ABSTRACT
Purpose
Multiple myeloma is still an incurable disease In the past decade, with the continuous progress of treatment methods, the progression-free survival of patients has been prolonged, but some patients still progress in the early stage of the disease. Our research analyses the clinical laboratory indicators of newly diagnosed multiple myeloma (NDMM) patients, to obtain the relevant factors of disease progression within one year in MM patients and to establish a prediction model.
Methods:
108 MM patients treated in our hospital from January 2015 to January 2020 were retrospectively analyzed. After univariate and multivariate logistic regression analyses, the related factors of disease progression within one year in NDMM patients were obtained, and a prediction model was established.
Results:
Treatment regimen containing at least two targeted drugs (OR = 0.226, 95% CI 0.068-0.753), increased lactate dehydrogenase(LDH, OR = 3.452, 95% CI 1.101-10.826) and increased serum corrected calcium(OR = 4.466, 95% CI 1.346-14.811) were identified as potential predictors by statistical analysis. The prediction model was obtained: x = −2.042-1.489 × treatment regimen (including at least two targeted drug assignment as 1, otherwise 0) + 1.239 ×LDH (U/L, lactate dehydrogenase elevation assignment as 1, normal as 0) +1.496 × serum corrected calcium (mmol/L, serum corrected calcium elevation assignment as 1, normal as 0). Receiver operating characteristic curve analysis showed that the model has good predictive performance.
Conclusion:
The possibility of disease progression within one year can be predicted by the prediction model. The model can be used as a reference for clinicians to make individualized treatment plans for patients so that patients can obtain better treatment effects.
1. Introduction
Multiple myeloma (MM) is a malignant proliferative disease of plasma cells. Malignant plasma cells undergo clonal proliferation in the bone marrow microenvironment and generate abnormal amounts of monoclonal immunoglobulin, which affects all organs of the body[Citation1]. The pathogenesis of MM is not clear and may be related to genetic factors, autoimmune diseases, ionizing radiation, viral infection, exposure to chemical components, chronic inflammation, etc. MM accounts for 10% of hematological malignancies, ranking second and slightly lower than lymphoma[Citation2–5]. According to global standards, the annual incidence rate of MM is 2.1 cases per 100,000, with a peak age of 50–60 years and slightly more males than females[Citation5–7]. With the aging of China's population, the incidence rate of myeloma is also increasing year by year, reaching 2.0 cases per 100,000, mostly middle-aged and elderly people, with slightly more males than females[Citation8].
The course of MM is generally slow, and it is still a malignant tumor that cannot be completely cured. In the past decade, due to the continuous maturation of autologous stem cell transplantation (ASCT) technology and the continuous progress of symptomatic support treatment, such as anti-rejection and anti-infection treatments after transplantation, the survival rate of MM patients has been significantly improved [Citation9,Citation10]. Based on the Surveillance, Epidemiology, and End Results (SEER) database, Siegal et al. found that the 5-year relative survival rate of MM increased from 25% in 1975–1977 and 27% in 1987–1989 to 49% in 2005–2011 [Citation4]. In a retrospective analysis of 117,711 patients with MM, Dawn Swan et al. found that the five-year progression-free survival (PFS) and overall survival (OS) increased from 28% to 31% and from 52% to 69% between 1995 and 2019 [Citation11]. According to a phase III trial, the median OS of patients with MM has increased from three years to the current eight years [Citation12]. Considering the application of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), the median survival time of MM patients has almost doubled. At present, bortezomib, lenalidomide and other drugs are still in the first-line position in the treatment of MM. With the application of monoclonal antibodies (mAbs), immune checkpoint inhibitors (ICIs) and other drugs in MM as well as chimeric antigen receptor T-cell (CAR T) immunotherapy, a large amount of clinical trial data have been obtained [Citation13,Citation14]. The survival of MM patients is expected to continue to be prolonged. However, some patients still have progressive disease (PD) in the early stage. Clinically, it was observed that PD occurred in some MM patients during induction treatment, mostly manifesting as bone pain at the new site and significantly decreased physical status. The progression of the disease not only reduces the quality of life of patients but also places a serious burden on the patient's finances and national medical care. Therefore, delaying disease progression and prolonging PFS is one of the goals of the treatment of MM.
