ABSTRACT
α-synuclein (α-syn) is a highly conserved and thermostable protein that is widely distributed in human brain. An intracellular aggregation of α-syn in dopaminergic neurons is the hallmark of a group of neurodegenerative diseases including Parkinson’s disease. Interestingly, α-syn is also highly expressed in red blood cells and is considered as one of the most abundant proteins in red blood cells. Moreover, α-syn is thought to play a regulatory role during normal erythropoiesis. However, whether α-syn participates in the pathogenesis of erythroid diseases has not been reported. In this review, we discuss the protein structure of α-syn and the importance of α-syn in erythropoiesis.
Introduction
The Synuclein protein was originally isolated from the Torpedo electric lobe and rat brain tissues in 1988 [Citation1]. α-synuclein (α-syn), a 14 kDa presynaptic protein, together with β-synuclein (β-syn) and γ-synuclein (γ-syn), belongs to the synuclein protein family. Synuclein genes are located on different chromosomes and encode peptides containing highly conserved amino terminal but different carboxyl terminal. α-syn has long been studied in Parkinson’s disease (PD), a neurodegenerative disorder pathologically characterized by the accumulation and aggregation of amyloid fibers of α-syn and the Lewy body (a large globular protein complex) formation [Citation2]. Overall, impaired α-syn metabolism and its abnormal aggregation in neuronal cells are considered as the key steps in the pathogenesis of PD [Citation3].
In addition to the central nervous system, α-syn also exists in a variety of non-neuronal tissues and cells [Citation4], including muscle [Citation5], kidney [Citation6], liver [Citation7], spleen [Citation7], lung [Citation8], bladder [Citation9], myocardial muscle [Citation8], bone marrow [Citation10], saliva [Citation11], blood cells [Citation12] and skin fibroblasts [Citation13]. Interestingly, α-syn is also abundantly expressed in erythroid cells and might function during erythropoiesis [Citation10,Citation14,Citation15]. Further experiments demonstrate that α-syn can cross the blood–brain barrier in two-way directions: brain-to-blood and blood-to-brain [Citation16]. Moreover, extracellular vesicles from erythrocytes contain α-syn and can be transported across the blood–brain barrier [Citation17]. These discoveries suggest that blood-borne α-syn might contribute to the pathogenesis of PD [Citation18]. In this review, we focus on elucidating the potential biological functions of α-syn in development of red blood cells. The overview regarding the role of α-syn in PD itself can be found in recent reviews [Citation19–21].
α-syn gene and protein
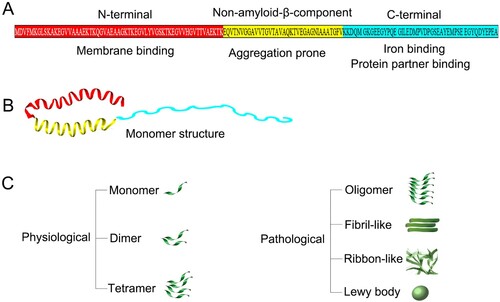
Genes encoding α-syn protein are located on human chromosome 4 and mouse chromosome 6. The genetic variability of SNCA genes affects the level of α-syn in the blood and brain [Citation22]. α-syn is a highly conserved small protein, whose amino acid sequence consists of three domains (): (1) the N-terminal domain is amphiphilic and lysine-rich, containing incomplete repetitive sequences (XKTKEGVXXXX) [Citation23] and thus can easily form α-helix. This domain commits the binding to membranes, preferentially to phospholipids [Citation24]. The physiological form of α-syn is N-terminal-acetylated after translation [Citation25]; (2) the non-amyloid-β-component domain is aggregation-prone, however, its residues are usually not exposed to cytoplasm, which counteracts spontaneous aggregation [Citation25]; (3) the C-terminal domain is an acidic carboxyl terminal that regulates the nuclear localization and interactions of α-syn with other protein partners or cellular components.
