ABSTRACT
Nucleophosmin 1 (NPM1, also known as B23) is a multifunctional protein involved in a variety of cellular processes, including ribosomal maturation, centrosome replication, maintenance of genomic stability, cell cycle control, and apoptosis. NPM1 is the most commonly mutated gene in adult acute myeloid leukemia (AML) and is present in approximately 40% of all AML cases. The underlying mechanisms of mutant NPM1 (NPM1mut) in leukemogenesis remain unclear. This review summarizes the structure and physiological function of NPM1, mechanisms underlying the pathogenesis of NPM1-mutated AML, and the potential role of NPM1 as a therapeutic target. It is reported that dysfunctional NPM1 might cause AML pathogenesis via its role as a protein chaperone, inhibiting differentiation of leukemia stem cells and regulation of non-coding RNAs. Besides conventional chemotherapies, NPM1 is a promising therapeutic target against AML that warrants further investigation. NPM1-based therapeutic strategies include inducing nucleolar relocalisation of NPM1 mutants, interfering with NPM1 oligomerization, and NPM1 as an immune response target.
1. Introduction
AML is a malignancy characterized by clonal proliferation originating from primitive hematopoietic stem or progenitor cells. According to the 2016 WHO classifications, AML is classified into 6 categories: AML with recurrent genetic abnormalities, AML with myelodysplasia related changes, therapy-related myeloid neoplasms, AML, not otherwise specified (NOS), myeloid sarcoma, and myeloid proliferations associated with Down syndrome[Citation1,Citation2]. As a distinct subtype of AML with recurrent genetic abnormalities, AML with NPM1mut accounts for about 40% of AML and 60% of cytogenetically normal AML (CN-AML) [Citation3]. The frequency of NPM1mut in the Asian population is relatively lower (about 20%) compared to that in the European and American populations [Citation4]. AML patients with NPM1mut show unique biological and clinical features; for example, NPM1mut is more common in subtypes M1 (42%), M4 (57%), M5a (49%), and M5b (70%), leukemic cells are often marked by a cup-like nuclear invagination. Low expression or loss of CD34 expression was observed in more than 95% of NPM1mut AML [Citation5,Citation6]. NPM1mut is closely associated with the pathogenesis and prognosis of AML [Citation7], NPM1mut predicts higher response rates to induction therapies and longer overall survival (OS) in AML [Citation8–12], especially in those without a high burden of FLT3-ITD (FLT3-ITD/FLT3wt > 0.5), DNMT3A mutations and IDH mutations [Citation13–15]. This review focuses on the mechanisms underlying the pathogenesis of NPM1mut AML and the potential role of NPM1 as a therapeutic target.
2. Structure and function of NPM1
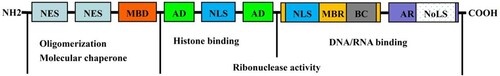
NPM1 is a multifunctional protein comprising 294 amino acid (AA) residues, it is mainly located in the nucleoli and is capable of shuttling between the nucleus and cytoplasm. It is encoded by the NPM1 gene located on chromosome 5q35 [Citation16]. NPM1 plays critical roles in ribosomal maturation, centrosome replication, maintenance of genomic stability, cell cycle control, and apoptosis. Inactivation of NPM1 in mice causes defects that lead to death in mid-pregnancy [Citation17]. In humans, NPM1 inactivation in adult hematopoietic stem cells (HSC) results in bone marrow failure [Citation18]. NPM1 exerts a chaperone-like activity via its N-terminal domain (NTD), thereby protecting the structure of multiple proteins to maintain their enzymatic activities during thermal denaturation [Citation19]. Two canonical nuclear export signals (NES) in the NTD of NPM1 () mediate its nuclear export by interacting with nuclear export protein 1 (XPO1) [Citation20] and are crucial for NPM1 function. In addition, NPM1 binds histones via the acidic domains (AD) of mimic DNA/RNA, which is rich in negatively charged aspartic acid and glutamic acid residues (acting as histone chaperones) and is further involved in nucleosome formation and chromatin remodeling [Citation21,Citation22]. The carboxy-terminal domain (CTD) of NPM1 is rich in hydrophobic aromatic amino acids (F268, Y271, F276, W288, and W290). Nucleolar localization signals (NoLS) located on the third helix (H3) of a special ‘three-helix bundle’ structure map of NPM1 in the nucleoli. Two tryptophan residues (W288 and W290) inside NoLS are essential in allowing NPM1 to maintain the ‘three-helix bundle’ structure [Citation20]. Between AD and CTD, NPM1 contains an alkaline fragment with more than 50 AA residues that binds to DNA/RNA. In addition, this region contains a nuclear localization sequence (NLS) and another NLS located between the two ADs. The two NLS act as importin α/β recognition sites, which mediate the nuclear localization of NPM1 () [Citation22].
Figure 1. Structure of NPM1. NPM1 is composed of an N-terminal oligomerization domain (NTD), a central region, and a C-terminal DNA/RNA binding domain (CTD). NTD contains two nuclear export signals (NES) and one metal binding domain (MBD), function as protein chaperone. The central region harbors two acidic domains (AD) and one nuclear localization signal (NLS), NPM1 binds histones via mimic DNA/RNA through its ADs. The CTD consists of another NLS, one moderately basic region (MBR), one basic cluster (BC), one aromatic region (AR), and one nucleolar localization signal (NoLS).
3. NPM1mut in AML
3.1. NPM1mut clone and AML pathogenesis
NPM1mut-AML was initially thought to be caused by haploinsufficiency of NPM1 [Citation23]. However, the identification of NPM1mut in CD14+ monocytes, CD66b+ granulocytes, CD19+ B cells, CD3-CD14-CD16+CD56+ NK cells as well as CD3+ T cells in AML patients harboring NPM1mut, suggested that NPM1mutAML originated from early-stage stem cells with the capacity to differentiate into any lineage [Citation24]. Moreover, NPM1mut was also found in leukemia stem cells (LSCs), and mice transplanted with CD34+ leukemic cells developed morphologically and immunohistochemically confirmed leukemia, predicting that NPM1mut is an initiating factor in the pathogenesis of AML [Citation5]. However, subsequent studies showed that a single NPM1 mutation only affected megakaryocyte development in mice and was not sufficient to cause AML [Citation25]. In addition, 36.5% of patients with relapsed NPM1mut-AML lost NPM1mut and developed new cytogenetic and molecular alterations [Citation26]. In recent years, it is hypothesized that different genetic events can cause leukemia transformation. Clinically, almost all NPM1mut were found to co-occur with mutations in genes involved in DNA methylation regulation (such as DNMT3A, TET2, IDH1 and IDH2), RNA splicing (SRSF2 and SF3B1) or genes associated with adhesive protein complexes (RAD21, SMC1A, SMC3 and STAG2) [Citation27]. Mutations associated with cell signaling pathways (FLT3, NRAS and PTPN11) also coexist with NPM1mut in AML [Citation27]. Furthermore, mice with DNMT3A-mutant clonal hematopoiesis (CH) progressed to myeloproliferative neoplasms (MPNs) after the introduction of NPM1mut, and more aggressive MPN was observed with a longer latency between the two mutations. MPN uniformly progressed to AML in secondary recipient mice. These results indicate that NPM1mut drove the evolution of DNMT3A-mutant CH to AML [Citation28]. Above all, these data suggest that NPM1mut might be a secondary molecular event in AML leukemogenesis.
