ABSTRACT
Background
Epstein Barr virus positive (EBV+) immunodeficiency-associated lymphoproliferative disorders (IA-LPD) are heterogeneous diseases with variable treatment strategies that are not well-defined.
Case presentation
A 68-year-old woman with systemic lupus erythematosus developed EBV+ B-cell polymorphic lymphoproliferative disease (LPD). Positron emission tomography computed tomography (PET/CT) showed a large nasopharyngeal mass, multiple pulmonary lesions, splenomegaly and disseminated lymphadenopathy. Plasma EBV DNA was grossly elevated to 1.5 × 104 IU/mL. There were three paraproteins. Treatment with O-CHOP (obinutuzumab, cyclophosphamide, adriamycin, vincristine, prednisolone) led to undetectable plasma EBV DNA, suggesting eradiation of the EBV-positive malignant clone. However, radiologic abnormalities were still present on PET/CT, and paraprotein persisted. A nasopharyngeal re-biopsy showed infiltration with EBV-negative plasma cells. On treatment with lenalidomide, she finally achieved complete metabolic response. Molecular analysis showed that the EBV+ B-cell LPD and the EBV– plasma cell lesion exhibited identical immunoglobulin gene rearrangements. Next generation sequencing revealed that the EBV+ B-LPD showed mutation in only one gene (TP53), a profile typical of EBV-driven lymphoid neoplasms. However, the EBV– plasma cell lesion showed mutations in five genes (TP53, SF3B1, STAT5B, CD79B and CRKL), suggesting that these mutations instead of EBV infection were the oncogenic driver.
Conclusion
The presence of both EBV+ and EBV– lesions, which showed different mutational profiles, indicated clonal heterogeneity that might be of biologic and therapeutic significance.
Introduction
Epstein Barr virus positive (EBV+) immunodeficiency-associated lymphoproliferative disorders (IA-LPD) are heterogeneous [Citation1,Citation2]. Histopathologic entities include B-cell hyperplasia, polymorphic B-cell proliferations, indolent B-cell lymphomas, and aggressive B-cell lymphomas (diffuse large B-cell lymphoma; high-grade B-cell lymphoma, not otherwise specified; Burkitt lymphoma; plasmablastic lymphoma; classical Hodgkin lymphoma) [Citation1]. Treatment strategies are not well-established. For iatrogenic EBV+ IA-LPD, reduction of immunosuppression is recommended. When immunodeficiency cannot be reversed, aggressive treatment including immunochemotherapy and radiotherapy is needed [Citation2].
Case presentation
A 68-year-old woman with a 13-year history of systemic lupus erythematosus treated with hydroxychloroquine (for three years) and methotrexate (for one month) presented with bilateral pulmonary masses and mediastinal lymphadenopathy. Biopsy of the pulmonary mass showed caseating granulomas negative for tuberculosis. Empirical treatment for actinomycosis was given.
She did not improve and was referred to our center. F-18 fluorodeoxyglucose (FDG) positron emission tomography computed tomography (PET/CT) showed a huge nasopharyngeal mass (arrow, A), significantly enlarged pulmonary lesions, splenomegaly and generalized lymphadenopathy. Biopsy of the nasopharyngeal mass showed a dense polymorphic infiltrate of CD20+ B-cells, admixed with CD3+ T-cells and mature plasma cells with lambda (λ) light chain restriction (A). In-situ hybridization (ISH) showed numerous cells positive for Epstein–Barr virus (EBV) encoded small RNA (EBER), which on combined ISH/immunophenotyping were found predominantly in the CD20+ population (A). Features were consistent with polymorphic EBV-associated B-cell LPD [Citation1], or polymorphic B-cell LPD, EBV+, iatrogenic/immune senescence [Citation2]. Serum immunoglobulin (Ig) electrophoresis showed three paraproteins, two quantifiable (IgAλ: 2.5 g/L; IgG kappa, IgGκ: 24.6 g/L), and one unquantifiable (IgGλ) (). Plasma EBV DNA was grossly elevated (1.5 × 104 IU/mL) (). Bone marrow trephine biopsy showed paratrabecular atypical B-cell aggregates, comprising predominantly CD20+ cells. Scattered CD138+ plasma cells were present, showing λ light chain restriction. Interestingly, EBER+ cells were substantially fewer than those in the nasopharyngeal biopsy (B).
