ABSTRACT
Objectives
The current study aimed to explore the incidence of MPL mutations and the clinical and molecular characteristics of AML with MPL mutation.
Methods
In total, 1509 patients with newly diagnosed AML were retrospectively analyzed between January 2017 and December 2020. MPL mutations were detected via next-generation sequencing. During the same period, we also enrolled 30 patients with other myeloid neoplasms (MNs) with MPL mutation, which included myelodysplastic syndrome (n = 15), myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) (n = 6), and MPN (n = 9). The clinical characteristics of MPL-mutated AML and other types of MNs or MPL-wide type (MPL-wt) AML were compared, and the spectrum of co-mutations and MPL mutation profiles in MPL-mutated AML were analyzed.
Results
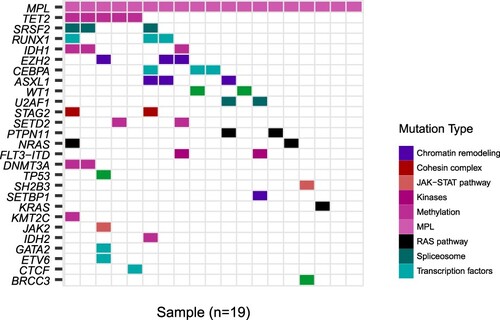
MPL mutations were identified in 19 (1.26%) of 1509 patients with AML. The waterfall diagram showed that the co-mutations were mainly epigenetic modifications (TET2, IDH1, and EZH2), spliceosomes (SRSF2), and transcription factors (RUNX1). The platelet count of the AML group was significantly lower than that of the MPN group (p = 0.001). MPL mutations were commonly observed in the intracellular region in AML but the transmembrane region in MPN (p = 0.013). The MPL-mutated AML group had a lower white blood cell count and a lower rate of complete remission than the MPL wild-type AML group (p = 0.037).
Conclusion
MPL mutations are clinically relevant in patients with AML, and they may be a novel subtype characterized by lower white blood cell counts and poor complete remission rates. However, further studies must be conducted to identify its correlated mechanism.
KEYWORDS:
1. Introduction
The myeloproliferative leukemia virus oncogene (MPL), which encodes the thrombopoietin receptor (TPO-R), plays vital roles in not only regulating megakaryopoiesis and platelet production but also supporting the maintenance and self-renewal of hematopoietic stem cells (HSCs) [Citation1]. Recent studies have identified multiple MPL mutations that can lead to severe hematological disorders [Citation2,Citation3]. For example, loss-of-function MPL mutations have been reported in familial aplastic anemia, and gain-of-function MPL mutations are associated with myeloproliferative disorders caused by constitutive signaling [Citation4]. Several studies have revealed that MPL is involved in maintaining the properties of leukemia stem cells (LSCs) and can be a candidate surface marker of LSCs [Citation5]. To the best of our knowledge, MPL mutations have rarely been reported in patients with acute myeloid leukemia (AML). The current study aimed to explore the incidence of MPL mutations and the clinical and molecular characteristics of AML with MPL mutation. Moreover, it emphasized the importance of identifying the characteristics of patients with MPL-mutated (MPL-mut) AML. However, further studies must be conducted to evaluate its correlated mechanism.
2. Materials and methods
2.1. Case identification
In total, 1509 patients with newly diagnosed AML were retrospectively analyzed between January 2017 and December 2020. AML was diagnosed according to the revised 2016 World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia [Citation6]. MPL mutations were detected via next-generation sequencing (NGS). During the same period, we also enrolled 30 patients with other types of MPL- mutated myeloid neoplasms (MNs), which included myelodysplastic syndrome (MDS, n = 15), myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN, n = 6), and myeloproliferative neoplasm (MPN, n = 9). Targeted sequencing of 172 known genes, which commonly mutate in myeloid neoplasms, was performed using Illumina Nextseq 550 sequencer (Illumina, San Diego, CA, the USA) (Supplementary Table 1).
2.2. Treatment methods
Induction chemotherapy regimens included standard chemotherapy (DA/IA, daunorubicin/idarubicin 60/10 mg/m2 d1-3, cytarabine 100 mg/m2 d1-7) or decitabine-based induction among older patients or those with underlying comorbidities. The postremission strategies comprise intensive chemotherapy and high-dose therapy, followed by autologous or allogeneic hematopoietic-cell transplantation (HCT). The current study was approved by the local ethics committee of the institution and was conducted in accordance with the Declaration of Helsinki.