In clinical work, we observed that the proportion of plasma cells and some biochemical indexes in patients with early progression of MM usually showed higher levels, including high levels of lactate dehydrogenase (LDH), hypercalcemia, elevated serum creatinine, hepatitis B virus positivity, etc. It is not clear whether these factors are related to the early progression of MM. We retrospectively analyzed the basic clinical characteristics and test indicators of 108 patients diagnosed with MM in our hospital, aiming to develop a new predictive model to evaluate whether patients with newly diagnosed multiple myeloma (NDMM) are at high risk of early progression and to help clinicians identify patients with early progression and intervene in advance.
2. Patients and methods
2.1 Patients
We comprehensively collected data from 108 MM patients admitted to the Department of Hematology, The People's Hospital of Shangrao City, Jiangxi Province, from 01/2015–01/2020 (). All patients met the Chinese Guidelines for the Diagnosis and Treatment of Multiple Myeloma (version 2020) [Citation15]. The general data of patients included age, proportion of plasma cells in bone marrow at the time of diagnosis, Durie-Salmon (DS) stage (stage I, II, III), International Staging System (ISS) stage (stage I, II, III) and treatment regimen. Clinical test data included neutrophil-to-lymphocyte ratio (NLR), LDH, serum corrected calcium, blood β2-microglobulin (β2-MG), serum creatinine (Scr), and hepatitis B surface antigen (HBsAg). The concept of MM progression was formulated according to the Chinese Guidelines for the Diagnosis and Treatment of Multiple Myeloma (version 2020) [Citation15]. The 108 MM patients were divided into a progressive group (n = 30) and a nonprogressive group (n = 78) according to their disease status within 1 year after diagnosis.
This research protocol was conducted following the Declaration of Helsinki and was approved by the Ethics Committee of The People's Hospital of Shangrao City. We reviewed and collected the relevant medical records and follow-up data after obtaining informed consent from the patients.
2.2 Statistical analysis
SPSS 25.0 statistical software was used for all data. The significance level of all statistical tests was set to 0.05, and all significance levels were two-sided. Univariate logistic regression analysis was performed with the progression of MM patients within 1 year as the dependent variable (Y) and age, clinical stage (DS stage, ISS stage), proportion of plasma cells in bone marrow, treatment regimens, NLR, LDH, serum corrected calcium, Scr, β2 microglobulin, and HBsAg as independent variables (X). If the analysis results showed that a variable had a significant statistical value (p < 0.05), then multifactor logistic regression analysis was performed. Based on the results of the analysis, a prediction model of disease progression within 1 year of MM diagnosis was established. Additionally, the validity of the model was verified by receiver operating characteristic (ROC) curves. The study design is summarized in .
3. Results
3.1 Characteristics of MM
We analyzed 108 MM patients admitted to Shangrao People's Hospital from 2015 to 2020. The assignments of dependent and independent variables are summarized in . The characteristics of the patients are summarized in .
Table 1. Variable assignments.
Table 2. Characteristics of MM patients.
3.2 Univariate logistic regression analysis
Age, sex, clinical stage (DS stage, ISS stage), proportion of plasma cells in bone marrow, treatment regimens, NLR, LDH, serum corrected calcium, Scr, β2-Mg, and HBsAg were included as independent variables (X), and disease status was included as a dependent variable (Y). SPSS 25.0 software was used for relevant statistical analysis, and the logistic regression method was used for single factor analysis. P < 0.05 was considered statistically significant, as shown in .
Table 3. Univariate logistic regression analysis.
The univariate analysis results () indicated that the proportion of plasma cells in bone marrow, whether the treatment regimen included at least two targeted agents, NLR, LDH, serum corrected calcium and Scr were significantly correlated with the progression of the disease (p < 0.05).
3.3 Multivariate logistic regression analysis
SPSS 25.0 statistical software was used to perform multivariate logistic regression analysis of variables with statistical significance in the univariate analysis. P < 0.05 was considered statistically significant ().
Table 4. Multivariate logistic regression analysis.
