ABSTRACT
Background
Peripheral morphological abnormalities play important roles in the early diagnosis and prognosis of the COVID-19 infection. The aim of the present study was to assess the morphological alterations in the peripheral blood (PB) cells in patients with COVID-19 infection, with special attention to a different group of atypical lymphocytes that had been observed in the PB of COVID-19 cancer and non-cancer patients.
Methods
The PB cells were examined in 84 COVID-19 positive cancer patients, and 20 COVID-19 positive non-cancer patients, compared to 30 healthy normal controls. The data were correlated to the disease severity, patients’ clinicopathological features, and outcomes.
Results
There was an increased incidence of giant platelets, neutrophils shifting left, and abnormal monocytes in the COVID-19 positive cancer and non-cancer patients compared to the control group (P < .001, P < .001 and P = .014; respectively). Neutrophils with abnormal toxic granulations, Pseudo Pelger-Heut abnormality, and reactive lymphocytes were significantly increased in COVID-19 cancer patients compared to COVID-19 non-cancer patients and the control group (P = .001, P < .001, and P < .001; respectively). An abnormal form of lymphocytes’ morphological changes (Covicytes) was significantly detected in COVID-19 cancer patients [60.7% (51/84)], and in COVID-19 non-cancer patients [55% (11/20)], while it was absent in the normal controls [0.0% (0/30), P < 0.001]. The presence of the Covicytes is associated significantly with a better prognosis in cancer and non-cancer COVID-19 patients.
Conclusion
Covicytes could be a useful marker supporting the diagnosis of SARS-COV-2 infection, and it is associated with a favorable prognosis.
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) or COVID-19 viral infection is a worldwide pandemic disease [Citation1]. It had been estimated that the total number of confirmed COVID-19 cases all over the world was 261,623,251 till 23rd November 2021, with 5,202,181 global deaths [Citation2]. Moreover, the COVID-19disease can be presented with different degrees of severity, ranging from mild symptoms (Influenza-like) to a severe infection, which can lead to life-threatening complications including acute respiratory distress (ARDS) and multiple organ failure [Citation3].
Many risk factors had been reported to increase the incidence of COVID-19 poor sequelae including old age, comorbidities like diabetes mellitus (DM), cardiovascular disease, respiratory system dysfunction, and cancer [Citation4–6]. Indeed, cancer patients are at a higher risk of a complicated course than non-cancer patients, which would result in higher mortality [Citation7–9]. This is because cancer patients suffered from immunodeficiency, which is due to the effect of the present malignancy itself, the administrated chemotherapy, radiotherapy, and/or the cytotoxic treatment, in addition to the frequent contact with the healthcare workers. All these factors contribute to the associated COVID-19 higher mortality and the poor clinical outcomes [Citation10,Citation11].
Clinical Laboratory medicine plays a pivotal role in the early detection, diagnosis, and management of COVID-19 disease [Citation12]. Many studies have addressed the clinical and laboratory effects associated with COVID-19 infection, with special attention paid to the peripheral blood cells and the morphological alterations that occurred during the course of infection [Citation13,Citation14]. The most common hematological changes that occur during the COVID-19 infection are lymphopenia, neutrophilia, and thrombocytopenia. In addition to the associated morphological abnormalities of the blood cells including large granular lymphocytes, atypical lymphocytes with abundant basophilic cytoplasm, and lymphoplasmacytic cells [Citation15–17]. The presence of the reactive and a typical lymphoid cell suggests an abundant production of virus-specific T cells, thus explaining the better outcome for patients [Citation18].
While the morphological abnormalities detected in the neutrophils in COVID-19 patients were toxic granulation, cytoplasmic vacuolization, aberrant nuclear projections, and/or abnormal nuclear shapes such as pi-shaped nuclei, fetus-shaped nuclei, C and donut-shaped nuclei. The pseudo-Pelger-Huet abnormality and smudged neutrophils were also seen [Citation14,Citation15,Citation19]. Regarding the monocytes, it showed large coalescing cytoplasmic vacuoles, clumped chromatin, and granulations [Citation15,Citation19,Citation20]. These changes were attributed to the infection of the virus to monocytes through the ACE2 receptors, and the response of monocytes to the virus by vacuolization and increased granulation is seen morphologically, in addition to the increased expression of CD80. The CD206 and secretion of IL6, IL10, and TNFα were detected as a part of the hyper inflammation state [Citation13]. While platelets appeared giant and clumping [Citation15,Citation20].