3.2. Underlying mechanisms of NPM1mut in AML pathogenesis
NPM1 exon 12 mutations are frequent in AML patients and are classified as either type A (NPM1mA) and non-Type A mutations. NPM1mA (tandem duplications of TCTG occur between 863–864 nucleotides resulting in AA chain extension) is the main type of mutation in adult AML patients (accounting for 75–80% of adult NPM1mut-AML). Non-type A NPM1 mutations are called ‘rare type’ and are mainly caused by insertion of different bases at the end of exon 12. There are more than 40 mutations in total, which were named alphabetically in the order of detection (B-ZY) [Citation29].
These mutations lead to frameshift mutations in the CTD of NPM1, thus inserting a new NES or leading to loss of W288 and W290 (or W290 alone) in the original NLS, resulting in ectopic localization of NPM1 in the cytoplasm of leukemia cells (also known as NPM1c+) [Citation30]. Polypeptides harboring NPM1mut have different kinetics and oligomerization levels and therefore have different abilities to form amyloid aggregates. Thus, like wild-type NPM1 (NPM1wt), NPM1mut may have a modulated function by regulation of oligomerization [Citation7]. Moreover, NPM1-CTD bound with high-affinity G-quadruplex DNA regions found in ribosomal DNA, while NPM1 variants completely lost this activity, which is crucial for its nucleolar localization [Citation31]. This suggests that the abnormal structure of NPM1mut might lead to its cytoplasmic localization. In addition, NPM1wt could also abnormally localize to the cytoplasm by forming a heterodimer with NPM1mut [Citation32].
Compared with NPM1wt-patients, NPM1mut-AML patients were more likely to express NPM1mut, suggesting that the expression preference of mutant transcripts may be related to the pathogenesis of AML [Citation33]. NPM1mut further induces AML through a variety of mechanisms. In unstressed cells, NPM1 disrupts the ARF-MDM2 interactions, thereby inhibiting p14ARF-dependent p53 activation. In response to cellular stress, NPM1 and ARF may relocate to the cytoplasm and competitively bind to MDM2. Similar to the above, the abnormal cytoplasmic localization of ARF was induced by NPM1mut, resulting in its ubiquitination and degradation, thus impairing the function of the tumor suppressor ARF-MDM2-p53 axis [Citation20].
Fbw7γ (a member of the F-Box protein family and an important component of the E3 ligase complex of the Myc oncoprotein) is degraded following its abnormal cytosolic delocalization by mutated NPM1, thus stabilizing Myc and further promoting the proliferation and survival of leukemia cells [Citation29,Citation34–36]. NPM1mut may also act as a ‘molecular partner’ to directly form a complex with multiple transcription factors (such as CTCF, MEF/ELF4 and NF-κB, etc.) to interfere with the transcription of downstream target genes (such as MDM2, etc.), thereby fine-regulating the pathogenesis of AML [Citation37–39].
Another key event in the leukemogenesis of AML is the failure of LSCs to differentiate into mature hematopoietic cells. In embryonic stem cells, downregulation of NPM1 increased the transcription of key genes involved in mesodermal differentiation, suggesting its fundamental role in the maintenance of the stem cell phenotype [Citation40]. In AML, NPM1mut regulates a series of differentiation-inducing genes such as homobox (HOX), PBX homobox 3 (PBX3) and Meis homobox 1 (Meis) genes. XPO1 inhibition induced the relocalisation of NPM1mut to the nucleus and degradation of NPM1mut; this led to the down-regulation of the expression of HOX and Meis gene, thereby further inducing differentiation of AML cells into mature cells and prolonging the survival of leukemia mice carrying NPM1mut. The DOT1L inhibitor EPZ5676 promoted the apoptosis of leukemia cells harboring NPM1mut by decreasing the expression of HOXA9 and PBX3. These results suggest that persistent cytoplasmic localization of NPM1mut is necessary for the maintenance of leukemia stem cells [Citation41–43]. However, it has been reported that co-overexpression of NPM1mA with Meis1 or HOXA9 was not sufficient to induce AML [Citation44], highlighting the necessity of prodromal molecular events before NPM1mut acquisition. In a mouse model of AML in which mice were transplanted with NPM1+/− hematopoietic stem/progenitor cells co-transduced with mutant genes (such as IDH2R140Q), conditional knockout of IDH2R140Q resulted in the failure of LSC maintenance and implantation, and promoted the apoptosis of NPM1mut cells. These results suggested that IDH2mut is critical for the development and maintenance of AML-LSCs [Citation45]. Both knock-in mice carrying double mutations (NPM1mA/+NRASG12D/+ and NPM1mAFLT3-ITD) had AML-like manifestations and shared common characteristics (overexpression of HOX gene, enhanced self-renewal ability, amplification of hematopoietic progenitor cells, and myeloid differentiation preference). In addition, NPM1 mutation has significant molecular synergies with both NRASG12D and FLT3-ITD. Moreover, HOX is necessary for survival of both types of dual-mutated AML [Citation46].
In addition, in recent years, aberrations in non-coding RNAs (e.g. overexpression of miR-21, miR-10a, and miR-10b) have been found to be closely related to the occurrence and development of NPM1mut-AML [Citation47]. Long non-coding RNA (lncRNA) HOXB-AS3 upregulated proliferation of NPM1mut-AML cells in vitro and vivo, regulates ribosomal RNA transcription and de novo protein synthesis, which may serve as a compensation mechanism for adequate protein production in leukemia cells [Citation48]. Overexpression of HOXBLINC (a HOXB gene-related lncRNA) in mice enhanced the self-renewal capacity of HSC and led to AML-like disease [Citation49].