Figure 1. Protein structure of α-syn. (A) α-syn is a highly conserved small protein, whose amino acid sequence consists of three domains: the N-terminal domain, the non-amyloid-β-component domain and the C-terminal domain; (B) Monomeric α-syn usually conforms into an α-helix structure; (C) Multiple forms of α-syn, among which the oligomer and the aggregated forms are usually pathogenic to brain tissues.
α-syn is a heat stable protein, a property that allows treatment at 100°C as part of its partial purification [Citation26]. The half-life of intracellular α-syn is about 50 h [Citation27]. α-syn has a wide variety of forms: folded or unfolded according to their structure; acetylated, phosphorylated, ubiquitinated or nitrated based on post-translational modification [Citation28]; as soluble oligomers or insoluble aggregates in terms of solubility. Therefore, the corresponding form of a α-syn protein should always be considered when judging the related function.
Structurally, there are multiple forms of α-syn: monomeric, tetrameric, oligomeric, fibrillary, ribbon-like and inclusion body, among which oligomers and fibrils seem to be the main toxic form () [Citation29]. Acetylated monomeric α-syn represents the predominant species in the cytoplasm of normal mammalian cells [Citation25]. Under specific stress conditions, the α-syn monomers interact with ligands, lipids, biofilms and other proteins to form stable polymers or show different conformations and structures [Citation24]. In vivo, factors such as post-translational modification, oxidative stress and proteolysis have been shown to influence the balance between monomer and oligomer states [Citation24]. Moreover, intracellular pH, salt concentration and calcium concentration also directly affect the conformations and structures of α-syn [Citation28]. Recent reports have described the physiological existence of α-syn in the form of helical folded tetramer [Citation30,Citation31], yet other follow-up studies support that α-syn exists mainly in the form of unfolded monomer [Citation32–34]. Such monomer-tetramer debate is still the center of continuous discussion about the natural structural state of α-syn.
Expression of α-syn in erythropoiesis
It is well known that α-syn is abundantly expressed in neuronal cells [Citation21]. Interestingly, α-syn is also expressed in blood cells including erythrocytes, megakaryocytes and platelets, T and B lymphocytes, natural killer (NK) cells, monocytes and macrophages. Particularly, erythroid cells express high levels of α-syn [Citation35,Citation36]. To date, the abundant presence of SNCA mRNA and protein in erythroid cells have been confirmed by various methods (quantitative PCR, microarray, western blot, immunohistochemistry, electrochemiluminescence and ELISA). In human peripheral blood, approximately 1% of α-syn is present in the plasma while the remainder exists in the cells [Citation35]. However, it is hard to distinguish whether the plasma α-syn originates from lysed erythrocytes when preparing samples or from microvesicles released from erythrocytes and non-erythroid cells. Among the cellular fractions of peripheral blood, erythroid cells contain the majority (>99%) of α-syn, while only a small share of the total α-syn is present in mononuclear cells (0.05%) and platelets (0.2%) [Citation35]. Although α-syn is found mainly in red blood cells, this does not mean that the concentration of α-syn in each red blood cell is higher than that in other cell types in blood. In fact, the highest amount of α-syn per milligram of cellular proteins is actually in platelets [Citation35]. For example, the concentration of α-syn in human platelets is about 264 nanogram per milligram of cellular protein (∼264 ng/mg), which is higher than that in peripheral mononuclear cells (155 ng/mg) and red blood cells (131 ng/mg) [Citation35].