Some studies have shown the impact of COVID-19 infection in cancer patients regarding the clinical and the laboratory parameters [Citation21,Citation22]. Yet, up to our knowledge, rare reports were published about the morphological abnormalities in the peripheral blood cells in cancer patients with COVID-19 infection. In the current study, we are discussing the morphological alterations in the peripheral blood cells with attention to a certain morphological abnormality in the lymphocytes observed in our cohort of cancer and non-cancer patients with COVID-19 infection. Therefore, we thought to assess this type of lymphocytes’ morphological abnormality (Covicytes) in COVID-19 positive patients with and without cancer in comparison to a normal healthy control group. The observed Covicytes were correlated to the patients’ clinicopathological features, COVID-19 severity, as well as patients’ outcomes in the form of intensive care unit (ICU) admission and mortality. This will add another diagnostic/prognostic marker for COVID-19 infection, that will help in better patients’ risk stratifications, and accordingly better management especially in cancer patients who are at a higher risk for COVID-19 complications.
Patients and methods
This is a retrospective cohort study conducted on patients with COVID-19 viral infection during the period between June 2020 and April 2021. The recruited cohort consisted of three groups: group 1 (G1) which was formed of 84 COVID-19 positive cancer patients who were presented to the National Cancer Institute (NCI), Cairo University. The patients were histo-pathologically and radiologically confirmed with cancer. Group 2 (G2) was formed of 20 COVID-19 positive non-cancer patients, who were the health care providers or their family members who proved to be positive for COVID19 infection. While group 3 (G3) was formed of 30 healthy age and sex-matched healthy volunteers who were presented to the hospital for blood donation and proved to be negative for COVID-19 by Rt–PCR. The study was conducted at the NCI, Cairo University, where all patients were confirmed with the diagnosis of COVID-19 through the clinical examination, radiological assessment (chest X-ray and chest CT), and laboratory assessments including positive viral RNA expression by RT–PCR, serum ferritin, D-dimer and C-reactive protein (CRP). All participating patients and control subjects did not receive any type of COVID-19 vaccine. The patients were assessed for the severity of SARS-COV-2 infection according to the world health organization (WHO) classification into patients with mild infection who had mild symptoms and normal imaging findings. Moderate COVID-19 infection in patients who had a fever and lung affection by chest X-ray and CT. While severe infection in patients who showed severe respiratory symptoms, respiratory rate >30/min and O2 saturation was <93% in the rest state [Citation23]. While pediatric cancer patients were categorized into asymptomatic infection in patients who had no symptoms of COVID-19 at any time point, mild infection in children who had mild symptoms with no hospitalization, moderate severity in patients who required hospitalization without ICU care, while severe infection in children who required ICU care for SARS-COV2 symptoms [Citation24].
Peripheral blood (PB) samples were obtained from all participating subjects at admission during the routine workup of the patients. The blood samples were collected in Ethylenediaminetetraacetic acid (EDTA) vacutainers and prepared for undergoing complete blood count (CBC) analysis. Also, differential total leukocyte count (TLC) was done using SYSMEX XN1000 and SYSMEX XT 1800 analyzers, which included an absolute count of lymphocytes, monocytes, and neutrophils, eosinophils, basophils, and immature granulocytes. The immature granulocytes represented an automated count of promyelocytes, myelocytes, and metamyelocytes in the peripheral blood. Peripheral blood (PB) smears were done by spreading one drop of the blood on a slide and stained with Leishman stain. The WBC morphology was analyzed as changes from normal expected/baseline morphology. The blood films were examined by two experienced hematopathologists using Lecia light microscope with 100× oil-immersed magnifications.
Other routine laboratory tests done for the patients and the control subjects were the assessment of the liver function tests, kidney function tests, markers of inflammation like C-reactive protein (CRP), lactate dehydrogenase (LDH), serum Ferritin, D Dimer, and finally coagulation profile.
Management of the patients
Most of the cancer patients 65 (77.4%) were receiving chemotherapy according to the assigned protocol, 36 (42.9%) patients were on induction chemotherapy, 11 (13.1%) were on maintenance therapy, and 8 (9.5%) patients were on consolidation chemotherapy. Also, there were 28 (33.3%) post-surgery, and only one was receiving radiotherapy, with 40 (47.6%) patients receiving intensified chemotherapy according to their risk stratification.