3.3. Therapy of NPM1mut-AML
Despite the high complete remission (CR) rate of NPM1mut-AML without FLT3 mutations after the standard ‘7 + 3’ IA regimen induction, all patients relapsed within 41 months. The CR rate and 3-year OS in patients who received fludarabine in combination with IA were significantly higher than those in the non-fludarabine group. These results suggested that AML patients with NPM1mut may benefit from intensive chemotherapy [Citation50,Citation51]. However, these patients did not benefit from allogeneic hematopoietic stem cell transplantation (allo-HSCT) compared with patients with autologous hematopoietic stem cell transplantation (ASCT) consolidation [Citation52,Citation53]. This contrasts with the results of the AML2003 trial, in which patients in the allo-HSCT arm had significantly better 3-year relapse-free survival (RFS) and OS than those in the non-allograft group (ASCT or chemotherapy consolidation). Among patients with normal karyotype and FLT3wt, the 3-year RFS of the allo-HSCT subgroup was still superior to that of the non-allo-HSCT group, but there was no significant difference in OS. This suggests that AML cases with NPM1mut, especially those with FLT3-ITD mutation, may still benefit from allo-HSCT [Citation54]. In addition, allo-HSCT is recommended as first-line consolidation therapy for NPM1mut-AML patients with other dismal prognostic factors, such as DNMT3AR882 mutation. If no suitable donor is available, these patients may still benefit from ASCT [Citation53,Citation55]. But allo-HSCT was not observed to improve the outcomes of patients with co-occurrence of FLT3-TKD and NPM1 mutations [Citation56]. In elderly or unfit patients, no significant difference was observed in OS between the NPM1mut and NPM1wt cohorts after azacytidine-containing induction therapy, suggesting that hypomethylating agents are unable to improve long-term survival when used in the first-line treatment of NPM1mut-AML [Citation57]. However, maintenance therapy with lenalidomide combined with azacytidine enhanced the function of cytotoxic T lymphocytes in AML patients with NPM1mut in remission, which further enabled sustained remission. Therefore, azacytidine/lenalidomide as maintenance therapy for high-risk AML deserves further study [Citation58].
4. NPM1 as a novel therapeutic target
Despite notable advances in the treatment of this frequent AML subtype in recent years, approximately 50% of AML patients with NPM1mut treated with conventional regimens eventually died of disease progression [Citation59]. In vitro, knockdown of NPM1 resulted in proliferation inhibition and increased the apoptosis of leukemia cells [Citation60]. The stable expression of NPM1 variants in NPM1mut-AML cells makes it a promising therapeutic target, and in recent years, an increasing number of therapies targeting NPM1 have been explored.
4.1. Nucleolar relocalisation of NPM1mut
Persistent cytoplasmic localization of NPM1mut is a key event in leukemia development [Citation41], therefore, interfering with NPM1mut mislocalisation is a feasible strategy for the treatment of NPM1-mutated AML. Both natural product avrainvillamide (AVA) and its fully synthetic AVA analog exerted potent anti-leukemia activities in vitro and vivo. And the NPM1-mutated cell line OCI-AML3 and primary AML cells were more sensitive to AVA than cells expressing NPM1wt, mainly due to AVA induced NPM1mut nuclear retention and enhanced proteasomal degradation of NPM1mut and XPO1 [Citation61]. Similarly, XPO1 inhibitor and EAPB0503 restored NPM1 nucleolar localization and promoted NPM1mut degradation. Furthermore, XPO1 inhibition led to the down-regulation of HOX, thus inducing AML cell differentiation, promoting apoptosis, and reducing the leukemia burden in xenograft mice [Citation41,Citation62].
4.2. Nucleolar stress induction
Actinomycin D induces nucleolar oxidation, while NPM1 is S-glutathionylated at Cys275 under stress, further leading to its dissociation from rDNA/rRNA and translocation to the nucleoplasm, thus interfering with ribosomal biogenesis. TP53 mutations or 17p deletion do not frequently co-occur with NPM1 mutations in AML, thus, p53-mediated nucleolar stress still exists, and there is still a low-level expression of NPM1wt in the nucleoli of NPM1mut-cells. It is speculated that the nucleoli of NPM1mut-AML cells may be sensitive to medicines that trigger nucleolar stress (such as actinomycin D). Falini et al. reported that a newly diagnosed 60-year-old AML patient with NPM1mut and FLT3wt who could not undergo intensive chemotherapy due to heart disease responded to Actinomycin D. Later, Guillaume et al. reported that 3/17 (18%) of relapsed/refractory (R/R) adult AML carrying NPM1 mutation achieved CR after 1 course of actinomycin [Citation9,Citation63]. In another phase II single-centre clinical trial, 10 adult patients with R/R NPM1 mutated AML were treated with actinomycin. All patients responded to the treatment, with 44% achieving CR/CRi, with good treatment tolerance. Compared with NPM1wt cells, low-dose actinomycin displayed a more effective stress response in NPM1mut-cells, suggesting that NPM1 mutated AML was more sensitive to nucleolar stress. Therefore, actinomycin is a potential treatment option for R/R NPM1 mutated AML and warrants further investigation [Citation64].
4.3. Interfere with NPM1 oligomerization
NSC348884 is a small molecule inhibitor that inhibits the oligomerization of NPM1, further inducing the apoptosis of OCI-AML3 cells and primary NPM1mut- AML cells sensitized by all-trans retinoic acid (ATRA). However, a recent study showed that NSC34884-mediated cytotoxicity in AML cells by regulating cell adhesion signaling rather than inhibiting NPM1 oligomerization [Citation65]. The 1-benzyl-2-methyl-3-indole-methyl-thiobarbituric acid analogs 7K and 7L were found to cause dose-dependent apoptosis in NPM1mut harboring OCI-AML3 cells. Molecular docking studies showed that they exert their potent anti-leukemia activities mainly by binding to the pocket in the central channel of the NPM1 pentameric structure [Citation66]. In irradiated cells, YTR107, a small molecule radiation sensitizer, suppressed the formation of the NPM1 pentamer by binding to NPM1, thus inhibiting SUMOylated NPM1 from associating with RAD51, preventing RAD51 foci formation and repair of DSBs. YTR107 can also acted synergistically with the PARP1/2 inhibitor ABT 888 to increase replication stress and radiation-induced cell lethality [Citation67].
The multivalent pseudopeptide N6L, a synthetic ligand of cell surface nucleolin, sensitized AML cells to doxorubicin and cytarabine treatment. N6L bound NPM1-NTD with high affinity and inhibits its phosphorylation. In AML cells, N6L co-localized with NPM1mut in the cytoplasm and interfered with its protein–protein associations, hence displaying its anti-leukemia activity [Citation68].
4.4. Down regulation of NPM1mut
Arsenic trioxide (ATO) and ATRA cause proteasome-dependent NPM1mut degradation, induce apoptosis of NPM1mut-AML cell lines and primary AML cells, and sensitize leukemia cells to daunomycin-containing chemotherapy. However, prolonged survival was not observed in NPM1 mutated AML treated with ATRA [Citation69,Citation70]. Both epigallocatechin3-gallate (EGCG) and deguelin (a rotenoid isolated from several plant species) inhibited the expression of NPM1, exhibited proliferation inhibition and apoptosis induction of NPM1-altered AML cells in vitro. However, deguelin has no such effect in OCi-AML2 cells (harboring NPM1wt), suggesting that these molecules are expected to be used in AML cases with NPM1 mutations [Citation71–73].
