ABSTRACT
Background
Whether combined CsA with androgen therapy was superior to androgen therapy alone in NSAA remains controversial. This study aimed to assess the efficacy and safety of combined therapy versus androgen therapy for NSAA patients using a meta-analytic approach.
Methods
An electronic database of PubMed, EmBase, Cochrane library, CNKI, VIP, and Wanfang was systematically searched for randomized controlled trials (RCTs) from their inception to February 2020. The primary endpoint was effective rate, while the secondary endpoints included white blood cell (WBC), hemoglobin, platelet, and potential adverse events. The pooled results from included trials were calculated with the random-effects model.
Results
Forty-three RCTs recruited 2610 NSAA patients for the final quantitative meta-analysis. We noted that combined therapy was associated with an increased incidence of effective rate than androgen therapy alone (relative risk [RR]: 1.35; 95% confidence interval [CI]: 1.29–1.41; P < 0.001). Moreover, patients treated with combined therapy were associated with higher WBC (weighted mean difference [WMD]: 1.22; 95%CI: 0.94–1.49; P < 0.001), hemoglobin (WMD: 12.93; 95%CI: 8.86–17.01; P < 0.001), and platelet (WMD: 8.65; 95%CI: 7.05–10.24; P < 0.001). Finally, the pooled incidence of hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage were 0.35 (95%CI: 0.22–0.48), 0.24 (95%CI: 0.15–0.32), 0.22 (95%CI: 0.10–0.35), 0.19 (95%CI: 0.14–0.25), and 0.06 (95%CI: 0.01–0.11), respectively.
Conclusions
This study found that combined CsA with androgen therapy was superior to androgen therapy alone for Chinese patients with NSAA, and the most common adverse of combined therapy included hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage.
Introduction
Aplastic anemia (AA) was characterized by hypocellular bone marrow and pancytopenia, which could be divided into non-severe AA (NSAA), severe AA, and very severe AA based on the degree of cytopenia. NSAA is an acquired condition with a diminished bone marrow activity, which could cause anemia, thrombocytopenia, and leukopenia. NSAA could be caused by a viral infection, medications, and other unknown causes [Citation1–3]. Patients with NSAA may not receive any intervention, and the immunosuppressive therapy or bone marrow transplantation is just given for patients who progress to severe AA. There was little attention paid to the treatment of NSAA.
Cyclosporine A (CsA), as an immunoagent, plays an important effect on lymphocyte proliferation and has significant application value in the treatment of aplastic anemia. Moreover, androgens are commonly used in the treatment of AA in China, promoting the production of erythropoietin and increasing the hematopoietic function of the bone marrow. However, whether the combined CsA with androgen therapy was superior to androgen therapy alone in NSAA is limited and unclear. Therefore, the current meta-analysis was conducted to assess the efficacy and safety of combined therapy in the treatment of NSAA in Chinese people compared with androgen therapy alone.
Methods
Data sources, search strategy, and selection criteria
This review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 [Citation4]. The study designed as randomized controlled trials (RCTs) and compared the efficacy and safety of combined CsA with androgen therapy versus androgen therapy alone for Chinese patients with NSAA was appropriate. The electronic databases of PubMed, EmBase, Cochrane library, CNKI, VIP, and Wanfang were systematically searched from their inception up to February 2020 and using the following core terms: aplastic anemia [MeSH] OR aplastic anemia AND cyclosporine AND RCT.
The literature search and study selection were conducted following the standard flow by two reviewers independently, and any disagreement was resolved by group discussion. The inclusion criteria of this meta-analysis are listed as follows: (1) patients: adults patients from China and diagnosed with NSAA; (2) intervention: combined CsA with androgen therapy; (3) control: androgen therapy; (4) outcomes: effective rate, white blood cell (WBC), hemoglobin, platelet, and potential adverse events; and (5) study design: the study had to have an RCT design.
Data collection and quality assessment
Two reviewers independently conducted the data abstracted and quality assessment, and any inconsistency was settled by an additional author reviewing the original article. The abstracted information included the first authors’ surname, publication year, sample size, mean age, percentage of males, intervention, control, treatment duration, and reported outcomes. The quality of included studies was assessed by the Jadad scale, which was based on randomization, concealment of the treatment allocation, blinding, completeness of follow-up, and the use of intention-to-treat analysis [Citation5].
