure 2. Comparison of overall survival (OS) and event-free survival (EFS) between HD-Mel and BEAM-like cohorts. (A) OS in patients treated with HD-Mel or BEAM-like, there were no significant differences (p 0.5); (B) Comparison of EFS in patients treated with HD-Mel or BEAM-like, there were no significant differences (p 0.5).
Discussion
Long-term survival in patients with R/R NHL/HL remains poor, even with the most aggressive salvage chemotherapy regimens [Citation1]. High-dose chemotherapy followed by autologous stem cell transplantation improves OS in many of these patients. Improvements in conditioning regimens, source of stem cells and supportive care have made ASCT a safe procedure endowed with a low transplant-related mortality [Citation1]. The high doses of chemotherapy are responsible for the positive effect of ASCT in lymphomas, the stem cell support being useful to minimize morbidity and mortality of the procedure and to shorten the cytopenic period [Citation1]. Accordingly, improvement in both OS and EFS in R/R lymphoma patients have been informed using different types of high-dose chemotherapy such as the combinations of BEAM [Citation4], FEAM [Citation5], TEAM [Citation6], thiotepa/cyclophosphamide [Citation7], busulfan/Mel/etoposide [Citation8], MEAM [Citation12], cyclophosphamide/etoposide [Citation1,Citation11]. Some of these combinations employ Mel in intermediate doses (70–140 mg/m2) and there is preliminary information about successful HD-Mel conditioning in RR HL patients [Citation13], but this approach has not been extensively studied, while it has been widely employed in autografting multiple myeloma patients [Citation9–11]. A single dose of Mel 200 mg/m2 has several advantages over other drug combinations: It can be delivered in a single day on an outpatient basis, its safety profile is good, it can be employed to graft patients without freezing the stem cells and there is plenty of worldwide experience with its use in multiple myeloma [Citation9–11]. In addition, the cost of generic melphalan is currently lower than that of the drugs employed in other combinations [Citation14], and it is variable throughout the world; indicates the cost of the drugs in several parts of the world, the 50 mg vial cost ranging from 67 to 2500 US dollars, being noteworthy that the generic Mel is biologically equivalent to the innovator [Citation15]. Accordingly, in some places, HD-Mel may be more affordable than BEAM-like preparative regimens. For all these reasons, HD-Mel seems to be a good option to conduct HSCT in resource-constrained settings [Citation16,Citation17].
Table 5. Costs of a 50 mg vial of melphalan, either innovator or generic (*) in different parts of the world.
Our results suggest that HD-Mel alone is as effective as other chemotherapeutic combinations traditionally employed in the setting of autologous HSCT in R/R both HL and NHL. Its use is endowed with several advantages over the other preparative regimen, one of them being its affordability and safety profile. Since it can be delivered on a single day it would appear to be the preferred preparative regimen when autografts are done with non-frozen cells, where short duration preparative regimens are needed [Citation10,Citation11,Citation16,Citation17]. The main limitations of this study are the relatively small numbers of patients included and that in one center, five of the patients were selected based on the necessity of a short conditioning because of religious reluctance to receive blood transfusions or to avoid pulmonary-related complications with conventional regimens. Of those, three were Jehovah witnesses, and interestingly, they did not require transfusions as a result of the previously described management. Furthermore, two patients grafted with HD-Mel had a high risk for developing pulmonary complications and none developed it. Although this heterogeneous sample poses a significant limitation, the matched pair analysis design helped to decrease bias.
Conclusions
Our results show promising results regarding the use of affordable methods, including single HD-Mel as chemotherapy for ASCT. No significant differences in terms of OS and EFS were noted, but costs and logistical related conditions could be simpler in selected patients. Data from other centers are needed to ratify or rectify this observation, which may have economic consequences: the simplification of the methods for conducting stem cell transplantation will result in its affordability and widespread use, with the goal of benefiting the largest number of patients worldwide [Citation16].