4.5. Therapeutic immune responses against the mutated NPM1
Somatic mutations in distinct cancers are a potential source of cancer-specific neoantigens, and NPM1mut is expected to be an ideal target for personalized immunotherapy due to its specificity in leukemia. Specimens of AML patients were stimulated with two endogenous HLA class I peptides (LAVEEVSLR and AVEEVSLRK) carrying NPM1mut originated from primary AML cells. ELISPOT assay revealed that interferon γ-secreting NPM1mut-specific T lymphocytes were observed in 50.6% of peripheral blood and 42.5% of bone marrow samples. The minimal residual disease (MRD) level in patients was negatively correlated with anti-leukemia specific T cells [Citation74]. Specific immunoresponsive CD8 cytotoxic T cells (CTLs) against NPM1 mutatants-derived epitopes may be involved in the clearance of MRD and is associated with a favorable prognosis and expected persistent MRD negative for this AML subtype [Citation75,Citation76]. These findings suggest that adoptive immunotherapy may be a promising treatment option for AML patients with NPM1mut, especially for maintenance therapy.
4.6. Factors co-expressed with NPM1 as therapeutic target
4.6.1. CD33
CD33 expression was significantly higher in newly diagnosed NPM1mut-AML patients than in NPM1wt patients, which laid a foundation for the application of anti-CD33 antibody (GO) in AML carrying NPM1mut [Citation77]. The CR/CRi rate and cumulative recurrence rate (CIR) of AML patients treated with a GO-containing regimen as initial induction therapy were significantly lower than those treated with standard induction group without GO [Citation78]. In the AMLSG 09-09 trial, the median NPM1mut copy number of patients treated with GO were significant lower across all treatment cycles, resulting in a significantly greater proportion of patients achieving MRD negativity. Moreover, addition of GO to the treatment of NPM1mut MRD-positive patients significantly reduced the NPM1mut copy number after two treatment cycles, thus significantly reducing CIR [Citation79].
4.6.2. Menin-MLL1
Overexpression of HOX genes is observed in almost all NPM1mut-AML patients. The histone modification enzymes MLL1 and DOT1L control the expression of HOX and FLT3 in NPM1-mutated AML, and the survival of NPM1mut-AML cells is particularly dependent on the Menin binding site in the MLL1 structure. In the NPM1-mutated AML mouse model, small-molecule inhibitors of Menin-MLL1 protein interaction (e.g. MI-3454) exhibit potent anti-leukemia effects. Blockage of both MLL1 and DOT1L led to significant inhibition of HOX and FLT3 expression, leukemia cell differentiation, and stronger anti-leukemia activity. In addition, MI-3454 was well tolerated and did not impair normal hematopoietic function in mice [Citation80,Citation81]. Menin inhibitor mainly targets CD34+CD38+ cells, while Venetoclax, a Bcl2 inhibitor, mainly targets CD34+CD38− cells. The combination of these two drugs may be effective at clearing hematopoietic stem/progenitor cells. Moreover, inhibition of Menin decreases the expression of a number of anti-apoptotic proteins and increases the expression of pro-apoptotic proteins. Therefore, in the AML PDX model carrying NPM1mutFLT3mut, treatment with a Menin inhibitor in combination with Venetoclax further improves mouse survival. The combination was more effective against primary AML cells harboring NPM1mutFLT3mut in vitro. On this basis, a triple combination regimen with FLT3 inhibitors may further enhance the efficacy [Citation82]. Therefore, Menin-MLL1 is a promising therapeutic target for NPM1-mutated AML. AML is often preceded by a premalignant state (clonal hematopoiesis or myelodysplastic syndrome), characterized by significant myeloid progenitor cell proliferation and self-renewal for a relatively long period. VTP-50469, a small molecule targeting Menin-MLL1, which reverses this self-renewal, might serve as a promising prophylactic treatment in the premalignant stage [Citation83].
4.6.3. Interleukin-3 receptor α chain (IL-3Rα, also known as CD123)
CD123 is overexpressed in NPM1mut-AML cells, especially in LSC and patients with FLT3mut. Tagraxofusp (SL401) formed by IL-3 fusion with diphtheria toxin has been approved for the treatment of patients with blastocytic plasmacytoid dendritic cell tumor (BPDCN). SL401 has strong cytotoxic effect on CD123+ AML and MDS-derived primary cells, and has shown efficacy in R/R AML patients. However, since significant cytotoxic activity against normal hematopoietic progenitor cells has been observed, adverse events might be the major limiting factor of its application [Citation84]. In December 2018, MB-102 (CD123 CAR-T) developed by Mustang Bio Inc. received the Orphan drug designation for the treatment of BPDCN. Subsequent studies have shown that MB-102 can be used as a novel immunotherapy agent for patients with R/R AML [Citation59,Citation85], and several clinical trials are currently underway. Therefore, CD123 as a valuable therapeutic target is worth further evaluation.
4.6.4. Bcl-2
Bcl-2 is found to be down-regulated by NPM1mut in AML cell lines [Citation86], however, in AML cases with NPM1mut or IDH2mut, treatment with the Bcl2 inhibitor venetoclax in combination with hypomethylated agents (HMA) or low-dose Ara-C results in a high response rate and durable molecular response, suggesting that patients with NPM1mut may benefit from venetoclax-containing chemotherapy [Citation87]. Venetoclax in combination with low-dose Ara-C or azacytidine induced durable molecular remission without transplantation in all AML patients with persistent NPM1mut-MRD. After 1–2 cycles of venetoclax, MRD turned negative in 85.7% (6/7) of patients with molecular relapses [Citation88]. Cell line OCI-AML3 is highly sensitive to ATO and moderately sensitive to venetoclax. However, ATO combined with venetoclax significantly inhibited cell proliferation and induced apoptosis without affecting NPM1 expression, suggesting that venetoclax and ATO have synergistic anti-leukemia effects. Subsequently, two R/R AML patients with NPM1mut were found to be sensitive to venetoclax in combination with oral arsenic [Citation89]. DiNardo et al reported that venetoclax plus azacitidine combination provided higher and more-durable response rates and an improved OS in previously untreated patients who were ineligible for standard induction therapy. Besides, in the cases with NPM1 mutations, venetoclax containing regimen was associated with a significantly higher incidence of composite CR rate (CR + CRi) than the control regimen (66.7% vs 23.5%, P = 0.012) [Citation90]. Similar results were observed in another study, significant improvement in OS was seen in NPM1mut-AML patients age >65 years treated with HMA+ venetoclax vs HMA or intensive chemotherapy. On account of the impressive results compared with traditional regimens, lower-intensity venetoclax combination therapies are largely considered an improved standard of care for older patients with NPM1mut-AML [Citation91].