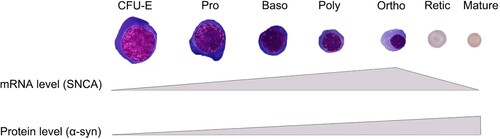
Nearly all erythroid precursors in human bone marrows strongly express α-syn [Citation37]. Further studies demonstrated that α-syn is differentially expressed during erythropoiesis. In culture of human CD34+ cells from peripheral blood, the α-syn gene starts transcription in the erythroid colony forming unit (CFU-E) stage and reaches peak levels in the late stage of erythroblasts (around orthochromatic stage), whereas protein levels from monomers reach the highest in terminal differentiation stages (enucleated cells) () [Citation14]. Interestingly, a high molecular protein band (over 100 kDa) can be detected in the early stage of erythroblasts (CFU-E and proerythroblast) but have not been confirmed as an oligomeric form of α-syn [Citation14]. Notably, the concentration of total α-syn (but not oligomeric α-syn) in peripheral erythrocytes in older people is relatively lower than that in younger individuals, possibly due to the DNA methylation of intron-1 of the SNCA gene [Citation38].
Figure 2. Differential expression of α-syn during human erythropoiesis. In culture of human CD34+ cells from peripheral blood, the α-syn gene starts transcription in the erythroid colony forming unit (CFU-E) stage and reaches peak levels in late stage of erythroblasts, whereas protein levels reach the highest in mature red blood cells.
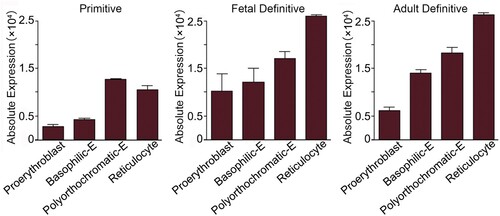
In murine erythrocytes, the concentration of α-syn is about half compared to that in human erythrocytes [Citation35]. Flow cytometry reveals that 80.0 ± 6.8% mouse bone marrow erythroid cells and almost all the peripheral erythrocytes express α-syn [Citation10]. The Erythron DB Resource (https://www.cbil.upenn.edu/ErythronDB/home.jsp) database at the University of Pennsylvania classifies α-syn mRNA levels in four developmental stages: proerythroblast, basophilic erythroblast, polyorthochromatic erythroblast and reticulocyte. In murine primitive erythropoiesis, the expression of SNCA mRNA reaches its peak in the polyorthochromatic stage, while in fetal definitive and adult definitive erythropoiesis, the expression of SNCA mRNA increases upon the maturation of erythroid cells ().
Figure 3. Differential expression of α-syn during mouse erythropoiesis. The Erythron DB Resource database classifies α-syn mRNA levels in four developmental stages. In primitive erythropoiesis, the levels of α-syn mRNA reaches its peak in the polyorthochromatic stage, while in fetal definitive and adult definitive erythropoiesis, the levels of α-syn mRNA are higher in late stage of erythroid cells (https://www.cbil.upenn.edu/ErythronDB/home.jsp).
Whether the alteration of α-syn levels is related with human erythrocyte-associated diseases have not yet been reported. One study demonstrated that α-syn is strongly expressed in neoplastic erythroid cells (erythroid leukemia cells) in a diffuse cytoplasmic pattern, but the α-syn-staining intensity in erythroid leukemia cells and erythroid cells from normal reactive bone marrows is very similar [Citation37]. Different neoplastic cells from the hemopoietic system differentially express α-syn [Citation37], and thus, α-syn may be an auxiliary indicator for distinguishing erythroid neoplastic diseases from neoplasms of non-erythroid hematopoietic cells.