Statistical analysis
The effective rate between combined therapy and androgen therapy was assigned as categories data, while the levels of WBC, hemoglobin, and platelet were assigned as continuous data. The relative risk (RR) and weighted mean difference (WMD) with a 95% confidence interval (CI) were applied to assess the pooled effect estimates for categories and continuous data, respectively. Moreover, the incidence of adverse events in patients treated with combined therapy was abstracted as the number of events and sample size. The pooled analyses in this study were conducted by the random-effects model [Citation6,Citation7]. Heterogeneity, among included trials, was assessed using I2 or Q statistic, and I2 > 50.0% or P < 0.10 was considered significant heterogeneity [Citation8,Citation9]. The robustness of pooled conclusions was assessed using sensitivity analysis [Citation10]. Subgroup analysis was conducted for effective rate based on publication year, mean age, percentage of males, control, and study quality, and the difference between subgroups was assessed using the interaction P test [Citation11]. Publication biases for effective rate, WBC, hemoglobin, and platelet were assessed by the funnel plot, Egger, and Begg test results [Citation12,Citation13]. The inspection level for all pooled results is 2-sided, and P < 0.05 was considered a significant difference. STATA software (version 10.0; Stata Corporation, College Station, TX, USA) was applied to analyze all data analyses in our meta-analysis.
Results
Literature search
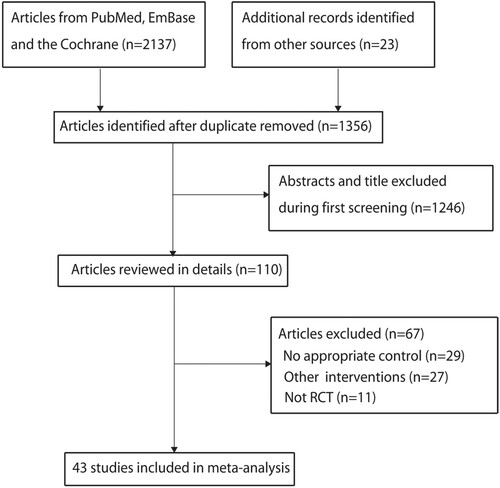
The initial electronic searches yielded 2137 records, and 781 were excluded because of duplicate titles. Additional 1246 articles were excluded due to the study reported irrelevant titles. A total of 110 studies were retrieved for further assessment, and 67 studies were excluded due to no appropriate control (n = 29); did not being treated with combined therapy (n = 27), and not RCT (n = 11). No new eligible study was found by reviewing the reference of retrieved studies. Finally, 43 studies involving 2610 NSAA patients from China were selected for this meta-analysis () [Citation14–56].
Study characteristics
The general characteristics of studies and patients are summarized in . The included studies published from 2001to 2020, and 30–120 patients were included in each included trial. The mean age of included patients ranged from 30.0 to 47.5 years, and the percentage of males ranged from 41.7 to 74.2 percent. A total of 36 studies compared combined therapy with stanozolol, while the remaining 7 studies compared combined therapy with other therapies, including testosterone undecanoate, and testosterone propionate, or did not define the type of androgen therapy.
Table 1. The baseline characteristics of included studies.
Primary endpoint
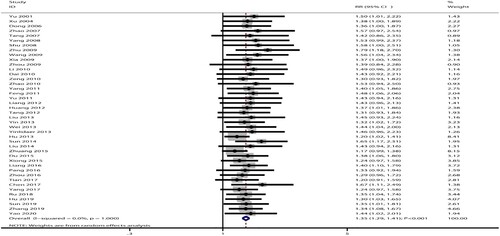
For the effect of combined therapy versus androgen therapy on the incidence of effective rate, data were reported in 42 trials. The pooled RR indicated combined therapy was associated with an increased incidence of effective rate (RR: 1.35; 95%CI: 1.29–1.41; P < 0.001; ), and no evidence of heterogeneity was seen across included trials (I2 = 0.0%; P = 1.000). The pooled conclusion was stable and not altered by sequential excluding individual trials (Supplemental 1). This significant difference remained in all subgroups ().
Table 2. Subgroup analysis for the effective rate.