4.6.5. Other NPM1-related therapeutic targets
Pediatric lymphoma driven by the NPM1-ALK fusion gene has a response rate to ALK inhibitors such as crizotinib of 54%–90%, and these can thus can be used as an alternative to standard chemotherapy with lower toxicity and side effects. However, a proportion of patients progress within merely 3 months after initial of treatment [Citation92]. The FLT3 inhibitor Gilteritinib inhibited the phosphorylation of the NPM1-ALK fusion kinase and hence blocked the downstream signaling pathway, further inducing G0-G1 cell cycle arrest, apoptosis, decreased c-Myc expression and down-regulated CD30 expression. Therefore, Gilteritinib can be used to treat NPM1-ALK positive anaplastic large cell lymphoma [Citation93]. In addition, there are many other molecular events related to NPM1, such as autophagy activation and co-expression of IDH1/2, which are expected to provide more targets for new targeted therapies for NPM1-mutated AML in the future [Citation94,Citation95].
5. NPM1mut as a MRD monitoring marker
NPM1 mutations are considered as an ideal marker for MRD monitoring in AML since they are stable over the course of disease including relapse in most cases, and can also express in the whole leukemic populations. The NPM1mut-MRD of AML in first CR (CR1) was positively correlated with their baseline NPM1mut VAF, suggesting that NPM1mut-MRD can be used as a good prognostic factor to guide post-remission treatment [Citation96]. Both achievement of MRD negativity and deep reduction in NPM1mut transcripts (>4-log) at multiple time points after conventional chemotherapy (especially after double induction therapy) predict low risk of relapse and high survival rates, even in FLT3-ITD mutated AML patients, independent of the allelic ratio [Citation97,Citation98]. After induction therapy, the cumulative 2-year CIR of patients with relative expression of NPM1mut higher than 0.01 was 77.8%, and the 2-year CIR of the remaining cases was 26.4% [Citation96,Citation99]. Among 152 NPM1mut-AML with evaluable MRD assessment in CR1 enrolled in ALFA-0702, patients who did not achieve a 4-log reduction of NPM1mut in peripheral blood after induction therapy had a higher CIR [Citation100]. However, Ing S. Tiong et al documented that patients with NPM1mut-MRD positivity after completing intensive chemotherapy had a variable course. One hundred NPM1mut-AML who received ≥2 cycles of intensive chemotherapy without allo-HSCT in CR1 were included if bone marrow was NPM1mut MRD positive at the end of treatment (EOT). After a median of 23.5-month follow-up, 42% remained free of progression at 1 year, either spontaneously achieving complete molecular remission (30%) or retaining low-level transcript in the bone marrow for ≥ 12 months (12%) [Citation101].
As ELN recommended, using a dynamic risk assessment approach including MRD monitoring, allo-HSCT is clearly indicated when the estimated leukemia relapse risk is above 35–40% [Citation100]. Cornellissen JJ et al reported that subjects who were MRD-test-positive pretransplant had a greatest benefit from a transplant than those who were MRD-test-negative pretransplant [Citation102]. However, pretransplant MRD positive was also found to be associated with increased overall mortality and recurrence rates [Citation103,Citation104]. Dillon et al showed that AML patients who had high NPM1mut transcripts before allo-transplant had a dismal 2-year survivals than those without or with low NPM1mut transcripts (13%, 83% and 63%, respective) [Citation105]. And, the prognostic MRD threshold is still controversial to date. Michal Karas et al reported that higher NPM1mut-MRD level with cut-off 0.1% in bone marrow before allo-HSCT was independent poor prognostic factor for OS [Citation103,Citation104].
Moreover, post-transplant MRD was a strong independent predictor of both relapse risk and 3-year OS (20% ± 17.9% versus 88.6% ± 7.8% for MRD-positive and MRD-negative patients, respectively) in adult NPM1-mutated AML patients [Citation104]. Platzbecker et al documented that in AML or advanced MDS cases who became MRD-positive in CR after either conventional chemotherapy only or consecutive allo-HSCT, pre-emptive 5-azacitidine resulted in overall response rates of 71% and 48% in patients with and without allo-HSCT, respectively. After a median follow-up of 13 months, OS rate was 76%, suggesting that MRD-guided therapy may prevent or delay morphologic relapse [Citation106]. However, the pre-emptive strategies are not currently standardized, which need to be further studied.
According to ELN recommendations, serial MRD monitoring at multiple timepoints was necessary. During active treatment phase, MRD should be measured at least at diagnosis, after two cycles of therapy and at the EOT. During follow-up period, MRD assessments is recommended every three months for the first two years. Thereafter, timing of MRD monitoring should be personalized according to relapse risk [Citation107]. Molecular NPM1mut MRD evaluation includes RNA-based (RT-qPCR) and DNA-based (Q-PCR, ddPCR and deep sequencing) levels. DNA-based methods show strong correlation (95% agreement). Although more sensitive, RT-qPCR failed to detect leukemic MRD in 10% of samples with detectable NPM1mut DNA, suggesting that DNA-based method may act as a complementary for RT-qPCR [Citation108]. Besides the standard molecular methods, flow cytometry and immunohistochemical (IHC) methods are also good choices for MRD detection. Flow cytometry is able to reliably detect NPM1mut populations of at least 10% by using the mean fluorescence intensity. The results were consistent with conventional PCR, and the advantage is the rapid determination of NPM1 status in newly diagnosed patients [Citation109]. Anita Chopra et al reported that ASO-PCR detected NPM1mut in 21 (60%) of the 35 AML patients, and IHC detected NPM1mut in 19 patients. 13/35 patients were negative for both tests, and one IHC positive patient was not detected by ASO-PCR. Compared with ASO-PCR, the sensitivity and specificity of IHC were 90% and 93%, respectively. Advantages of IHC include the ectopic expression of cytoplasmic NPM1mut could be determined, and IHC is critical for diagnosis of AML cases presented with myeloid sarcoma [Citation110].
However, 13.5% (14/104) cases relapsed with NPM1wt-AML based on analysis of paired bone marrow samples from NPM1mut-AML patients [Citation111]. Moreover, the predictive value of NPM1mut MRD was influenced by DNMT3A mutation status in elderly AML patients. DNMT3A status exerted no impact on the probability of having a > 4-log MRD reduction after induction. But post-induction NPM1mut MRD reduction was not predictive of OS and LFS in DNMT3Amut patients, predicting that the presence of DNMT3A mutation seems to abrogate the predictive value of NPM1mut good molecular responses. Therefore, as a monitoring marker for MRD in AML patients, NPM1mut should be analyzed together with other markers [Citation98].
6. Conclusion
In conclusion, the structural complexity and functional diversity of NPM1 can lead to several molecular abnormalities, including the formation of fusion genes and gene mutations that play an important role in the occurrence and development of AML. Further elucidation of the underlying mechanism of NPM1 in AML is expected to provide a better theoretical basis for finer clinical prognostic stratification and targeted therapy with new medications.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (13.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Falini B, Nicoletti I, Martelli MF, et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–885.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405.