Function of α-syn in erythropoiesis
α-syn is transcriptionally activated by the master erythroid regulator GATA1
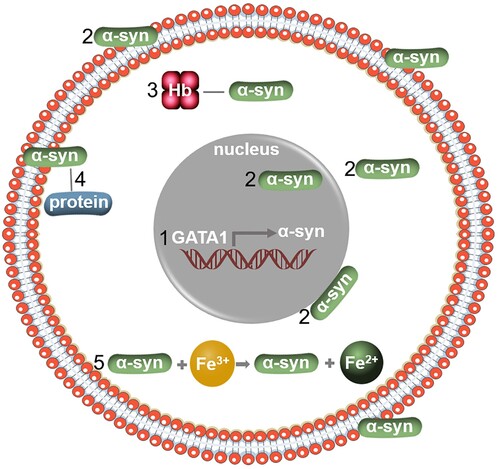
GATA-1 is a master transcription factor induced by erythropoietin secreted from the neuronal crest cells of the kidney [Citation39] and is a key erythroid regulatory factor that directly controls thousands of genes [Citation40,Citation41]. It has been found that GATA-1 directly regulates the expression of α-syn [Citation15]. In erythroid precursor cells, GATA-1 activates SNCA transcription by occupying the conserved intron-1 sequence of the SNCA gene () [Citation36]. Compared with uninduced erythroid progenitor cells, the levels of SNCA mRNA and α-syn protein induced by GATA-1 increase up to 62-fold and 7-fold, respectively [Citation36]. These studies strongly suggest that α-syn might play a role in erythropoiesis.
Figure 4. Expression and potential function of α-syn in erythroid cells. (1) α-syn can be transcribed by GATA1, a master transcription factor in erythroid cells; (2) Location of α-syn in erythroid cells; (3) α-syn binds to hemoglobin to form a protein complex; (4) α-syn acts as a chaperone to bind to other cellular proteins; (5) α-syn regulates iron homeostasis in erythroid cells.
Function in erythroid cells
The N-terminal of α-syn protein binds to the red blood cell membrane [Citation14,Citation42] (). Through this binding, α-syn might increase the mechanical strength of the membrane, thereby regulating the membrane fluidity to protect erythroid cells from being damaged by various mechanical pressures [Citation43]. Meanwhile, it is possible that the C-terminal of α-syn acts as a chaperone to bind to other cellular proteins and this might attribute to the fact that α-syn can prolong the life span of erythrocytes () [Citation43].
In α-syn knockout (KO) mouse models, the deletion of α-syn gene in mice does not affect basic brain functions at baseline and overall survival [Citation44]. Erythropoiesis in general was not disturbed compared with that in wild-type (WT) mice [Citation10,Citation15]. However, several groups demonstrate mild anemia at baseline erythropoiesis in α-syn KO mice, which is characterized by decreased red blood cell count, lower hemoglobin levels and lower hematocrit [Citation7,Citation45,Citation46]. Part of the reason might be the defect of enucleation [Citation14] though the reactive oxygen species (ROS) levels in these cells are even lower than that in WT mice [Citation15]. Whether severe phenotype changes under stress conditions (such as acute blood loss, acute hemolysis, or chemotherapeutic-induced anemia) in α-syn KO mice occur remains to be explored. Whether overexpression of α-syn causes defect of erythropoiesis is yet to be determined.
α-syn regulates iron homeostasis in red blood cells
Most of the iron in erythrocytes exists in heme that is essential for hemoglobin to carry oxygen [Citation47]. The unique properties of iron make it not only an electronic carrier, but also a catalyst for redox reaction [Citation48]. In erythroid cells, SNCA is co-expressed and co-induced with three heme metabolism genes ferrochelatase (FECH), 5-aminolevulinate synthase 2 (ALAS2) and biliverdin reductase B (BLVRB) [Citation36]. It has been demonstrated that hemoglobin (Hb) and α-syn form a complex that exists in peripheral red blood cells () [Citation49]. Likewise, iron is the mediator between α-syn and Hb. The main role of Fe(II) in human blood cells is to bind and transport oxygen, while Fe(III) does not have the ability to carry oxygen. α-syn is able to reduce Fe(III) into Fe(II) by upregulating the expression and activity of a cellular iron-reducing protein ferrireductase () [Citation50]. Meanwhile, deletion of the α-syn gene results in decreased ferritin and transferrin receptors in the brain, spleen, and neuroretina, which leads to disturbance of the iron homeostasis and thus less hemoglobin production [Citation7]. All these studies suggest a biological connection between α-syn and hemoglobin production [Citation51].