White blood cell
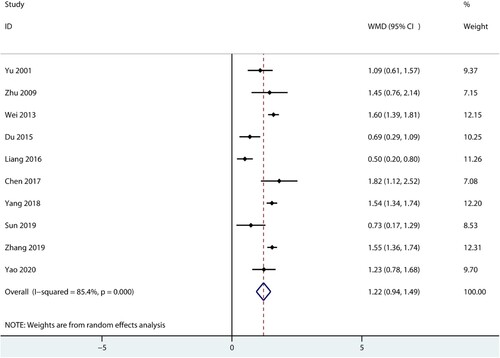
Data for the effect of combined therapy versus androgen therapy on WBC were reported in 10 trials. We noted that combined therapy was associated with high WBC levels compared with androgen therapy alone (WMD: 1.22; 95%CI: 0.94–1.49; P < 0.001; ), and significant heterogeneity was detected among included trials (I2 = 85.4%; P < 0.001). Sensitivity analysis indicated the pooled conclusion was not changed by sequential excluding individual trials (Supplemental 1).
Hemoglobin
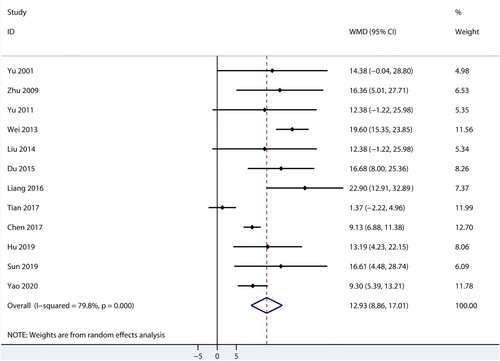
Data for the effect of combined therapy versus androgen therapy on hemoglobin were reported in 12 trials. The pooled result found patients treated with combined therapy were associated with a high level of hemoglobin (WMD: 12.93; 95%CI: 8.86–17.01; P < 0.001; ), and significant heterogeneity was seen across included trials (I2 = 79.8%; P < 0.001). This conclusion was robust and not altered by sequential excluding included trials (Supplemental 1).
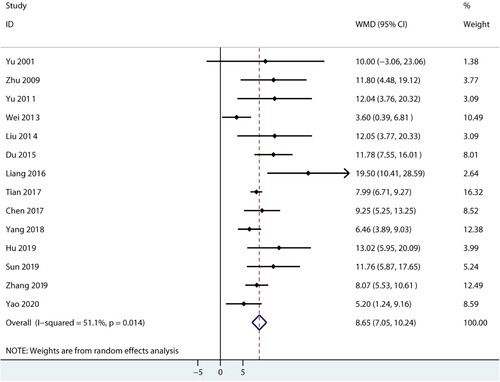
Platelet
Data for the effect of combined therapy versus androgen therapy on platelet were reported in 14 trials. We noted that combined therapy was associated with high platelet levels compared with androgen therapy alone (WMD: 8.65; 95%CI: 7.05–10.24; P < 0.001; ), and significant heterogeneity was found among included trials (I2 = 51.1%; P = 0.014). The conclusion was stable and not altered by excluding any particular trial (Supplemental 1).
Adverse events
The distribution of adverse events of hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage in patients treated with combined therapy is listed in . The pooled incidence of hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage for patients treated with combined therapy was 0.35 (95%CI: 0.22–0.48), 0.24 (95%CI: 0.15–0.32), 0.22 (95%CI: 0.10–0.35), 0.19 (95%CI: 0.14–0.25), and 0.06 (95%CI: 0.01–0.11), respectively.
Table 3. The results of adverse events in patients treated with combined therapy.
Publication bias
Publication biases for effective rate, WBC, hemoglobin, and platelet were assessed and displayed in Supplemental 2. Although no significant publication biases for WBC (PEgger: 0.204; PBegg: 0.721), hemoglobin (PEgger: 0.154; PBegg: 0.837), and platelet (PEgger: 0.074; PBegg: 0.101) were detected, we noted significant publication bias for effective rate (PEgger: < 0.001; PBegg: < 0.001). However, the conclusion was not altered by adjusting the trim and fill method [Citation57].