- Schneider F, Hoster E, Schneider S, et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol. 2012;91:9–18.
- Zhang M, Yin J, He Q, et al. Chinese and Europeans with acute myeloid leukemia have discordant mutation topographies. Leuk Res. 2018;70:8–12.
- Martelli MP, Pettirossi V, Thiede C, et al. CD34 + cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice. Blood. 2010;116:3907–3922.
- Rose D, Haferlach T, Schnittger S, et al. Subtype-specific patterns of molecular mutations in acute myeloid leukemia. Leukemia. 2017;31:11–17.
- La Manna S, Florio D, Di Natale C, et al. Conformational consequences of NPM1 rare mutations: An aggregation perspective in acute myeloid leukemia. Bioorg Chem. 2021;113:104997.
- Jeon Y, Seo SW, Park S, et al. Identification of two novel NPM1 mutations in patients with acute myeloid leukemia. Ann Lab Med. 2013;33:60–64.
- Beziat G, Tavitian S, Bertoli S, et al. Dactinomycin in acute myeloid leukemia with NPM1 mutations. Eur J Haematol. 2020;105:302–307.
- Helbig G, Wozniczka K, Wieclawek A, et al. Clinical relevance of mutant NPM1 and CEBPA in patients with acute myeloid leukemia - preliminary report. Contemp Oncol (Pozn). 2014;18:241–245.
- Döhner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–3746.
- Juliusson G, Jädersten M, Deneberg S, et al. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020;4:1094–1101.
- Cappelli LV, Meggendorfer M, Dicker F, et al. DNMT3A mutations are over-represented in young adults with NPM1 mutated AML and prompt a distinct co-mutational pattern. Leukemia. 2019;33:2741–2746.
- Yang X, Shi J, Zhang X, et al. Biological and clinical influences of NPM1 in acute myeloid leukemia patients with DNMT3A mutations. Cancer Manag Res. 2018;10:2489–2497. doi:10.2147/CMAR.S166714.
- Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643.
- Mitrea DM, Grace CR, Buljan M, et al. Structural polymorphism in the N-terminal oligomerization domain of NPM1. Proc Natl Acad Sci U S A. 2014;111:4466–4471.
- Grisendi S, Bernardi R, Rossi M, et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437:147–153.
- Nachmani D, Bothmer AH, Grisendi S, et al. Germline NPM1 mutations lead to altered rRNA 2'-O-methylation and cause dyskeratosis congenita. Nat Genet. 2019;51(10):1518–1529.
- Szebeni A, Olson MO. Nucleolar protein B23 has molecular chaperone activities. Protein Sci. 1999;8:905–912.
- Luchinat E, Chiarella S, Franceschini M, et al. Identification of a novel nucleophosmin-interaction motif in the tumor suppressor p14arf. FEBS J. 2018;285:832–847.
- Lindström MS. NPM1/B23: a multifunctional chaperone in ribosome biogenesis and Chromatin remodeling. Biochem Res Int. 2011;2011:195209.
- Cela I, Di Matteo A, Federici L. Nucleophosmin in its interaction with ligands. Int J Mol Sci. 2020;21:4885.
- Liso A, Castiglione F, Cappuccio A, et al. A one-mutation mathematical model can explain the age incidence of acute myeloid leukemia with mutated nucleophosmin (NPM1). Haematologica. 2008;93:1219–1226.
- Dvorakova D, Racil Z, Borsky M, et al. Clonal heterogeneity in patients with cytogenetically normal acute myeloid leukemia with NPM1 mutations. Leuk Lymphoma. 2013;54:1056–1060.
- Sportoletti P, Varasano E, Rossi R, et al. The human NPM1 mutation A perturbs megakaryopoiesis in a conditional mouse model. Blood. 2013;121:3447–3458.
- Martínez-Losada C, Serrano-López J, Serrano-López J, et al. Clonal genetic evolution at relapse of favorable-risk acute myeloid leukemia with NPM1 mutation is associated with phenotypic changes and worse outcomes. Haematologica. 2018;103:e400–e403.
- Patel JL, Schumacher JA, Frizzell K, et al. Coexisting and cooperating mutations in NPM1-mutated acute myeloid leukemia. Leuk Res. 2017;56:7–12.
- Loberg MA, Bell RK, Goodwin LO, et al. Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis. Leukemia. 2019;33:1635–1649.
- Alpermann T, Schnittger S, Eder C, et al. Molecular subtypes of NPM1 mutations have different clinical profiles, specific patterns of accompanying molecular mutations and varying outcomes in intermediate risk acute myeloid leukemia. Haematologica. 2016;101:e55–e58.
- Falini B, Bolli N, Shan J, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood. 2006;107:4514–4523.
- Chiarella S, De Cola A, Scaglione GL, et al. Nucleophosmin mutations alter its nucleolar localization by impairing G-quadruplex binding at ribosomal DNA. Nucleic Acids Res. 2013;41:3228–3239.
- Kunchala P, Kuravi S, Jensen R, et al. When the good go bad: mutant NPM1 in acute myeloid leukemia. Blood Rev. 2018;32:167–183.
- Bailey GD, Doolan L, Baskar A, et al. Preferential transcription of the mutated allele in NPM1 mutated acute myeloid leukemia. Sci Rep. 2020;10:17695.
- Tang Y, Tao Y, Wang L, et al. NPM1 mutant maintains ULK1 protein stability via TRAF6-dependent ubiquitination to promote autophagic cell survival in leukemia. FASEB J. 2021;35:e21192.
- Wang L, Yang L, Yang Z, et al. Glycolytic enzyme PKM2 mediates autophagic activation to promote cell survival in NPM1-mutated leukemia. Int J Biol Sci. 2019;15:882–894.
- Di Matteo A, Franceschini M, Paiardini A, et al. Structural investigation of nucleophosmin interaction with the tumor suppressor Fbw7γ. Oncogenesis. 2017;6:e379.
- Sportoletti P, Celani L, Varasano E, et al. GATA1 epigenetic deregulation contributes to the development of AML with NPM1 and FLT3-ITD cooperating mutations. Leukemia. 2019;33:1827–1832.
- Wang AJ, Han Y, Jia N, et al. NPM1c impedes CTCF functions through cytoplasmic mislocalization in acute myeloid leukemia. Leukemia. 2020;34:1278–1290.
- Ando K, Tsushima H, Matsuo E, et al. Mutations in the nucleolar phosphoprotein, nucleophosmin, promote the expression of the oncogenic transcription factor MEF/ELF4 in leukemia cells and potentiates transformation. J Biol Chem. 2013;288:9457–9467.
- Johansson H, Simonsson S. Core transcription factors, Oct4, Sox2 and Nanog, individually form complexes with nucleophosmin (Npm1) to control embryonic stem (ES) cell fate determination. Aging (Albany NY). 2010;2:815–822.