α-syn in other hematopoietic cells
α-syn also plays an important role in the development and function of other hematopoietic cells besides erythroid lineage cells [Citation10,Citation52,Citation53]. In leukocytes, deletion of α-syn is associated with ultrastructural changes including cell size and microvesicle shape. An increase in the number of smooth-endoplasmic reticulum (SER), specific granules (SG) and inclusion bodies is also observed [Citation46]. α-syn is essential for the maturation and normal function of B lymphocytes. In the absence of α-syn, the number of B lymphocytes decrease and the production of immunoglobulin is insufficient, indicating a role of α-syn in B lymphocyte-mediated immunity [Citation45]. Similarly, α-syn is important to the development and immune response of T-lymphocytes. α-syn depletion results in a defect of T-cell differentiation characterized by significant increases of in the number of CD4 and CD8 double negative cells and significant decreases in the number of CD4 single positive and CD8 single positive T lymphocytes; as a result, the number of circulating T cells in blood is markedly reduced [Citation54]. Moreover, while splenic CD4 and CD8 positive cells are hyperactivated in α-syn KO mice, the Th2 response in these mice is defective [Citation54]. α-syn is also expressed in NK cells to regulate NK cell function through interaction with soluble N-ethylmalemide-sensitive factor attachment protein receptor (SNARE) complexes [Citation55]. In transgenic mice overexpressing α-syn, macrophages show impaired phagocytosis, interruption of cytokine production and defective fragment clearance [Citation56]. The highest concentration of α-syn per milligram of cellular proteins is actually in platelets [Citation35]. In α-syn KO mice, the mean platelet volume is significantly reduced [Citation45]. Moreover, α-syn acts as a calcium-dependent negative regulator for the release of α-granule by platelets, while platelets abnormalities are found in patients with neurodegenerative diseases [Citation57]. The functional partners of α-syn might exist, hinted by the similar domains of this protein in platelets as seen in brain tissues [Citation58]. Interestingly, α-syn levels in plasma increase over time during platelet storage [Citation59], although it has not been determined whether this concentration increase correlates with biological activities such as the negative regulation of granule release from platelets. Elaborating the mechanism of how α-syn affects hematopoiesis requires additional investigations.
Erythroid α-syn in neurodegenerative diseases
The presence of anemia or low hemoglobin levels are often associated with PD [Citation51,Citation60]. A recent study demonstrated that patients with newly diagnosed anemia have higher risk developing PD after four years [Citation61]. By observing the ultrastructure of erythrocytes in patients with PD, the shape of erythrocytes is found to be changed, including phospholipid membrane scrambling, membrane blebbing and cell shrinkage, all of which are the characteristics of eryptosis, a class of programed cell death [Citation62].
So far, α-syn has been studied most in neurodegenerative diseases including Alzheimer’s disease (AD), PD, Huntington’s disease (HD), motor neuron disease (MND), frontotemporal dementia (FTDS), multiple system atrophy (MSA) [Citation63] and prion diseases [Citation64]. In some neurodegenerative diseases, α-syn in cerebrospinal fluid (CSF) is used as a clinical indicator for diagnosis [Citation65]. In terms of biomarkers, however, α-syn in erythrocytes have more advantages than CSF since its acquisition is much less invasive. Specifically, oligomer/total protein ratio of α-syn in erythrocytes could be a diagnostic biomarker for PD [Citation65]. The levels of α-syn dimer and the ratio of dimer/monomer in erythrocyte membrane might also be useful indicators of PD and related diseases [Citation66]. In addition, phosphorylated α-syn can be detected in both the erythrocytic fractions [Citation67] and the blood plasma of PD [Citation68]. Nevertheless, the levels of α-syn in erythrocytes vary in different neurodegenerative disorders, suggesting that erythrocyte is an ideal source for the study of neurodegenerative diseases [Citation69,Citation70].