2.3. Clinical end-points and definitions
Complete remission was defined as bone marrow blasts of < 5%, absence of circulating blasts and blasts with Auer rods and extramedullary disease, absolute neutrophil count of > 1.0 × 109/L, and platelet count of > 100 × 109/L. Hematologic relapse was defined as bone marrow blasts of ≥ 5%, the reappearance of blasts in the blood, or the development of extramedullary disease. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death from any cause. Patients without data about death during the last follow-up were censored on the date that they were last known to be alive.
2.7. Statistical analysis
Median values (and ranges) were calculated for non-normally distributed data. Frequencies were compared using the chi-square test or the Fisher’s exact test for categorical variables after cross tabulation. The non-normally distributed data of the different groups were compared using the Kruskal–Wallis H test and the Bonferroni adjusted test. P value of < 0.05 was considered statistically significant. Statistical analyses were conducted using the Statistical Package for the Social Sciences software version 22 for Windows (IBM Corp.).
3. Results
3.1. Clinical characteristics of MPL-mut AML
MPL mutations were identified in 19 (1.26%) of 1509 patients with AML. The median age of 19 patients with MPL-mut AML was 48 (range: 18–84) years, and the female-to-male ratio was 1.1:1. Among 19 patients, 15 presented with de novo AML, 3 with secondary AML (2 from myelodysplastic syndrome, 1 from chronic myeloid leukemia), and 1 with treatment-related AML.
The NGS test was performed on 30 patients with other types of MPL-mut MNs. The platelet count, number of co-mutated genes, and location of MPL mutations significantly differed between the four groups (). Moreover, the platelet count of the AML group was significantly lower than that of the MPN group (p = 0.001). MPL mutations were most commonly observed in the intracellular region in AML but the transmembrane region in MPN (p = 0.013).
Table 1. Clinical characteristics of MPL-mutated MN patients.
The propensity score-matched method was used to match 19 patients with AML according to sex and age and patients with MPL wide-type (MPL-wt) AML. The MPL-mut group had a lower white blood cell (WBC) count (p = 0.001) and a lower rate of complete remission (CR) (p = 0.025) than the MPL-wt group ().
Table 2. Clinical characteristics of MPL-mutated and MPL-wt AML patients.
3.2. Spectrum of co-mutations and MPL mutation profiles in MPL-mut AML
To investigate the spectrum of co-mutations in MPL-mut AML, 172 known genes were selected for a complete mutational screening in 19 patients with MPL-mut AML. shows the waterfall diagram of MPL-mut AML. NGS showed that the co-mutations were mainly associated with epigenetic modifications (TET2, IDH1, and EZH2), spliceosomes (SRSF2), and transcription factors (RUNX1).
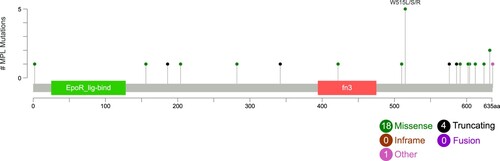
Among them, three patients had four MPL mutations (W515R/L/S and Y591D), and five presented with two types (with W515S as the dominant clone and Q586* as the subclone). In total, 23 kinds of mutations were divided into missense (n = 18), nonsense (n = 3), frameshift (n = 1), and others (n = 1). According to the location, mutations were classified into extracellular domain mutation (n = 7), transmembrane domain mutation (n = 1) and intracellular domain mutation (n = 15). MPL mutational profiles in AML patients are described in [Citation7].
3.3. Treatment and follow-up
Among the 19 MPL-mut AML patients, 2 only received supportive therapy, 11 received standard chemotherapy regimen(DA/IA), and the remaining 6 cases received decitabine-based induction due to unfit conditions:In total, 6 (31.58%) of 19 patients achieved CR after the first cycle of induction chemotherapy. In post-remission treatment, 10 patients received Allo-HCT. Among them, eight received haploidentical HCT and two 10/10 HLA-matched HCT. During the follow-up, one patient died, and 13 survived. The median OS was 17.5 (range: 2–37) months. Among patients with MPL-wt AML, 15 received standard chemotherapy regimen (DA/IA), and the remaining four received decitabine-based induction. Further, 13 (68.42%) of 19 patients achieved CR after the first cycle of induction chemotherapy. In post-remission treatment, 14 patients received Allo-HCT. Among them, eight patients received haploidentical HCT and six 10/10 HLA-matched HCT. During the follow-up, one patient died, and the median OS was 15 (range: 5–26) months.