Acknowledgements
This study was approved by the Institutional Review Board from Centro de Hematología y Medicina Interna, the Hospital Universitario de Nuevo León and the Instituto de Cancerología, Clínica Las Americas.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Gutiérrez-Aguirre CH, Ruiz-Argüelles G, Cantú-Rodríguez OG, et al. Outpatient reduced-intensity allogeneic stem cell transplantation for patients with refractory or relapsed lymphomas compared with autologous stem cell transplantation using a simplified method. Ann Hematol. 2010;89:1045–1052.
- Sánchez-Valledor LF, Habermann TM, Murrieta-Alvarez I, et al. Long-term results of the treatment of Hodgkin’s lymphoma in a resource-constrained setting: real-world data from a single center. World J Clin Oncol. 2021;12:800–807.
- Appelbaum FR, Herzig GP, Ziegler JL, et al. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95.
- Anderson CC, Goldstone AH, Souhami RL, et al. Very high dose chemotherapy with autologous bone marrow rescue in adult patients with resistant relapsed lymphoma. Cancer Chemother Pharmacol. 1986;16:170–175.
- Olivieri J, Mosna F, Pelosini M, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: An observational study on 1038 patients from Fondazione Italiana Linfomi. Biol Blood Marrow Transpl. 2018;24:1814–1822.
- Joffe E, Rosenberg D, Rozovski U, et al. Replacing carmustine by thiotepa and cyclophosphamide for autologous stem cell transplantation in Hodgkin’s and non-Hodgkin’s B-cell lymphoma. Bone Marrow Transpl. 2021;53:29–33.
- Marchesi F, Capria S, Pedata M, et al. BEAM conditioning regimen ensures better progression-free survival compared with TEAM but not with FEAM in lymphoma patients undergoing autologous stem cell transplant. Leukemia Lymph. 2020;61:2238–2241.
- Kim K, Kim W, Kim S, et al. Treatment with intravenous busulfan, melphalan, and etoposide followed by autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma: a multicenter study from the consortium for improving survival of lymphoma. Transpl Int. 2020;33:1211–1219.
- López-Otero A, Ruiz-Delgado GJ, Ruiz-Argüelles GJ. A simplified method for stem cell autografting in multiple myeloma: a single institution experience. Bone Marrow Transplant. 2009;44:715–719.
- Ruiz-Argüelles GJ, Gómez-Rangel D, Ruiz-Delgado GJ, et al. Results of an autologous noncryopreserved, unmanipulated peripheral blood hematopoietic stem cell transplant program: a single-institution, 10-year experience. Acta Haematol. 2003;110:179–183.
- Karduss-Urueta A, Gale RP, Gutiérrez-Aguirre CH, et al. Freezing the graft is not necessary for autotransplants for plasma cell myeloma and lymphomas. Bone Marrow Transplant. 2018;53:457–460.
- Sugimoto M, Ito S, Mashima K, et al. Retrospective evaluation of the MEAM regimen as a conditioning regimen before autologous peripheral blood stem cell transplantation for lymphoma in two centers with different dosing schedules of melphalan. Ann Hematol. 2016;95(9):1513–1519.
- Al Hashmi H, Kaloyannidis P, Kafnar S, et al. Single-agent high-dose melphalan as conditioning regimen in autologous hematopoietic stem cell transplantation for Hodgkin’s lymphoma: safety, and long-term efficacy. Biol Blood Marrow Transpl. 2018;24:S132–S1S3.
- Ruiz-Argüelles GJ. Whither the bone marrow transplant? Hematology. 2010;15:1–3.
- Pai AA, Devasia AJ, Panetta JC, et al. Pharmacokinetics and efficacy of generic melphalan is comparable to innovator formulation in patients with multiple myeloma undergoing autologous stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2020;20:130–135.
- Ruiz-Argüelles GJ. Lessons learned starting a bone marrow transplantation programme in a resource-constrained setting. Lancet Haematol. 2020;7:e509–e10.
- Gale RP, Ruiz-Argüelles GJ. The big freeze may be over: a contracting universe for cryopreservation? Bone Marrow Transpl. 2018;53:947–948.