Conclusion and future perspective
Evidence has shown the participation of α-syn in erythropoiesis. In addition, blood-borne α-syn could be a useful biomarker for anemia and neurodegenerative diseases. Considering the abundance of α-syn in erythroid cells, however, lots of questions related to the functions of α-syn and the mechanism in erythropoiesis are yet to be answered. For instance, is the natural α-syn in erythroid cells is monomer or tetramer? Does α-syn impact stress erythropoiesis? Does α-syn affect disease development of erythroid cells? Do synergistic effects of α-syn with other molecules exist during erythroid development? Answering these questions should identify new cellular pathways that are amenable to manipulation by standard pharmacologic approaches to treat erythroid diseases.
URL
Erythron DB Resource, https://www.cbil.upenn.edu/ErythronDB/home.jsp.
Acknowledgements
We are grateful to Yuanjun Yu for language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8(8):2804–2815.
- Araki K, Yagi N, Aoyama K, et al. Parkinson’s disease is a type of amyloidosis featuring accumulation of amyloid fibrils of α-synuclein. Proc Natl Acad Sci USA. 2019;116(36):17963–17969.
- Emelyanov A, Kulabukhova D, Garaeva L, et al. SNCA variants and alpha-synuclein level in CD45 + blood cells in Parkinson’s disease. J Neurol Sci. 2018;395:135–140.
- Gelpi E, Navarro-Otano J, Tolosa E, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Movement Disord. 2014;29(8):1010–1018.
- Khoshi A, Goodarzi G, Mohammadi R, et al. Reducing effect of insulin resistance on alpha-synuclein gene expression in skeletal muscle. Diabetol Metab Syndr. 2019;11:99.
- Lee BR, Kamitani T. Improved immunodetection of endogenous alpha-synuclein. PLoS One. 2011;6(8):e23939.
- Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: implications for visual manifestations of Parkinson’s disease. Free Radical Bio Med. 2016;97:292–306.
- Berger-Sieczkowski E, Lutz MI, Auff E, et al. Gaucher cells are not associated with alpha-synuclein neuropathology in infants. Clin Neuropathol. 2016;35(3):122–128.
- Barkovits K, Kruse N, Linden A, et al. Blood contamination in CSF and its impact on quantitative analysis of alpha-synuclein. Cells (Basel, Switzerland). 2020;9(2):370.
- Nakai M, Fujita M, Waragai M, et al. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358(1):104–110.
- Abd-Elhadi S, Basora M, Vilas D, et al. Total α-synuclein levels in human blood cells, CSF, and saliva determined by a lipid-ELISA. Anal Bioanal Chem. 2016;408(27):7669–7677.
- Miller DW, Hague SM, Clarimon J, et al. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62(10):1835–1838.
- Mizuta I, Tsunoda T, Satake W, et al. Calbindin 1, fibroblast growth factor 20, and alpha-synuclein in sporadic Parkinson’s disease. Hum Genet. 2008;124(1):89–94.
- Araki K, Sugawara K, Hayakawa EH, et al. The localization of alpha-synuclein in the process of differentiation of human erythroid cells. Int J Hematol. 2018;108(2):130–138.
- Renella R, Schlehe JS, Selkoe DJ, et al. Genetic deletion of the GATA1-regulated protein alpha-synuclein reduces oxidative stress and nitric oxide synthase levels in mature erythrocytes. Am J Hematol. 2014;89(10):974–977.
- Peelaerts W, Bousset L, Van der Perren A, et al. Alpha-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522(7556):340–344.
- Matsumoto J, Stewart T, Sheng L, et al. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017;5(1):71.
- Sui Y, Bullock KM, Erickson MA, et al. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides. 2014;62:197–202.
- Alegre-Abarrategui J, Brimblecombe KR, Roberts RF, et al. Selective vulnerability in α-synucleinopathies. Acta Neuropathol. 2019;138(5):681–704.