- Brunetti L, Gundry MC, Sorcini D, et al. Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell. 2018;34:499–512. e9.
- Mullighan CG, Kennedy A, Zhou X, et al. Pediatric acute myeloid leukemia with NPM1 mutations is characterized by a gene expression profile with dysregulated HOX gene expression distinct from MLL-rearranged leukemias. Leukemia. 2007;21:2000–2009.
- Zhang W, Zhao C, Zhao J, et al. Inactivation of PBX3 and HOXA9 by down-regulating H3K79 methylation represses NPM1-mutated leukemic cell survival. Theranostics. 2018;8:4359–4371.
- Wiktorin HG, Nilsson T, Jansson A, et al. Mutated NPM1 in combination with overexpression of Meis1 or Hoxa9 is not sufficient to induce acute myeloid leukemia. Exp Hematol Oncol. 2015;5:25.
- Ogawara Y, Katsumoto T, Aikawa Y, et al. IDH2 and NPM1 mutations cooperate to activate Hoxa9/Meis1 and hypoxia pathways in acute myeloid leukemia. Cancer Res. 2015;75:2005–2016.
- Dovey OM, Cooper JL, Mupo A, et al. Molecular synergy underlies the co-occurrence patterns and phenotype of NPM1-mutant acute myeloid leukemia. Blood. 2017;130:1911–1922.
- Havelange V, Ranganathan P, Geyer S, et al. Implications of the miR-10 family in chemotherapy response of NPM1-mutated AML. Blood. 2014;123:2412–2415.
- Papaioannou D, Petri A, Dovey OM, et al. The long non-coding RNA HOXB-AS3 regulates ribosomal RNA transcription in NPM1-mutated acute myeloid leukemia. Nat Commun. 2019;10:5351.
- Zhu G, Luo H, Feng Y, et al. HOXBLINC long non-coding RNA activation promotes leukemogenesis in NPM1-mutant acute myeloid leukemia. Nat Commun. 2021;12:1956.
- Tiribelli M, Geromin A, Damiani D, et al. Impact of fludarabine-based induction therapy on outcome of FLT3-/NPM1 + cytogenetically normal acute myeloid leukemia. Am J Hematol. 2012;92:E45–E47.
- Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089.
- Guièze R, Cornillet-Lefebvre P, Lioure B, et al. Role of autologous hematopoietic stem cell transplantation according to the NPM1/FLT3-ITD molecular status for cytogenetically normal AML patients: a GOELAMS study. Am J Hematol. 2012;87:1052–1056.
- Huang Y, Hu J, Lu T, et al. Acute myeloid leukemia patient with FLT3-ITD and NPM1 double mutation should undergo allogeneic hematopoietic stem cell transplantation in CR1 for better prognosis. Cancer Manag Res. 2019;11:4129–4142.
- Röllig C, Bornhäuser M, Kramer M, et al. Allogeneic stem-cell transplantation in patients with NPM1-mutated acute myeloid leukemia: results from a prospective donor versus no-donor analysis of patients after upfront HLA typing within the SAL-AML 2003 trial. J Clin Oncol. 2015;33:403–410.
- Gorin NC, Labopin M, Meloni G, et al. Impact of FLT3 ITD/NPM1 mutation status in adult patients with acute myelocytic leukemia autografted in first remission. Haematologica. 2013;98:e12–e14.
- Boddu P, Kantarjian H, Borthakur G, et al. Co-occurrence of FLT3-TKD and NPM1 mutations defines a highly favorable prognostic AML group. Blood Adv. 2017;1:1546–1550.
- Prata PH, Bally C, Prebet T, et al. NPM1 mutation is not associated with prolonged complete remission in acute myeloid leukemia patients treated with hypomethylating agents. Haematologica. 2018;103:e455–e457.
- Wei A, Tan P, Perruzza S, et al. Maintenance lenalidomide in combination with 5-azacitidine as post-remission therapy for acute myeloid leukemia. Br J Haematol. 2015;169:199–210.
- Perriello VM, Gionfriddo I, Rossi R, et al. CD123 is consistently expressed on NPM1-mutated AML cells. Cancers (Basel). 2021;13:496.
- Qin FX, Shao HY, Chen XC, et al. Knockdown of NPM1 by RNA interference inhibits cells proliferation and induces apoptosis in leukemic cell line. Int J Med Sci. 2011;8:287–294.
- Andresen V, Erikstein BS, Mukherjee H, et al. Anti-proliferative activity of the NPM1 interacting natural product avrainvillamide in acute myeloid leukemia. Cell Death Dis. 2016;7:e2497.
- Nabbouh AI, Hleihel RS, Saliba JL, et al. Imidazoquinoxaline derivative EAPB0503: A promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia. Cancer. 2017;123:1662–1673.
- Yang K, Wang M, Zhao Y, et al. A redox mechanism underlying nucleolar stress sensing by nucleophosmin. Nat Commun. 2016;7:13599.
- Gionfriddo I, Brunetti L, Mezzasoma F, et al. Dactinomycin induces complete remission associated with nucleolar stress response in relapsed/refractory NPM1-mutated AML. Leukemia. 2021;35:2552–2562.
- Šašinková M, Heřman P, Holoubek A, et al. NSC348884 cytotoxicity is not mediated by inhibition of nucleophosmin oligomerization. Sci Rep. 2021;11:1084.
- Penthala NR, Ketkar A, Sekhar KR, et al. 1-Benzyl-2-methyl-3-indolylmethylene barbituric acid derivatives: anti-cancer agents that target nucleophosmin 1 (NPM1). Bioorg Med Chem. 2015;23:7226–7233.
- Traver G, Sekhar KR, Crooks PA, et al. Targeting NPM1 in irradiated cells inhibits NPM1 binding to RAD51, RAD51 foci formation and radiosensitizes NSCLC. Cancer Lett. 2021;500:220–227.
- De Cola A, Franceschini M, Di Matteo A, et al. N6l pseudopeptide interferes with nucleophosmin protein-protein interactions and sensitizes leukemic cells to chemotherapy. Cancer Lett. 2018;412:272–282.
- Martelli MP, Gionfriddo I, Mezzasoma F, et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood. 2015;125:3455–3465.
- Nazha A, Bueso-Ramos C, Estey E, et al. The addition of All-trans retinoic acid to chemotherapy may not improve the outcome of patient with NPM1 mutated acute myeloid leukemia. Front Oncol. 2013;3:218.
- Chi HT, Ly BT, Vu HA, et al. Down-regulated expression of NPM1 in IMS-M2 cell line by (-)-epigallocatechin-3-gallate. Asian Pac J Trop Biomed. 2014;4:570–574.