All patients were treated for COVID-19 infection according to the guidelines of the WHO [Citation25]. Remdesivir was administrated in some selected severe cases, and in other cases started Oseltamivir if Remdesvir was not available. Steroids were administrated for all severe, critical cases and those who were admitted to the ICU with proper tapering or dose escalation according to the clinical status and response. Some cases with Moderate COVID-19 benefited from Steroids for five to seven days. Moreover, patients were monitored for associated complications, such as acute liver injury, acute kidney injury, ARDS, disseminated intravascular coagulation (DIC), and/or shock.
Statistical analysis
Data were reported from the COVID-19 and cancer registry medical records at the NCI, Cairo University. Data management and analysis were performed using the statistical software package SPSS, version 22 (IBM, Armonk, NY, U.S.A.). Quantitative data were presented as median and interquartile ranges (IQR) or mean and standard deviation (SD) according to the performed normality test. The qualitative data were presented as frequency and percentages. The comparison between groups was performed using the Chi-square test and/or Fisher exact test which is appropriate. Mann–Whitney test and the independent T-test was used for comparing numerical variables between the patients’ groups. Kruskal Wallis test was used to compare the clinicopathological parameters among the different patient groups. All tests of hypotheses were performed at the alpha level of 0.05, with a 95% confidence interval.
Results
The median age of the assessed patients’ groups was 38 (range: 1–71years) in G1, 42 (range: 26–61 years) in G2, and 46 (range: 37–57 years) in G3. The cancer patients included in G1 showed 53.7% (45/84) patients with solid tumors and 46.4% (39/84) with hematological malignancies. There were 24 (28.6%) patients in remission disease, while 60 (71.4%) patients had progressive cancer disease. Twenty-six (31%) cancer patients were admitted to the ICU, 18 (21.4%) of them needed mechanical ventilation, and finally, 15 (17.9%) patients died out of concurrent COVID-19 infection ().
Table 1. Clinical features of the cancer patients with COVID-19.
Assessment of the clinical and laboratory markers among the patients’ groups
Overview of the CBC parameters of the three groups revealed that COVID-19 patients (both G1 and G2) had a significantly decreased Hb concentration level, increased red cell distribution width (RDW), and increased TLC compared to the control group; G3 (P < .001, P < .001 and .005; respectively). Increased neutrophil count and relative neutrophilia were significantly observed in G2 in comparison to G1 and G3 (P = .001 and P < .001; respectively). Absolute lymphopenia was markedly detected in COVID-19 patients (G1 and G2) in comparison to G3 (P = .001), while relative lymphopenia was significantly found in G2 (10.6, IQR: 1.7–35) compared to G1 and G3 [22.9 (IQR: 3–93), and 38.7 (IQR: 26–57); respectively, P < .001]. Similarly, the absolute and relative monocyte counts were significantly decreased in G2 compared to G1 and G3 (P = .026 and P < .001; respectively). Also, eosinophils and basophils counts were significantly decreased in COVID-19 patients (G1 and G2) in comparison to the normal subjects (P = .001 and P < .001; respectively, ).
Table 2. Assessment of the clinical and laboratory markers among the three patients’ groups.
Regarding the biochemical analysis of the serum markers of the examined groups, serum ferritin, LDH and CRP were significantly increased in COVID-19 patients (G1 and G2) compared to the control group (P < .001 for both). The alanine transaminase (ALT) was significantly increased in COVID-19 cancer patients (G1) compared to the other group (G2 and G3, P = .021, ).