The proportion of plasma cells in bone marrow, treatment plan, NLR, LDH, serum corrected calcium and Scr were included to construct a multivariate logistic regression equation. The multivariate analysis results (Table ) showed that at least two targeted drugs included in the treatment regimen was related to a reduced risk of MM progression, with statistical significance (OR = 0.226, 95% CI 0.068-0.753, p <0.05). The increase in LDH levels increased the risk of disease progression within 1 year in MM patients and was statistically significant (OR = 3.452, 95% CI 1.101-10.826, p < 0.05). The increase in serum corrected calcium levels increased the risk of disease progression within 1 year in MM patients and was statistically significant (OR = 4.466, 95% CI 1.346-14.811, p < 0.05).
3.4 Establishment of a prediction model
According to the principle of multifactor logistic regression analysis, the regression equation of MM disease progression within 1 year was obtained: X = −2.042-1.489× treatment regimen (including at least two targeted drugs is 1, otherwise 0) + 1.239 × LDH level (U/L, increased is 1, normal is 0) + 1.496 × serum corrected calcium level (mmol/L, increased is 1, normal is 0). A prediction model was established to estimate the risk of disease progression within 1 year of NDMM patients.
3.5 Evaluation of the accuracy of the model
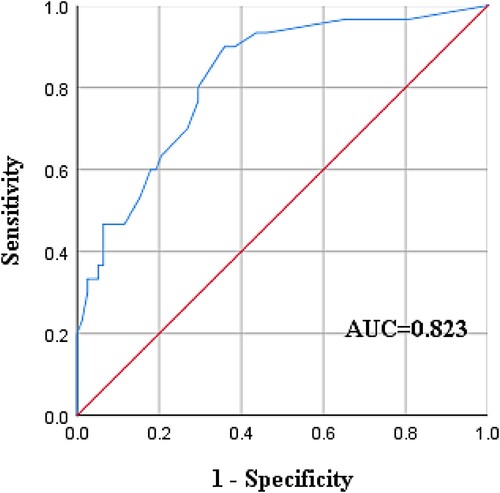
In multivariate analysis, the prediction probability (PRE) was obtained, and ROC curve analysis was carried out based on the PRE value. The results showed that the area under the curve (AUC) was 0.823 > 0.75, p < 0.001, and the 95% CI was 0.737-0.909, suggesting that the model had good prediction performance ()
The 108 patients were subdivided based on the equation ‘X = −2.042-1.489× treatment regimen (including at least two targeted drugs is 1, otherwise 0) + 1.239 × LDH level (U/L, increased is 1, normal is 0) + 1.496 × serum corrected calcium level (mmol/L, increased is 1, normal is 0)’, with the x value obtained by calculation. The data were imported into SPSS 25.0 software for ROC curve analysis, and the results showed that the AUC was 0.786, p < 0.001, and the 95% CI was 0.693-0.879. The optimal critical value of the corresponding model based on the maximum approximation index was −1.699. The corresponding sensitivity and specificity were 100.0% and 93.3%, respectively. Therefore, x = −2.0385 was used as the dividing line, x≥−2.0385 was judged as disease progression within 1 year in MM patients, and x <−2.0385 was judged as no progression. A four-lattice table was constructed (), with a sensitivity of 63.33% and a specificity of 80.70%. In summary, it suggested that the model had a good fitting degree.
Table 5. Prediction model evaluated by the four-cell table.
Discussion
In recent years, the treatment of MM has been progressing continuously. Nevertheless, MM is still incurable, and the majority of patients who eventually progress experience relapse. Once the first progression or relapse occurs, the duration of remission will gradually shorten, the treatment difficulty of patients will increase, and the drug efficacy response will worsen, which seriously affects the quality of life of patients and increases their economic burden. We predicted the risk of progression of NDMM patients by assessing their clinical characteristics and laboratory indicators and identified patients with a high risk of progression. Our study found that the application of targeted drugs can reduce the risk of disease progression. Elevated LDH and hypercalcemia were risk factors for early disease progression.