Figure 1. Positron emission tomography computed tomography. (A) On presentation, showing disseminated hypermetabolic lymphadenopathy, pulmonary lesions, a huge nasopharyngeal mass (arrow), and splenomegaly with abdominal lymphadenopathy. (B) End-of-treatment, showing residual nasopharyngeal mass of reduced size and metabolic activity (arrows). (C) After four 21-day cycles of lenalidomide, with complete resolution of the nasopharyngeal mass. There was some residual lymphadenopathy (arrow) with metabolic activity lower than that of the liver.
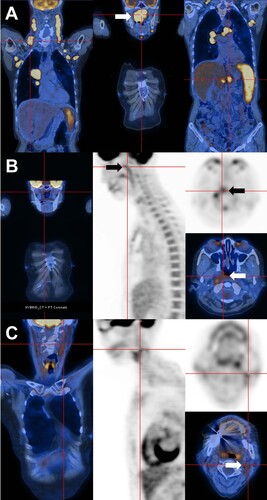
Figure 2. Histopathologic features. (A) Nasopharynegal biopsy on presentation, showing a polymorphic infiltrate of B-cells admixed with plasma cells and reactive T cells. The proportions of CD20-positive B-cells and CD138-positive plasma cells were about equal. Plasma cells showed lambda light chain restriction. In-situ hybridization for Epstein Barr virus encoded small RNA (EBER) showed numerous positive cells. Combined immunohistochemical staining and in situ hybridization showed that EBER expression was predominantly found in CD20-positive cells (original magnifications: hematoxylin eosin: 400; others: 200X). (B) Bone marrow biopsy on presentation, showing paratrabecular lymphoid infiltrates comprising predominantly CD20+ B-cells. CD138+ plasma cells were fewer, showing lambda light chain restriction. Different from the nasopharyngeal biopsy, there were only very few EBER+ cells (original magnifications: hematoxylin eosin: 800X; CD20: 400X; CD 138: 400X; kappa and lambda: 800X; EBER: 200X). (C) Nasopharyngeal biopsy after six courses of chemotherapy, showing a predominant mature-looking plasma cell infiltrate with very few CD3+ and CD20+ cells. Plasma cells were lambda light chain restricted. Practically no EBER+ cells were found (original magnification: hematoxylin eosin: 400X; others: 200X).
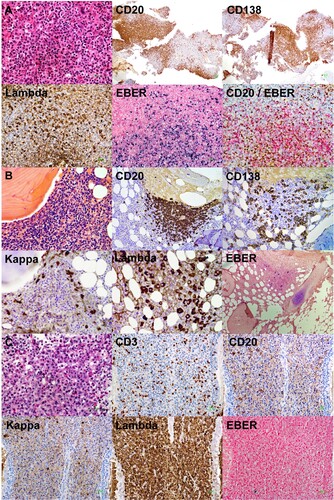
Figure 3. Evolution of laboratory parameters with treatment. On presentation, there were three paraproteins, two of which were quantifiable (immunoglobulin A lambda, IgAλ; immunoglobulin G kappa, IgGκ). EBV DNA was grossly elevated to > 104 IU/mL. After three cycles of O-CHOP (obinutuzumab, cyclophosphamide, adriamycin, vincristine, prednisolone), EBV DNA became undetectable. At the same time, paraprotein 1 had become undetectable. However, paraprotein 2 was still detectable after completion of six cycles of O-CHOP. With commencement of lenalidomide, paraprotein 2 gradually decreased, and became undetectable after 4 monthly cycles of lenalidomide. X axis (time) was the same for the paraprotein and EBV DNA.
Six cycles of obinutuzumab, cyclophosphamide, doxorubicin, vincristine and prednisolone were given. Plasma EBV DNA became undetectable after three cycles (). IgAλ became unquantifiable, whereas IgGκ was reduced but still present. End-of-treatment PET/CT showed persistent nasopharyngeal mass, although reduced in size and metabolic activity (arrows, B); associated with residual lymphadenopathy and pulmonary lesions of low-grade FDG-avidity. Re-biopsy of the nasopharynx showed a predominant infiltrate of EBER-negative (EBER–) plasma cells with λ-light chain restriction, and very few CD20+ cells (C).