Morphological changes in the blood cells among the patients groups
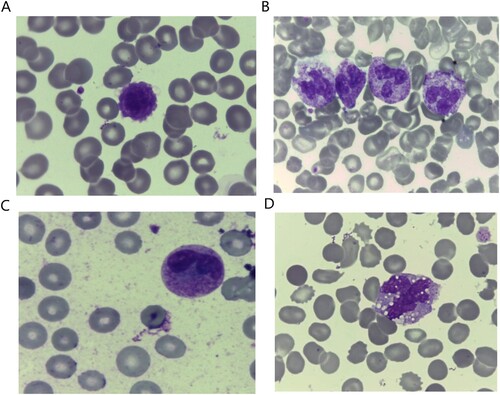
In the context of the morphological examination of the blood cells, there was an increased incidence of giant platelet forms in COVID-19 positive cancer (G1) and non-cancer (G2) patients in comparison to the control group [57.1% (48/85), 55.0% (11/20), and 13.3% (4/20); respectively, P < .001, (A)]. Neutrophils with toxic granulations were predominantly present in COVID-19 cancer patients [G1, 32.1% (27/84)] compared to the other groups [G2: 0.0% [0/20] and G3: 6.7% (2/30), P = .001, (B)]. Similarly, Pseudo Pelger-Heut abnormality was significantly detected in G1 [61.9% (52/84)], while it was present in 20% (4/20)in G2, and it was absent in G3 [0% (0/30), P < .001]. It was defined as abnormal granulocytes with bilobed or dumbbell shaped nucleus and coarse chromatin, ((C)). Neutrophils left shift were significantly present in COVID-19 positive patients [G1: 21.4% (18/84) and G2: 60% (18/20)], and it was absent in G3 (0/30 patients, P < 0.001). Abnormalities in Monocytes morphology in the form of vacuolations and aggressiveness are predominantly observed in COVID-19 positive patients [G1: 64.3% (54/84)] and G2: 55% (11/20), compared to G3: 33.3% (10/30), (P = .014, (D)).
Figure 1. Morphological characteristics of the peripheral blood cells in COVID-19 cancer patients showing: (A) Giant platelets. (B) Neutrophils with toxic granulations, bright mode. (C) Neutrophils with Pelger-Huet abnormality, bright mode. (D) Large sided aggressive looking monocytes with cytoplasmic vacuolations, 100× magnification.
While for the examined lymphocytes, a certain type of abnormal lymphocytes’ morphological changes was significantly detected in cancer patients withCOVID-19 infection [G1: 60.7% (51/84)], and in non-cancer patients with COVID-19 [G2: 55% (11/20)], while it was not detected in normal control subjects [G3: 0.0% (0/30), P < .001, (A)]. These morphological changes included lymphocytes with clumpy chromatin, basophilic abundant granular cytoplasm, and a characteristic cytoplasmic tail (Covicytes, (B–E)). Other lymphocytes with morphological reactive changes detected were plasmacytoid lymphocytes which was a reactive mature looking lymphocyte with dark basophilic cytoplasm and a slightly eccentric nucleus. It was defined as an intermediate stage between plasma cells and lymphocytes. The plasmacytoid lymphocytes were abundantly encountered in G1 [73.8% (62/84)], compared to G2 [35% (7/20)], and G3 [13.3% (4/30), P < .001, (A)]. Similarly, monocytoid and ballerina morphological changes of lymphocytes were significantly present in COVID-19 cancer patients [G1: 54.8% (46/84)], compared to the other groups [G2: 0% (0/20), and G3: 6.7% (2/30), P < .001]. The monocytoid and ballerina looking lymphocytes were reactive lymphocytes that characteristically scalloped the neighboring cells ((B)). The other morphological and laboratory changes among the assessed patients’ groups were illustrated in .
Figure 2. (A) Assessment of the Covicytes in patients’ groups. (B–E) Atypical lymphocytes with clumpy chromatin and basophilic cytoplasm with large granular tail (Covicytes), 100× magnification.
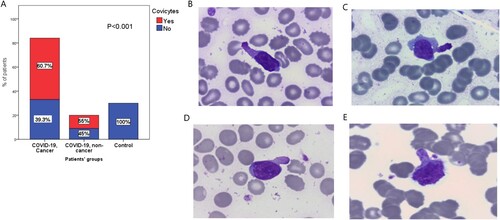
Figure 3. (A) The plasmacytoid lymphocyte, atypical lymphocyte with dark blue cytoplasm and slightly eccentric nucleus. (B) The monocytoid and ballerina looking lymphocytes were reactive lymphocytes that characteristically scalloping the neighboring cells, 100× magnification. (C) and (D) NK/T-cell lymphoma in leukaemic phase described by Leach M and Bain BJ, cited from the book ‘From the Image to the Diagnosis’, 1st edition. Page 58 [Citation25].