- Ray B, Mahalakshmi AM, Tuladhar S, et al. “Janus-Faced” α-synuclein: role in Parkinson’s disease. Front Cell Dev Biol. 2021;9:673395.
- Bougeaa A. Synuclein in neurodegeneration. Adv Clin Chem. 2021;103:97–134.
- Fuchs J, Tichopad A, Golub Y, et al. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008;22(5):1327–1334.
- Kessler JC, Rochet JC, Lansbury PJ. The N-terminal repeat domain of alpha-synuclein inhibits beta-sheet and amyloid fibril formation. Biochemistry-US. 2003;42(3):672–678.
- Lashuel HA, Overk CR, Oueslati A, et al. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48.
- Theillet F, Binolfi A, Bekei B, et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature. 2016;530(7588):45–50.
- Abd-Elhadi S, Honig A, Simhi-Haham D, et al. Total and proteinase K-resistant alpha-synuclein levels in erythrocytes, determined by their ability to bind phospholipids, associate with Parkinson’s disease. Sci Rep. 2015;5:11120.
- Okochi M, Walter J, Koyama A, et al. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J Biol Chem. 2000;275(1):390–397.
- Stephens AD, Zacharopoulou M, Kaminski Schierle GS. The cellular environment affects monomeric α-synuclein structure. Trends Biochem Sci. 2019;44(5):453–466.
- Cascella R, Bigi A, Cremades N, et al. Effects of oligomer toxicity, fibril toxicity and fibril spreading in synucleinopathies. Cell Mol Life Sci. 2022;79(3):174.
- Bartels T, Choi JG, Selkoe DJ. α-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110.
- Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797–17802.
- Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345–15364.
- Burré J, Vivona S, Diao J, et al. Properties of native brain α-synuclein. Nature (London). 2013;498(7453):E4–E6.
- Araki K, Yagi N, Nakatani R, et al. A small-angle X-ray scattering study of alpha-synuclein from human red blood cells. Sci Rep. 2016;6:30473.
- Barbour R, Kling K, Anderson JP, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5(2):55–59.
- Scherzer CR, Grass JA, Liao Z, et al. GATA transcription factors directly regulate the Parkinson’s disease-linked gene α-synuclein. Proc Natl Acad Sci USA. 2008;105(31):10907–10912.
- Maitta RW, Wolgast LR, Wang Q, et al. Alpha- and beta-synucleins are new diagnostic tools for acute erythroid leukemia and acute megakaryoblastic leukemia. Am J Hematol. 2011;86(2):230–234.
- Daniele S, Costa B, Pietrobono D, et al. Epigenetic modifications of the alpha-synuclein gene and relative protein content are affected by ageing and physical exercise in blood from healthy subjects. Oxid Med Cell Longev. 2018;2018:3740345.
- Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589(6):1251–1258.
- Yu M, Riva L, Xie H, et al. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell. 2009;36(4):682–695.
- Cheng Y, Wu W, Kumar SA, et al. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19(12):2172–2184.
- Moraitou M, Dermentzaki G, Dimitriou E, et al. Alpha-synuclein dimerization in erythrocytes of Gaucher disease patients: correlation with lipid abnormalities and oxidative stress. Neurosci Lett. 2016;613:1–5.
- Witt SN. Molecular chaperones, alpha-synuclein, and neurodegeneration. Mol Neurobiol. 2013;47(2):552–560.
- Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252.
- Xiao W, Shameli A, Harding CV, et al. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by α-synuclein, a key player in Parkinson’s disease. Immunobiology. 2014;219(11):836–844.
- Tashkandi H, Shameli A, Harding CV, et al. Ultrastructural changes in peripheral blood leukocytes in alpha-synuclein knockout mice. Blood Cells Mol Dis. 2018;73:33–37.
- Scott C, Arora G, Dickson K, et al. Iron chelation in local infection. Molecules. 2021;26(1):189.
- Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723–736.