Because the residual lesion comprised predominantly plasma cells, she was treated with lenalidomide (25 mg/day). The IgGκ paraprotein gradually declined (). PET/CT after four 21-day cycles of lenalidomide showed resolution of the nasopharyngeal mass (C), and complete metabolic remission of the pulmonary masses and lymphadenopathy. IgGκ paraprotein had also become unquantifiable (). At her latest follow-up twenty-two months after starting lenalidomide, there was no quantifiable paraprotein. EBV DNA remained undetectable, with the latest PET/CT confirming continuous remission.
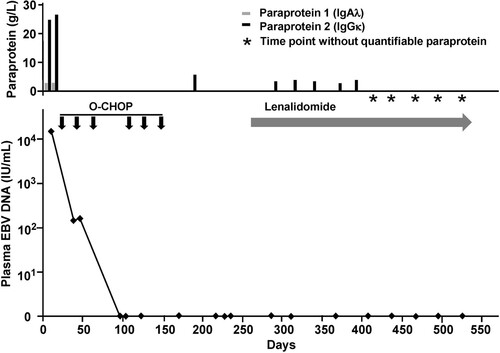
Polymerase chain reaction (PCR) for immunoglobulin heavy chain, and κ and λ light chain genes (IGH, IGK, IGL) was performed. The EBV+ B-cell LPD (first nasopharyngeal biopsy) and the EBV– plasma cell lesion (second nasopharyngeal biopsy) showed identical IGH, IGK and IGL rearrangements (). PCR for IGH in the marrow aspirate showed a clonal pattern, but the amplification peaks were weak and could not be conclusively shown to be identical with those of the nasopharyngeal biopsies. Next generation sequencing (NGS) of 35 lymphoid-relevant genes (ALK, ATM, BCL10, BCL2, BCL6, BIRC3, BTK, CARD11, CD79A, CD79B, CRKL, DNMT3A, EZH2, FBXW7, IDH1, IDH2, IRF4, JAK1, JAK2, JAK3, MALT1, MTOR, MYC, MYD88, NOTCH1, POT1, RHOA, SETD2, SF3B1, STAT3, STAT5A, STAT5B, TET2, TP53, and XPO1) was performed (Illumina MiSeq, Illumina, San Diego, CA, U.S.A.). The EBV+ B-cell LPD showed mutations in only one gene (TP53). On the other hand, the EBV– plasma cell lesion showed mutations in five different genes (TP53, SF3B1, STAT5B, CD79B and CRKL) ().
Figure 4. Polymerase chain reaction for IGH, IGK and IGL genes. The first and second nasopharyngeal biopsies showed identical amplification peaks.
Table 1. Next generation sequencing of sequential nasopharyngeal biopsies of a case of immunodeficiency-associated B-cell lymphoproliferative disease (B-LPD).
Discussion and conclusion
IA-LPD can be both EBV+ and EBV– [Citation2], their pathogenesis conventionally regarded to be due to impaired immunosurveillance. In EBV+ IA-LPD, EBV is considered a key driver, with oncogenesis mediated through the anti-apoptosis and transforming properties of EBV oncoproteins [Citation3]. Accordingly, EBV infection of lymphoid cells should precede cellular transformation. For EBV– IA-LPD, pathogenetic mechanisms are more diverse, including genotoxicity of previous immunosuppression/chemotherapy; opportunistic infection by other oncogenic viruses; or a hypothetical ‘hit-and-run’ process, whereby an initial EBV infection resulting in transformation is followed by loss of the virus [Citation2].
In our case, however, pathogenesis appears to be more complicated. The EBV+ B-cell LPD and the EBV– plasma cell lesion were derived from the same mutated B-cell clone, as evidenced by identical rearrangements of IG genes. However, they exhibited different mutational profiles. The EBV+ B-LPD showed a profile typical of EBV+ IA-LPD. In a previous NGS study of a 68-gene panel, for EBV+ posttransplant LPDs, the median number of genes mutated was only one, with TP53 most commonly mutated (36% of cases) [Citation4]. These findings implied that EBV oncoproteins including LMP1 might directly activate NF-κB, hence forgoing other genetic mutations, in the process of B-cell transformation. Such genetic features were recapitulated in the first nasopharyngeal biopsy (EBV+ B-cell LPD). On the other hand, the EBV– plasma cell lesion showed five gene mutations, with SF3B1, STAT5B and CD79B connected with NF-κB activation. These features showed that in the second nasopharyngeal biopsy (EBV– plasma cell lesion), genetic mutations independent of EBV infection might be responsible for the malignant phenotype.