![Figure 3. (A) The plasmacytoid lymphocyte, atypical lymphocyte with dark blue cytoplasm and slightly eccentric nucleus. (B) The monocytoid and ballerina looking lymphocytes were reactive lymphocytes that characteristically scalloping the neighboring cells, 100× magnification. (C) and (D) NK/T-cell lymphoma in leukaemic phase described by Leach M and Bain BJ, cited from the book ‘From the Image to the Diagnosis’, 1st edition. Page 58 [Citation25].](/cms/asset/c4ee8707-c33f-444e-8d23-900f4c3b349d/yhem_a_2089830_f0003_oc.jpg)
Association between the presence of Covicytes and the clinical features of COVID-19 positive cancer patients
The Covicytes were significantly observed in patients with increased platelets and increased eosinophil counts (P < .001 and P = .004; respectively). Cancer patients who showed Covicytes had mild normocytic anemia (Hb concentration was 10.8 ± 2.3 gm/dL) compared to the cancer patients without Covicytes who had moderate anemia (Hb concentration was 8.6 ± 2 gm/dL, P < .001). Similarly, cancer patients with Covicytes showed increased lymphocyte count in contrast to those without Covicytes [1.7 (0.1–4.4), and 1.3 (0.3–3.3); respectively, P = .023]. In addition, the presence of Covicytes in COVID-19 cancer patients was associated significantly with decreased immature granulocytes, LDH, CRP, ALT, and urea (P = .019, .035, .049, .28, and .046; respectively). The incidence of bloodstream infections was decreased in patients positive for Covicytes [5.9% (3/51)], in relation to the cancer patients negative for Covicytes who had an increased incidence of bloodstream infection [21.2% (7/33), P = .044].
Abnormalities in platelet morphology were observed significantly in 70.6% (36/51) of patients who showed Covicytes, compared to 36.4% (12/33) who didn’t show these cells (P = .003). Similarly, abnormalities in neutrophils morphology in the form of Pseudo Pelger-Heut appearance were associated significantly with the presence of Covicytes, in comparison to those who didn’t have Covicytes [76.5% (39/51) and 39.4% (13/33); respectively, P = 0.001]. In addition, the presence of Covicytes in COVID-19 cancer patients was associated significantly with the increased mature lymphocytes and plasmoid cell counts (P = .001 and P < .001; respectively). Moreover, monocytoid and ballerina lymphocytes were associated significantly with the presence of Covicytes in relation to the cancer patients who didn’t have Covicytes [70.6% (36/51), and 30.3% (10/33); respectively, P < .001]. Also, abnormalities in monocytes morphology in the form of vacculated and aggressive monocytes were significantly detected in COVID-19cancer patients positive for Covicytes compared to those negative for Covicytes [76.5% (39/51) and 45.5% (15/33); respectively, P = .005, ].
Table 3. Association between the Covicytes and the clinical and laboratory features of the cancer COVID-19 patients.
Association between the presence of Covicytes and the clinical features of the non-cancer COVID-19 positive patients
The presence of Covicytes in COVID-19 patients was associated with mild anemia with normal RDW, in comparison to the COVID-19 patients negative for Covicytes who had moderate anemia with increased RDW (P < .001). There was a significant increase in the platelet count in COVID-19 patients with Covicytes in relation to COVID-19 patients without Covicytes (P = .02). In addition, patients with Covicytes showed a significant decrease in the relative neutrophil count and a significant increase in the absolute and relative lymphocyte count, compared to the other patients without Covicytes (P = .05, P < .001 and P = .003; respectively), though both groups showed neutrophilia as well as lymphopenia. Also, there was a significant decrease in the PTT, serum ferritin, and ALT in patients with Covicytes, compared to those without Covicytes (P = .001, P = .01, and P = .016; respectively).
Abnormalities in the cellular morphology were observed significantly in COVID-19 patients with Covicytes. These cellular abnormalities included Giant platelets which were present in 81.8% (9/11) of patients with Covicyte, compared to 22.2% (2/9) in those without Covicytes (P = .022). Also, plasmacytoid lymphocytes and vacuolated, aggressive monocytes were present significantly in COVID-19 patients with Covicytes [63.6% (7/11), and 81.8% (9/11); respectively], compared to COVID-19 patients without Covicytes who did not show plasmacytoid cells, and had only abnormal monocytes in 22.2% (2/9) of the patients. On the other hand, all patients who had Covicytes did not show Pseudo Pelger-Heut cells in their peripheral blood (P = .026, ).
Table 4. Association between Covicytes and clinical features of the non-cancer COVID-19 patients.