Some studies have found that lenalidomide and bortezomib share pathways including caspase-mediated apoptosis and inhibition of the NF-kappa B signaling pathway, so there is a synergistic effect between them [Citation16–18]. These two drugs also enhance the activity of dexamethasone, so the combination of the three agents in the VRd regimen (bortezomib, lenalidomide, and dexamethasone) may enhance the efficacy [Citation19,Citation20]. According to guidelines [Citation15], the most widely used induction treatment scheme at present is the two or three combination scheme based on PIs or/and IMiDs combined with alkylating agents and glucocorticoids. SWOG S0777 demonstrated the significant superiority of the VRd regimen. In the PETHEMA/Gem2012 study, the results showed a very good partial response (VGPR). With the extension of the treatment cycle, there was a deeper efficacy response [Citation21]. Leo Sekine et al. conducted a meta-analysis on the selection of induction therapy for NDMM patients who were not eligible for transplantation and showed that the regimen containing lenalidomide and bortezomib was better than the thalidomide regimen, and the regimen containing mAbs showed significant superiority in PFS. It also confirmed the obsolescence of classical therapies such as the MP regimen (melphalan and dexamethasone), suggesting that the current triple or quadruple regimen combining different novel drugs has significant advantages in OS and PFS [Citation22].
The widespread clinical application of PIs and IMiDs has almost doubled the median survival time of MM patients [Citation23]. The development of clinical trials of mAbs and CAR-T immunotherapy may further prolong the survival time and improve the quality of life of MM patients. With the discovery of multiple effective targets for the treatment of MM and the development of targeted drugs, by comprehensively considering the efficacy of drugs and overcoming adverse reactions, we will continue to design and find more brand-new chemicals and will develop new drugs with better curative effects and less toxicity and side effects in the future.
Calcium ions (Ca2+) are ubiquitous second messengers that play an important role in regulating the physiological functions of normal cells [Citation24]. The change in Ca2 + concentration is closely related to gene expression, cell proliferation, cell death, cell cycle migration and control. [Citation25,Citation26]. In addition to the role of Ca2 + in normal cells, a large number of studies have also shown that Ca2+-dependent signaling pathways are also involved in tumor proliferation, differentiation, migration, invasion, metastasis and apoptosis, so it is often found that the expression of the Ca2 + regulatory network in tumor cells is significantly higher than that in normal cells [Citation25–27]. It has been reported that the Ca2 + signaling pathway affects the prognosis of solid tumors, such as lung cancer, breast cancer, cervical cancer, esophageal cancer and colon cancer, and hematological tumors, such as MM, by influencing tumor growth, proliferation, metastasis and survival [Citation26,Citation28,Citation29]. Wisloff et al. found that hypercalcemia can be a separate adverse factor affecting the quality of life of MM patients [Citation30].
Blood calcium levels are also closely related to kidney function. The destruction of MM bone leads to high calcium, and calcium salts can be deposited in the glomerulus, thus affecting the filtration function of the glomerulus [Citation31] and even causing acute renal impairment [Citation32]. In clinical work, blood calcium levels should be dynamically monitored to control them, reduce renal burden, reduce the Ca2 + regulatory network, and improve the prognosis and survival of MM patients. The results of this small clinical study showed that serum corrected calcium was associated with the progression and recurrence of MM and was a risk factor for the progression of MM.
The typical characteristic of malignant tumors is infinite proliferation, which requires a large amount of energy to support the infinite expansion of cells. LDH is a key enzyme in glycolysis, so LDH may be closely correlated with tumors. LDH has been confirmed to be correlated with the prognosis of hepatocellular carcinoma (HCC), neuroblastoma, esophageal cancer, melanoma and other tumors [Citation33–39]. In these tumors, LDH is highly expressed with increased lactic acid and has poor sensitivity to chemotherapy and radiotherapy [Citation40,Citation41]. Other studies have confirmed that LDH is an independent risk factor for hematological malignancies such as leukemia and lymphoma, that LDH levels can reflect the severity of the disease, and that LDH levels will decrease significantly with the improvement of the disease [Citation34–38]. A high level of LDH is associated with adverse reactions of sarcomas, lymphomas and other solid tumors to radiotherapy and chemotherapy [Citation42], and an elevated LDH may lead to drug resistance of tumors during standard chemotherapy [Citation43], while a low level of LDH is associated with a better response to treatment [Citation42,Citation44].