Discussion
This meta-analysis based on published RCTs assesses the efficacy and safety of combined therapy versus androgen therapy for Chinese patients with NSAA. This is the first meta-analysis focused on this topic and given the accurate effect estimates regarding the outcomes of effective rate, WBC, hemoglobin, platelet, and potential adverse events. This study found that combined therapy versus androgen therapy could significantly improve effective rate, WBC, hemoglobin, and platelet for Chinese patients with NSAA. Moreover, the most common adverse events in patients treated with combined therapy were hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage. Finally, the treatment effect of combined therapy versus androgen therapy on effective rate does not differ when stratified by publication year, mean age, percentage of males, control, and study quality.
The causes of NSAA are complex and varied, and are closely related to the injury of hematopoietic stem cells, immune-mediated response, and abnormal blood microcirculation function. T cell immune-mediated dysfunction could exert an inhibitory effect on the function of hematopoietic stem cells and cause the normal hematopoietic function of the body, which is associated with various anemia symptoms [Citation58]. Although traditional treatment strategies could improve the hematological parameters, they are prone to adverse reactions such as abnormal hair, skin rash, abnormal liver function, and gingival hyperplasia, which seriously affect the improvement effect of anemia symptoms.
The pooled results of this study found the effective rate, WBC, hemoglobin, and platelet could significantly be improved in NSAA patients treated with combined therapy. The potential reason for this could be that CsA as an immunosuppressive agent could significantly relieve the immune response by inhibiting the of negative factors (interleukin-interferon and tumor necrosis factor) by the effective regulation of the proportion of T cell subsets and reducing the influence of negative factors on hematopoietic function [Citation59,Citation60]. Moreover, androgen therapy could significantly increase the erythropoietin, enhance the differentiation and proliferation of stem cells, and regulate immune function [Citation61]. Therefore, the combination of hematopoietic and immunosuppressants could enhance hematopoietic function.
Subgroup analyses found a significant difference between combined therapy and androgen therapy in effective rate in all subsets. However, we noted the treatment effect is more evident when trials published before 2010, mean age <35.0 years, a percentage of males <60.0%, used other androgen agents as background therapies, or studies reported the type of randomization with relatively high quality. These results could be explained by the treatment effect of combined therapy that was more suitable for younger male patients, and used testosterone undecanoate and testosterone propionate or did not define the type of androgen therapy as background therapies. Moreover, the physical examination was more standardized, and NSAA patients could help with early diagnosis.
The most common adverse events for NSAA patients treated with combined therapy were hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage. The timely treatment strategies were given for patients with adverse events which could be effectively alleviated. Therefore, we should pay more attention to the physical health and mental health of our NSAA patients during the treatment process. Moreover, communications with patients should enhance their attitude and confidence when suffering from the disease and result in the recovery of the disease. Finally, the family members of patients should be involved in the treatment process, which could play a supervisory role in the drugs and quantity to ensure the treatment effect and improve the prognosis of NSAA.
Several strengths and limitations in this meta-analysis should be highlighted: (1) the analysis of this study was based on RCTs, and the evidence level was relatively high; (2) the pooled results were based on a large sample size, and the results of this study are more robust than any individual trial; (3) subgroup analysis for effective rate was conducted to assess the treatment effect of combined therapy versus androgen therapy in specific subpopulations; (4) substantial heterogeneity for WBC, hemoglobin, and platelet were detected; (5) the study quality of included trials was low, and the blindness, concealment of the treatment allocation, and the use of intention-to-treat analysis were not reported in nearly all included trials; and (6) the analysis based on published articles, and publication bias was an inevitable problem.
Conclusion
This study found that combined therapy was significantly associated with the improvement in effective rate, WBC, hemoglobin, and platelet than androgen therapy alone for Chinese patients with NSAA. Moreover, the most common adverse events in patients treated with combined therapy were hirsutism, handshake, gingiva hyperplasia, liver function damage, and renal function damage. Further large-scale RCT should be conducted to assess other effective treatment strategies for patients with NSAA.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this study does not directly involve human subjects.
Supplemental Material
Download MS Word (334.1 KB)Supplemental Material
Download MS Word (697.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Rosenfeld S, Follmann D, Nunez O, et al. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135.
- Young NS, Calado RT, Scheinberg P. Current concepts in pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519.
- Maciejewski JP, O ‘Keefe C, Gondek L, et al. Immune-mediated bone marrow failure syndromes of progenitor and stem cells: molecular analysis of cytotoxic T cell clones. Folia Histochem Cytobiol. 2007;45:5–14.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6:e1000097.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Mak. 2005;25:646–654.
- Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions 501. The Cochrane Collaboration; 2008.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560.
- Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:17.
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editor. Systematic reviews in health care: metaanalysis in context. 2nd ed. London: BMJ Books; 2001. p. 312.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101.
- Yu G, Zhang C, Li D. Effect of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. Heilongjiang Med Pharm. 2001;24:21.
- Xu H, Chen T, Zhang X, et al. Effect observation of cyclosporine A combined with androgen in the treatment of chronic aplastic anemia. Chin J Hemorh. 2004;14:477–478.
- Dong J, Xu H, Fang F, et al. Observing the effect of cyclosporine A combined androgen on chronic aplastic anemia. Hebei Med. 2006;12:1156–1157.
- Zhao Y, Zhu X, Yu T, et al. Cyclosporine A combined with testosterone undecanoate in the treatment of chronic aplastic anemia. Chin Hydro Med. 2007;5:270–271.
- Tang X, Zeng Y, He W, et al. Oserving the effect of cyclosporine A combined stanozolol on chronic aplastic anaemia. West China Med J. 2007;22:528.
- Yang G. Clinical observation of 35 cases of chronic aplastic anemia treated by cyclosporine A combined with kanglilong. Shandong Med J. 2008;48:92–93.
- Shu H, Fang T, Ge S. Effect of cyclosporine soft capsule combined with kanglilong in the treatment of chronic aplastic anemia. Chin J Misdiag. 2008;8:3577–3578.
- Zhu W, Li L. Therapeutic effect of cyclosporin A combined with testosterone propionate on chronic aplastic anemia. Prac Prev Med. 2009;16:1557–1558.
- Wang P, He Y, Fu H. Effect of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. Chin Prac Med. 2009;4:161–162.
- Xia Z. Effect observation of cyclosporine A combined with kangilong in the treatment of 29 cases of chronic aplastic anemia. Huazhong Med J. 2009;33:345–346.
- Zhou Z, Wang Z, Zhao X. Clinical observation of cyclosporine A combined therapy for chronic aplastic anemia. J Qiqihar Med Coll. 2009;30:2763–2764.
- Li Y, Pan H. Effect of cyclosporine A combined with testosterone undecatrate in the treatment of chronic aplastic anemia. Youjiang Med. 2010;38:427–428.
- Dai Q, Xu X, Meng W, et al. Effect of cyclosporine A combined with kanglilong in the treatment of chronic aplastic anemia. J Clin Med Prac. 2010;14:52–53.
- Zeng X. Observation on the curative effect of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. Hebei Med J. 2010;32:37–38.
- Zhao J. Observation on the curative effect of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. Chin Commun Phys. 2010;15:16.
- Yang J. Effect of cyclosporine A combined with kanglilong in the treatment of 31 cases of chronic aplastic anemia. Guide Chin Med. 2011;9:72–73.
- Feng G. The effect of cyclosporine A combined with kangilong in the treatment of 52 cases of chronic aplastic anemia. Chin Prac Med. 2011;6:135–136.
- Yu H, Zhang Z, Wu T. The clinical observation of cyclosporine A combined with stanozolol used in chronic aplastic anemia. Chin Mod Doct. 2011;49:52–53.
- Liang Y, Lu L, Cui X, et al. Clinical observation of cyclosporine A combined with stazolium in the treatment of chronic aplastic anemia. Chin J Chin Ration Drug Use. 2012;5:65–66.
- Huang W. Evaluation of efficacy on stanozolol tablets joint ciclosporine capsules in the treatment of 30 patients with chronic aplastic anemia. Chin Med Pharm. 2012;2:84–85.
- Tang W, Chen J. Evaluation of efficacy on stanozolol tablets joint cyclosporine capsules in the treatment of 50 patients with chronic aplastic anemia. Chin Foreign Med. 2012;32:73–74.
- Liu X. Effect of cyclosporine A combined with kanglilong in the treatment of chronic aplastic anemia. J Med Theor Prac. 2013;26:2185–2186.
- Yin Y. Cyclosporin A joint stanozolol tablets in treatment of chronic aplastic anemia clinical curative effect. Chin J Trau Disab Med. 2013;21:19–21.