4. Discussion
The MPL was first reported in 1992. Thereafter, MPL mutations in congenital amegakaryocytic thrombocytopenia (CAMT) and MPN were discussed. However, these mutations are extremely rare in patients with AML. CAMT is a rare inherited bone marrow failure syndrome presenting as isolated thrombocytopenia at birth that progresses to pancytopenia due to the exhaustion of hematopoietic progenitors [Citation8]. It is commonly caused by deleterious homozygous or compounds heterozygous mutations in MPL, which are often found in the extracellular domain. In our cases, all seven extracellular domain mutations were heterozygous, with VAFs of < 60%. However, there was no antecedent thrombocytopenia nor a significant family history. Hence, the likelihood of a pre-existing CAMT is not possible. W515R/L/S mutations are mainly found in ET and PMF in MPN [Citation9], and patients with ET with MPL mutations have a higher risk of secondary myelofibrosis and transformation to acute leukemia. Three patients presented with hotspot mutations (W515R/L/S). Among them, case three was secondary to MDS and case five was treatment-related AML,and the other patient with no previous hematology disease.In patients with prior MPLW515L-mutant myeloproliferative neoplasm, leukemic transformation was accompanied by MPL-mutant leukemic blasts [Citation10]. Since there was no previous NGS results in the 2 cases,the clonal evolution could not be completely assessed.
In 2016,studies assessed a thrombopoietin receptor antagonist, referred to as LCP4, which can deplete myelofibrosis hematopoietic stem and progenitor cells [Citation11]. Then, in 2020,some other researchers subsequently discovered that partial agonistic diabodies, by reducing the strength of the TPO-R signal, not only preserved HSCs in culture, but also blocked oncogenic signaling in ET.This finding has the potential to improve HSC cultures for transplants, as well as serve as a unique therapeutic approach for MPL-mut diseases [Citation12].
The current study had several limitations. That is, the sample size was small because of the low frequency of MPL mutations. Hence, it was challenging to analyze prognostic significance. Nevertheless, further studies should be conducted to assess the functions of the abovementioned modifications to validate the mechanism of leukemogenesis with MPL mutations and the role of small molecular–targeting drugs.
In conclusion, our study highlights the clinical relevance of MPL mutations in patients with AML. Our findings may be attributed to the higher number of patients in the adverse risk group and more patients were given the decitabine-based regimen instead of standard chemotherapy regimen. Moreover, MPL mutation may play an important role in it.
Novelty statements
MPL mutations in patients with acute myeloid leukemia (AML) are rarely reported. The current study explored the incidence of MPL mutations and the clinical and molecular characteristics of AML with MPL mutation. This research emphasized the importance of identifying the characteristics of AML with MPL mutation. Nevertheless, further studies must be conducted to identify the associated mechanism.
Supplemental Material
Download MS Word (15.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mignotte V, Vigon I, de Crèvecoeur EB, et al. Structure and transcription of the human c-mpl gene (MPL). Genomics. 1994;20(1):5–12.
- Jacob G, Jyoti N, Joanna B E, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379(15):1416–1430.
- Fox NE, Chen R, Hitchcock I, et al. Compound heterozygous c-Mpl mutations in a child with congenital amegakaryocytic thrombocytopenia: functional characterization and a review of the literature. Exp Hematol. 2009;37(4):495–503.
- Deutsch VR, Tomer A. Advances in megakaryocytopoiesis and thrombopoiesis: from bench to bedside. Br J Haematol. 2013;161(6):778–793.
- Huan L, Na Z, Yihui L, et al. c-MPL Is a candidate surface marker and confers self-renewal, quiescence, chemotherapy resistance, and leukemia initiation potential in leukemia stem cells. Stem Cells. 2018;9999:1–12.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional Cancer Genomics data. Cancer Discov. 2012;2(5):401–404.
- Germeshausen M, Ballmaier M. CAMT-MPL: congenital amegakaryocytic thrombocytopenia caused by MPL mutations- heterogeneity of a monogenic disorder - comprehensive analysis of 56 patients. Haematologica. 2021;106(9):2439–2448.
- Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112(1):141–149.
- Beer PA, Ortmann CA, Stegelmann F, et al. Molecular mechanisms associated with leukemic transformation of MPL-mutant myeloproliferative neoplasms. Haematologica. 2010;95(12).
- Wang X, Haylock D, Hu CS, et al. A thrombopoietin receptor antagonist is capable of depleting myelofibrosis hematopoietic stem and progenitor cells. Blood. 2016;127(26):3398–3409.
- Cui L, Moraga I, Lerbs T, et al. Tuning MPL signaling to influence hematopoietic stem cell differentiation and inhibit essential thrombocythemia progenitors. Proc Natl Acad Sci. 2021;118(2):e2017849118.