LDH is closely related to tumor proliferation, so LDH may be a potential target for effective tumor treatment and may provide a new idea for tumor treatment. LDHB shows diversity in different types of tumors; for example, it is highly expressed in cervical cancer [Citation45], oral squamous cell carcinoma and other tumor cells [Citation46], while it is downregulated in highly metastatic prostate cancer and other tumor cells [Citation47]. Therefore, there are few studies on LDHB-targeting drugs. LDHA is highly expressed in most tumors. Qiu Jinmei et al. used LDHA as the target for antitumor research. Targeting LDHA will reduce the transformation process of pyruvate to lactic acid in tumor cells and then cut off the energy supply to tumor cells. However, normal human cells can directly use pyruvate to cycle tricarboxylic acid in mitochondria to synthesize a large amount of ATP. Therefore, the energy supply for normal cell growth is less affected [Citation48]. Long-term inhibition of LDHA can lead to the genetic deletion of LDHA, but it is only found to cause non-fatal pustular psoriasis [Citation49]. Therefore, antitumor therapy by inhibiting LDHA and cutting off the tumor energy supply can be a new target for tumor prevention and treatment, with high feasibility and a relatively low frequency of treatment-related adverse reactions [Citation48,Citation49]. LDH has been shown to be a strong survival predictor of MM in several studies during the conventional chemotherapy period [Citation37,Citation38,Citation50–52], which is consistent with the finding of this study.
A large number of studies have confirmed that increased NLR is associated with the poor prognosis of tumors [Citation53–57], and the hypothesis of the relationship between the two was first proposed by Galenus, indicating that tumors mainly originate from inflammatory tissue injury [Citation58]. Subsequently, Virchow and Dvorak confirmed the above hypothesis [Citation59]. An increase in NLR indicates an increase in neutrophils, while a decrease in lymphocytes indicates high inflammatory activity but weak tumor lethality [Citation60], which leads to the tumor-promoting effect on inflammatory activities, indicating that high NLR expression is correlated with poor tumor prognosis [Citation61]. Many studies have also confirmed that increased NLR is associated with a poor prognosis of malignant tumors, which has been confirmed in solid tumors such as esophageal cancer, gastric cancer, pancreatic cancer, HCC, rectal cancer, lung cancer, breast cancer and renal cell carcinoma [Citation53–55]. Zhuangzhuang Cui et al. [Citation56] and Juyong Sun et al. [Citation62] all showed that increased NLR was associated with the poor prognosis of MM patients. In this study, logistic regression analysis was conducted to analyze the correlation between NLR and the progression of MM, but multivariate analysis showed no statistical significance, which may be related to the small sample size and single-center nature of the study. However, active and effective anti-inflammatory therapy is necessary in MM patients with high NLR levels.
This study identified three factors as potential predictors and constructed a prediction model for disease progression within one year to help clinicians identify and predict MM patients with a high risk of progression as soon as possible so that they can evaluate and formulate appropriate treatment plans. NDMM patients should be treated with the appropriate targeted drugs as much as possible, dynamically rechecking electrolytes and adjusting blood calcium levels in time. Dynamic reexamination of LDH should be performed, and those with elevated LDH levels should start antitumor treatment as soon as possible, increase the reexamination frequency, and understand the changes in the disease over time. The study of new targets of LDHA may further prolong the survival time of patients.
The sample size of this study is limited, and it is a single-center study, so the results are not widely representative. Due to the limited number of cases, this model has not been externally validated, so clinical data need to be collected to further verify the predictive performance of this model. In addition, more research on cytogenetics and molecular biology can further establish a more representative, comprehensive and accurate risk assessment model for early progression.
Conclusion
It is very important to identify MM patients with early progression as soon as possible in clinical work. Our prediction model shows that MM patients with high levels of LDH and high blood calcium are more likely to have early disease progression. The use of targeted drugs can reduce the risk of early disease progression, help patients obtain better treatment effects, create opportunities for maintenance treatment, hematopoietic stem cell transplantation and CAR-T therapy, and bring longer PFS times to patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–1873.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060.
- Teras L-R, Carol-E D, James-R C, et al. 2016 US lymphoid malignancy statistics by world health organization subtypes. Ca-Cancer J Clin. 2016;66(6):443–459.
- Siegel R-L, Kimberly-D M, Ahmedin J. Cancer statistics, 2016. Ca-Cancer J Clin. 2016;66(1):7–30.
- Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016;43(6):676–681.
- Liu Y. Multiple myeloma. China Academic Journal Electronic Publishing House. 2020.