- Wei T, Gong Z. Clinical effects of ciclopirin A combined with stanozolol in treatment of chronic aplastic anemia. Chin J Coal Indus Med. 2013;16:1802–1804.
- Yilidaer A, Dilinazi A, Hao J, et al. Clinical treatment of chronic aplastic anemia. Med Infor. 2013;26:223.
- Hu H. Clinical analysis of stanzole combined with cyclosporine A in the treatment of chronic aplastic anemia. Chin Prac Med. 2013;8:112–113.
- Sun T, Yang L, Zhang Y. Clinical efficacy of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. J Aerospace Med. 2014;25:1705–1706.
- Liu W, Wu R, Li L. Effect analysis of cyclosporine A combined with kangilong in the treatment of chronic aplastic anemia. Chin J Prev Contr Chron Dis. 2014;22:106–107.
- Zhuang W. Clinical analysis of 90 cases of chronic aplastic anemia. Med Infor. 2015;28:228.
- Du Z, Dai C. Effects of cyclosporine A combined with kangilong on bone marrow stromal cell-related cytokines in patients with chronic aplastic anemia. Chin J Gerontol. 2015;35:955–957.
- Xiong H. Clinical observation of cyclosporine A combined with statazole in the treatment of non-severe aplastic anemia. Chin Prac Med. 2015;10:134–135.
- Liang Y, Zhang Y, Qin Y, et al. The treatment effect and adverse reaction of cyclosporin A combined testosterone undecanoate on chronic aplastic anemia. J Clin Exper Med. 2016;15:1604–1606.
- Pang H. Clinical effect of cyclosporine A in the treatment of chronic aplastic anemia (CAA). Chin Health Stand Manag. 2016;7:95–96.
- Zhou S. The clinical value of stazoll and cyclosporine A in the treatment of chronic aplastic anemia. Chin J Trau Disab Med. 2016;24:25–26.
- Tian J. Analysis of treatment and outcome of non-severe aplastic anemia. World Clin Med. 2017;11:39–40.
- Chen Q. Clinical observation of cyclosporine A combined with kanglilong in the treatment of patients with chronic aplastic anemia. Med Front. 2017;7:87–88.
- Yang X. Observation on the effect of cyclosporine A combined with kanglilong in the treatment of patients with chronic aplastic anemia. Med Front. 2017;7:226–227.
- Ru W. Study on the clinical effect of kangilong combined with cyclosporine A in the treatment of chronic aplastic anemia. Clin Res. 2018;26:100–101.
- Yang Q. Treatment of chronic aplastic anemia with stanzole and cyclosporine A. Shenzhen J Integrat Tradit Chin West Med. 2018;28:162–163.
- Hu X. Effect observation and effect analysis of effectiveness of kangilong combined with cyclosporine A in the treatment of chronic aplastic anemia. J Clin Med. 2019;6:29–30.
- Sun D, Zhang Z. Effect of cyclosporine A combined with kanglilong in the treatment of chronic aplastic anemia. Guide Chin Med. 2019;17:42–43.
- Zhang Z, Li Z, Yang X, et al. Efficacy of cyclosporine A combined with stazolium in the treatment of patients with chronic aplastic anemia. Prac Clin J Integrat Tradit Chin West Med. 2019;19:24–26.
- Yao C. Clinical observation of cyclosporine A combined with stazolium in the treatment of chronic aplastic anemia. Public Med Forum Magaz. 2020;24:79–80.
- Duvall S, Tweedie R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98.
- Kwon JH, Kim I, Lee YG, et al. Clinical course of non-severe aplastic anemia in adults. Int J Hematol. 2010;91:770–775.
- Matsuda K, Koya J, Arai S, et al. Cyclosporine therapy in patients with transfusion-independent non-severe aplastic anemia: a retrospective analysis. Intern Med. 2019;58:355–360.
- Mandal PK, Baul S, Dolai TK, et al. Outcome of cyclosporine monotherapy in patients of aplastic anemia: experience of a tertiary care hospital in eastern India. Indian J Hematol Blood Transfus. 2017;33:144–147.
- Piedras J, Hernández G, López-Karpovitch X. Effect of androgen therapy and anemia on serum erythropoietin levels in patients with aplastic anemia and myelodysplastic syndromes. Am J Hematol. 1998;57:113–118.