The presence on presentation of three paraproteins, two with lambda light chain and one with kappa light chain, was also of interest. Intriguingly, the nasopharyngeal and marrow lesions all showed lambda-light chain restriction, which corresponded to the circulating IgGλ and IgAλ paraproteins. However, there was another IgGκ paraprotein that persisted for a considerable duration after chemotherapy. The origin of this paraprotein remained obscure, but could conceivably be from a non-biopsied lesion shown on PET/CT. This lesion was likely to be EBV-negative, as plasma EBV DNA remained undetectable during persistence of the IgGκ paraprotein. Biclonal paraproteinemia in plasma cell malignancies is uncommon, reported to occur only in 0.2% of 1027 cases in one series [Citation5]. The presence of multiple paraproteins with different light chains further underlined the clonal heterogeneity present in our case.
These observations suggested in our case, a common transformed B-cell gave rise to two subclones. EBV infected only one subclone and might not, therefore, be the initiating oncogenic event. This heterogeneity was not merely a histopathologic curiosity, but was clinically relevant. The initial immunochemotherapy eradicated the EBV+ subclone. However, the EBV– subclone did not fully respond. As it showed a predominant plasma cell differentiation, we surmised that a change in treatment strategy was needed. Lenalidomide exerts pleiotropic effects on B-cell and T-cells [Citation6]. Anecdotal data showed that lenalidomide might be effective in EBV+ B-cell and T-cell lymphomas [Citation7–9]. In EBV+ B-cell lymphomas responding to lenalidomide, plasmacytic derivation appeared to be a common denominator [Citation9]. The switch to lenalidomide successfully induced a remission of the EBV– subclone in our patient.
Treatment of EBV-related IA-LPD varies from conservative (with reduction of immunosuppression) as in EBV-positive mucocutaneous lesions [Citation10, Citation11] to combination chemotherapy in aggressive lymphomas. As shown in this case, detailed analysis of clonal structure of IA-LPD is of biologic and pathogenetic interest, with potential implications on treatment.
Informed consent
The patient gave informed consent to treatment.
Author contributions
Y.Y. Hwang: treated the patient, wrote and approved the manuscript; R. Au-Yeung: performed the histopathologic studies, wrote and approved the manuscript; R. Y.Y. Leung: performed the histopathologic studies, wrote and approved the manuscript; E. Tse: performed the laboratory tests, wrote and approved the manuscript; Y.L. Kwong: treated the patient, wrote and approved the manuscript.
Acknowledgement
The authors have no acknowledgements to make
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Natkunam Y, Goodlad JR, Chadburn A, et al. EBV-Positive B-Cell proliferations of varied malignant potential: 2015 SH/EAHP workshop report-part 1. Am J Clin Pathol. 2017;147(2):129–152.
- Natkunam Y, Gratzinger D, Chadburn A, et al. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood. 2018;132(18):1871–1878.
- Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17(11):691–700.
- Menter T, Juskevicius D, Alikian M, et al. Mutational landscape of B-cell post-transplant lymphoproliferative disorders. Br J Haematol. 2017;178(1):48–56.
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33.
- Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell Non-Hodgkin lymphoma. J Clin Oncol. 2015;33(25):2803–2811.
- Kwong YL. Radiologic and molecular remission of follicular T cell lymphoma treated with lenalidomide. Ann Hematol. 2017;96(3):513–515.
- Chan TSY, Mak V, Kwong YL. Complete radiologic and molecular response of HIV-negative primary effusion lymphoma with short-course lenalidomide. Ann Hematol. 2017;96(7):1211–1213.
- Hwang YY, Au-Yeung R, Lau HP, et al. Complete response of age-related Epstein-Barr virus-associated polymorphic nodal lymphoproliferative disease of plasmacytic type to low-dose lenalidomide. Ann Hematol. 2018;97(2):363–366.
- Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405–417.
- Zanelli M, Sanguedolce F, Palicelli A, et al. EBV-Driven Lymphoproliferative disorders and lymphomas of the gastrointestinal tract: a spectrum of entities with a common denominator (part 1). Cancers (Basel). 2021;13(18):4578.