- Cowan A-J, Christine A, Aleksandra B, et al. Global burden of multiple myeloma. JAMA Oncol. 2018;4(9):1221.
- Wang D, Hao Y, Meng X, et al. Epidemiology and etiology of multiple myeloma. Int J Epidem and Infect Dis. 2018;45(04):277–280.
- Kristinsson S-Y, Landgren O, Dickman P-W, et al. Patterns of survival in multiple myeloma: A population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993–1999.
- Turesson I, Velez R, Kristinsson S-Y, et al. Patterns of improved survival in patients With multiple myeloma in the twenty-first century: A population-based study. J Clin Oncol. 2010;28(5):830–834.
- Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Advances. 2017;1(4):282–287.
- Swan D, Hayden PJ, Eikema D, et al. Trends in autologous stem cell transplantation for newly diagnosed multiple myeloma: changing demographics and outcomes in European society for blood and marrow transplantation centres from 1995 to 2019. Br J Haematol. 2022.
- Jennifer N. Brudno I-M-S-D, Dalia-Salem-Constance-Yuan S-S, Steven-A-Feldman-David M, et al. T cells genetically modified to express an anti – B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36.
- Sanchez E, Emily-J S, Moryel-A Y, et al. The role of B-cell maturation antigen in the biology and management of, and as a potential therapeutic target in, multiple myeloma. Target Oncol. 2018;13(1):39–47.
- Chinese Society of Hematology. Multiple myeloma professional committee of Chinese medical doctor association. hematologist branch of Chinese medical doctor association. Guidelines for diagnosis and Treatment of Multiple Myeloma in China (revised in 2020). Chinese J Intern Med. 2020;59(5):341–346.
- Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076.
- Mitsiades N, Mitsiades C-S, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379.
- Mitsiades N, Mitsiades C-S, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99(12):4525–4530.
- Harousseau J-L, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–1505.
- Lacy M-Q, Gertz M-A, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival With lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82(10):1179–1184.
- Rosinol L, Oriol A, Rios R, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134(16):1337–1345.
- Sekine L, Klarmann Ziegelmann P, Manica D, et al. Upfront treatment for newly diagnosed transplant-ineligible multiple myeloma patients: A systematic review and network meta-analysis of 14,533 patients over 29 randomized clinical trials. Crit Rev Oncol Hematol. 2019;143:102–116.
- Schaapveld M, Otto V, Sabine S, et al. Improved survival among younger but not among older patients with multiple myeloma in the Netherlands, a population-based study since 1989. Eur J Cancer. 2010;46(1):160–169.
- Berridge M-J, Lipp P, Bootman M-D. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21.
- Berridge M-J, Bootman M-D, Roderick H-L. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529.
- Chen Y-F, Chen Y-T, Chiu W-T, et al. Remodeling of calcium signaling in tumor progression. J Biomed Sci. 2013;20:23.
- Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16(3):107–121.
- Chen Y-F, Lin P-C, Yeh Y-M, et al. Store-Operated Ca2+ entry in tumor progression: From molecular mechanisms to clinical implications. Cancers (Basel). 2019;11(7):899.
- Fiorio P-A, Kondratska K, Prevarskaya N. STIM and ORAI proteins: crucial roles in hallmarks of cancer. Am J Physiol Cell Physiol. 2016;310(7):C509–C519.
- Finn W-F, Kristin-Kvam A, Hjorth M, et al. Serum calcium is an independent predictor of quality of life in multiple myeloma. Eur J Haematol. 2007;78(1).
- Corso A, Zappasodi P, Lazzarino M. Urinary proteins and renal dysfunction in patients with multiple myeloma. Biomed Pharmacother. 2002;56(3):139–143.
- Prakash J, Mandal A-K, Vohra R, et al. Renal disease Is a prodrome of multiple myeloma: An analysis of 50 patients from eastern india. Ren Fail. 2009;31(4):267.
- Miao P, Sheng S, Sun X, et al. Lactate dehydrogenase a in cancer: A promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904–910.
- Dorneburg C, Matthias F, Thomas-F-E B, et al. LDHA in neuroblastoma Is associated with poor outcome and Its depletion decreases neuroblastoma growth independent of aerobic glycolysis. Clin cancer res. 2018;24(22):5772–5783.