- Yi S, Wen L, He J, et al. Deguelin, a selective silencer of the NPM1 mutant, potentiates apoptosis and induces differentiation in AML cells carrying the NPM1 mutation. Ann Hematol. 2015;94:201–210.
- Zhang X, Zhao Z, Yi S, et al. Deguelin induced differentiation of mutated NPM1 acute myeloid leukemia in vivo and in vitro. Anticancer Drugs. 2017;28:723–738.
- Forghieri F, Riva G, Lagreca I, et al. Characterization and dynamics of specific T cells against nucleophosmin-1 (NPM1)-mutated peptides in patients with NPM1-mutated acute myeloid leukemia. Oncotarget. 2019;10:869–882.
- Greiner J, Schneider V, Schmitt M, et al. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut). Blood. 2013;122:1087–1088.
- Casalegno-Garduño R, Meier C, Schmitt A, et al. Immune responses to RHAMM in patients with acute myeloid leukemia after chemotherapy and allogeneic stem cell transplantation. Clin Dev Immunol. 2012;2012:146463.
- De Propris MS, Raponi S, Diverio D, et al. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica. 2011;96:1548–1551.
- Schlenk RF, Paschka P, Krzykalla J, et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: early results from the prospective randomized AMLSG 09-09 phase III study. J Clin Oncol. 2020;38:623–632.
- Kapp-Schwoerer S, Weber D, Corbacioglu A, et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: results from the AMLSG 09-09 trial. Blood. 2020;136:3041–3050.
- Klossowski S, Miao H, Kempinska K, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Invest. 2020;130:981–997.
- Kühn MW, Song E, Feng Z, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. 2016;6:1166–1181.
- Carter BZ, Tao W, Mak PY, et al. Menin inhibition decreases Bcl-2 and synergizes with venetoclax in NPM1/FLT3-mutated AML. Blood. 2021;138:1637–1641.
- Uckelmann HJ, Kim SM, Wong EM, et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. 2020;367:586–590.
- Pemmaraju N, Konopleva M. Approval of tagraxofusp-erzs for blastic plasmacytoid dendritic cell neoplasm. Blood Adv. 2020;4:4020–4027.
- Testa U, Pelosi E, Castelli G. CD123 as a therapeutic target in the treatment of hematological malignancies. Cancers (Basel). 2020;11:1358.
- Zhang S, Qin F, Yang L, et al. Nucleophosmin mutations induce chemosensitivity in THP-1 leukemia cells by suppressing NF-κB activity and regulating Bax/Bcl-2 expression. J Cancer. 2016;7:2270–2279.
- DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803.
- Tiong IS, Dillon R, Ivey A, et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukemia. Br J Haematol. 2021;192:1026–1030.
- Cho H, Jang JE, Eom JI, et al. Arsenic trioxide synergistically promotes the antileukaemic activity of venetoclax by downregulating Mcl-1 in acute myeloid leukemia cells. Exp Hematol Oncol. 2021;10:28.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Lachowiez CA, Loghavi S, Kadia TM, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020;4(7):1311–1320.
- Prokoph N, Probst NA, Lee LC, et al. IL10RA modulates crizotinib sensitivity in NPM1-ALK+ anaplastic large cell lymphoma. Blood. 2020;136:1657–1669.
- Kuravi S, Cheng J, Fangman G, et al. Preclinical evaluation of Gilteritinib on NPM1-ALK-driven anaplastic large cell lymphoma cells. Mol Cancer Res. 2021;19:913–920.
- Huang M, Garcia JS, Thomas D, et al. Autophagy mediates proteolysis of NPM1 and HEXIM1 and sensitivity to BET inhibition in AML cells. Oncotarget. 2016;7:74917–74930.
- Mason EF, Kuo FC, Hasserjian RP, et al. A distinct immunophenotype identifies a subset of NPM1-mutated AML with TET2 or IDH1/2 mutations and improved outcome. Am J Hematol. 2018;93:504–510.
- Patel SS, Pinkus GS, Ritterhouse LL, et al. High NPM1 mutant allele burden at diagnosis correlates with minimal residual disease at first remission in de novo acute myeloid leukemia. Am J Hematol. 2019;94(8):921–928.
- Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: A study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716.
- Heiblig M, Duployez N, Marceau A, et al. The impact of DNMT3A status on NPM1 MRD predictive value and survival in elderly AML patients treated intensively. Cancers (Basel). 2021;13(9):2156.
- Hubmann M, Köhnke T, Hoster E, et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica. 2014;99(8):1317–1325.
- Balsat M, Renneville A, Thomas X, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French Association Group. J Clin Oncol. 2017;35(2):185–193.
- Tiong IS, Dillon R, Ivey A, et al. Clinical impact of NPM1-mutant molecular persistence after chemotherapy for acute myeloid leukemia. Blood Adv. 2021;5(23):5107–5111.
- Cornelissen JJ, Versluis J, Passweg JR, et al. Comparative therapeutic value of post- remission approaches in patients with acute myeloid leukemia aged 40-60 years. Leukemia. 2015;29:1041–1050.
- Karas M, Steinerova K, Lysak D, et al. Pre-transplant quantitative determination of NPM1 mutation significantly predicts outcome of aIlogeneic hematopoietic stem cell transplantation in patients with normal karyotype AML in complete remission. Anticancer Res. 2016;36(10):5487–5498.
- Delsing Malmberg E, Johansson Alm S, Nicklasson M, et al. Minimal residual disease assessed with deep sequencing of NPM1 mutations predicts relapse after allogeneic stem cell transplant in AML. Leuk Lymphoma. 2019;60(2):409–417.
- Dillon R, Hills RK, Freeman SD, et al. Molecular MRD status and outcome after transplantation in NPM1 mutated AML: results from the UK NCRI AML17 study. Blood. 2020;135(9):680–688.
- Platzbecker U, Middeke JM, Sockel K, et al. Minimal-residual disease guided treatment with azacitidine in MDS/AML patients at imminent risk of relapse: results of the prospective RELAZA2 trial. Blood. 2017;130:565.
- Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD working party. Blood. 2018;131:1275–1291.
- Pettersson L, Johansson Alm S, Almstedt A, et al. Comparison of RNA- and DNA-based methods for measurable residual disease analysis in NPM1-mutated acute myeloid leukemia. Int J Lab Hematol. 2021;43(4):664–674.
- Du Pisani LA, Shires K. Development of a flow cytometric method to detect the presence of mutated nucleophosmin 1 in acute myeloid leukemia. Hematol Oncol Stem Cell Ther. 2015;8(3):106–114.
- Chopra A, Soni S, Pati H, et al. Nucleophosmin mutation analysis in acute myeloid leukemia: immunohistochemistry as a surrogate for molecular techniques. Indian J Med Res. 2016;143(6):763–768.
- Höllein A, Meggendorfer M, Dicker F, et al. NPM1 mutated AML can relapse with wild-type NPM1: persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018;2(22):3118–3125.