- Yang W, Li X, Li X, et al. Hemoglobin-α-synuclein complex exhibited age-dependent alterations in the human striatum and peripheral RBCs. Neurosci Lett. 2020;736:135274.
- Brown DR. α-synuclein as a ferrireductase. Biochem Soc T. 2013;41(6):1513–1517.
- Santiago JA, Potashkin JA. Blood transcriptomic meta-analysis identifies dysregulation of hemoglobin and iron metabolism in Parkinson’s disease. Front Aging Neurosci. 2017;9:73.
- Pei Y, Maitta RW. Alpha synuclein in hematopoiesis and immunity. Heliyon. 2019;5(10):e2590.
- Shin EC, Cho SE, Lee DK, et al. Expression patterns of alpha-synuclein in human hematopoietic cells and in drosophila at different developmental stages. Mol Cells. 2000;10(1):65–70.
- Shameli A, Xiao W, Zheng Y, et al. A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology. 2016;221(2):333–340.
- Chiang SC, Theorell J, Entesarian M, et al. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood. 2013;121(8):1345–1356.
- Gardai SJ, Mao W, Schüle B, et al. Elevated alpha-synuclein impairs innate immune cell function and provides a potential peripheral biomarker for Parkinson’s disease. PLoS One. 2013;8(8):e71634.
- Park SM, Jung HY, Kim HO, et al. Evidence that alpha-synuclein functions as a negative regulator of Ca++-dependent alpha-granule release from human platelets. Blood. 2002;100(7):2506–2514.
- Stefaniuk CM, Schlegelmilch J, Meyerson HJ, et al. Initial assessment of α-synuclein structure in platelets. J Thromb Thrombolysis. 2021. doi:10.1007/s11239-021-02607-z.
- Stefaniuk CM, Hong H, Harding CV, et al. α-synuclein concentration increases over time in plasma supernatant of single donor platelets. Eur J Haematol. 2018;101(5):630–634.
- Savica R, Grossardt BR, Carlin JM, et al. Anemia or low hemoglobin levels preceding Parkinson disease: a case-control study. Neurology. 2009;73(17):1381–1387.
- Hong CT, Huang YH, Liu HY, et al. Newly diagnosed anemia increases risk of Parkinson’s disease: a population-based cohort study. Sci Rep-UK. 2016;6:29651.
- Pretorius E, Swanepoel AC, Buys AV, et al. Eryptosis as a marker of Parkinson’s disease. Aging (Albany, NY.). 2014;6(10):788–819.
- Liu G, Tian C, Gao L, et al. Alpha-synuclein in erythrocyte membrane of patients with multiple system atrophy: a pilot study. Parkinsonism Reatl D. 2019;60:105–110.
- Tofaris GK, Buckley NJ. Convergent molecular defects underpin diverse neurodegenerative diseases. J Neurol Neurosurg Psychiatry. 2018;89(9):962–969.
- Wang X, Yu S, Li F, et al. Detection of alpha-synuclein oligomers in red blood cells as a potential biomarker of Parkinson’s disease. Neurosci Lett. 2015;599:115–119.
- Papagiannakis N, Koros C, Stamelou M, et al. Alpha-synuclein dimerization in erythrocytes of patients with genetic and non-genetic forms of Parkinson’s disease. Neurosci Lett. 2018;672:145–149.
- Tian C, Liu G, Gao L, et al. Erythrocytic α-synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener. 2019;8:15.
- Foulds PG, Mitchell JD, Parker A, et al. Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J. 2011;25(12):4127–4137.
- Daniele S, Frosini D, Pietrobono D, et al. α-synuclein heterocomplexes with beta-amyloid are increased in red blood cells of Parkinson’s disease patients and correlate with disease severity. Front Mol Neurosci. 2018;11:53.
- Klatt S, Roberts A, Lothian A, et al. Optimizing red blood cell protein extraction for biomarker quantitation with mass spectrometry. Anal Bioanal Chem. 2020;412(8):1879–1892.