- Ferrara F, Salvatore M, Vittorina Z, et al. Acute myeloid leukemia in the elderly: A critical review of therapeutic approaches and appraisal of results of therapy. Leuk Lymphoma. 1998;29(3-4):375–382.
- Zhang C. Changes of lactate dehydrogenase levels in patients with acute leukemia. Asia-pacific Traditional Medicine. 2011;7(07):91–92.
- Bart Barlogie MD, Smallwood Leslie AE. High serum levels of lactic dehydrogenase identify a. American College of Physicians. 1989;110(7):521–525.
- Simonsson B, Brenning G, Et A. Prognostic value of serum lactic dehydrogenase(S-LDH)in multiple myeloma. Eur J Clin Invest. 1987;17(4):336–339.
- Koukourakis M-I, Giatromanolaki A, Sivridis E, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17(14):4892–4900.
- Urbanska K, Orzechowski A. Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int J Mol Sci. 2019;20(9).
- Koukourakis M-I, Giatromanolaki A, Panteliadou M, et al. Lactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapy. Br J Cancer. 2014;110(9):2217–2223.
- Koukourakis M-I, Giatromanolaki A, Sivridis E, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17(14):4892–4900.
- Ward P-S, Thompson C-B. Metabolic reprogramming: A cancer hallmark even warburg Did Not anticipate. Cancer Cell. 2012;21(3):297–308.
- Yao J-C, Phan A-T, Chang D-Z, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced Low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318.
- Wang S, Xie J, Li J, et al. Cisplatin suppresses the growth and proliferation of breast and cervical cancer cell lines by inhibiting integrin beta5-mediated glycolysis. Am J Cancer Res. 2016;6(5):1108–1117.
- Sun W, Zhang X, Ding X, et al. Lactate dehydrogenase B is associated with the response to neoadjuvant chemotherapy in oral squamous cell carcinoma. PLoS One. 2015;10(5):e0125976.
- Leiblich A, Cross S-S, Catto J-W, et al. Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer. Oncogene. 2006;25(20):2953–2960.
- Jin Mei Qiu, Juan Gong, Qing Chi Xie, et al. The role of lactate dehydrogenase in tumor metabolism and the development of antitumor drugs targeting lactate dehydrogenase. Cancer Res Prev Treat. 2020;47(12):82–87.
- Takeo N, Fujiwara S, Sakai T, et al. Hereditary lactate dehydrogenase M-subunit deficiency with late-developing pustular psoriasis-like lesions. J Dermatol. 2016;43(12):1429–1432.
- Dimopoulos M-A, Barlogie B, Smith T-L, et al. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med. 1991;115(12):931–935.
- Suguro M, Kanda Y, Yamamoto R, et al. High serum lactate dehydrogenase level predicts short survival after vincristine-doxorubicin-dexamethasone (VAD) salvage for refractory multiple myeloma. Am J Hematol. 2000;65(2):132–135.
- Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol. 2005;75(5):370–375.
- Feng J-F, Huang Y, Liu J-S. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–1612.
- de Martino M, Allan-J P, Sebastian H, et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urology. 2013;190(6):1999–2004.
- Xue T-C, Lan Z, Xiao-Yin X, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: A meta-analysis. PloS one. 2014;9(5):e96072.
- Zhuangzhuang Cui, Yuanyuan Zhang, Ping Bei, et al. Prognostic significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio for overall survival and progression-free survival in newly diagnosed multiple myeloma patients. J PLA med college. 2019;40(2):121–126.
- Wang J, Zhou X, Liu Y, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma: A meta-analysis. PLoS One. 2017;12(4):e176008.
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706.
- Dvorak H-F. Tumors: Wounds that Do Not heal. N Engl J Med. 1986;315(26):1650–1659.
- Kokcu A, Kurtoglu E, Celik H, et al. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer?. Asian Pac J Cancer Prev. 2014;15(22):9781–9784.
- Greenberg A-J, Vachon C-M, Rajkumar S-V. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia. 2012;26(4):609–614.
- Sun J, Mou N, Mou J, et al. Significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio changes in patients with multiple myeloma. Chinese J Exper Hematology. 2019;27(04):1185–1189.