Discussion
The current study demonstrated the different morphological changes in the PB cells in both cancer and non-cancer COVID-19 patients in relation to the control subjects. Indeed, there were many morphological abnormalities associated with COVID-19 infection had been reported in the literature. However, in the present study, we mainly throw light on a different group of atypical lymphocytes that was uncommon to our knowledge. These cells are characterized by abundant granular cytoplasm and a characteristic cytoplasmic tail ending with granules. Our data revealed that this type of cell was found significantly in 60.7% of cancer patients with COVID-19, and in 55% of non-cancer patients with COVID-19 infection, however, it was not detected in the control healthy group. Therefore, we named it as a ‘Covicytes’, because its presence in the blood film supports COVID-19 infection, but its absence could not exclude infection. Though there were many forms of reactive lymphocytes associated with SARS-COV-2 infection, however, Covicyte was the most prominent form of reactive lymphocytes detected in our cohort of COVID-19 patients. Actually, these cells could be similar to that described by Leach and Bain in their recently published book (2022). They found a population of large pleomorphic lymphoid cells with an ovoidnucleus and basophilic vacuolated cytoplasm with prominent projections (as shown in (C,D)). Astonishingly, it was described as a rare type of cancer, as they attributed these cells as a leukemic phase of extranodal natural killer (NK) T-cells lymphoma [Citation26]. Moreover, another morphological abnormality of lymphocytes was described by Pezeshki et al. [Citation17], who found an increased number of large granular lymphocytes (LGL) with large course azurophilic cytoplasmic granules in the peripheral blood of SARS-COV-2 patients. They proposed that Cytotoxic T lymphocytes (CTLs) and NK cells can morphologically be similar to these LGLs. As CTLs and NK cells have an important role in the cytotoxic response against viral infection like that seen in Epstein-bar virus and cytomegalovirus infection [Citation27]. In addition, we detected these Covicytes in the bone marrow of COVID-19 patients, but its incidence was much lower than that in the PB, so the PB film could be sufficient for confirming COVID-19 diagnosis and prognosis using these Covicytes. However, we wouldn’t be able to perform further characterization due to its very low number in the blood film.
Upon our extensive study on the Covicytes, we tried to assess the prognostic and the predictive role of these cells in COVID-19 cancer and non-cancer patients. The present results showed that COVID-19 cancer patients positive for Covicytes had a better prognosis in the form of a mild degree of normocytic anemia, increased lymphocytes, platelets, and eosinophils counts compared to cancer patients without Covicytes. Also, patients with Covicytes showed a significant decrease in the LDH, CRP, ALT, and urea, as well as a decreased incidence of bloodstream infections. Accordingly, the presence of Covicytes may reflect the host cellular immunity and inflammation status, and it could be considered as a part of the secondary body immune response to viral infection, owing to the lower levels of CRP, LDH, and decreased lymphopenia encountered in those patients. All these could possibly confer a better prognostic role of these cells in COVID-19 cancer and non-cancer patients. Adding to that, All the participated patients and control subjects were not vaccinated against COVID-19 infection. Therefore, we can assume that this would be useful to diminish the interlacing factors that could affect the inflammatory response of the body, which would result in the appearance of different aberrant morphological cells including the Covicytes.
In the context of the association of Covicytes with the other blood cells’ morphological abnormality, the present data showed that Covicytes associated significantly with the presence of giant platelets, Pseudo Pelger-Heut cells, vacuolated, and aggressive monocytes. Additionally, increased mature lymphocyte, plasmacytoid cells, as well as monocytoid and ballerina lymphocytes were significantly associated with the presence of Covicytes in COVID-19 cancer patients compared to those who didn’t have Covicytes. In addition to the previously mentioned results, the presence of Covicytes in COVID-19 non-cancer patients was associated significantly with the decreased serum ferritin, PTT, as well as a decreased degree of neutrophilia. It had been proposed by Pozdnyakova et al. [Citation19], that the abnormal monocytes and lymphocyte morphology found in the COVID-19 patients was significantly associated with a mild disease course. while these morphological changes were lost with the disease progression. Therefore, Covicytes could be considered a good prognostic factor for COVID-19 severity in both cancer and non-cancer patients.
Regarding the other lymphocytes’ morphological changes detected in the assessed patients’ groups, the current study demonstrated that Plasmacytoid lymphocytes were the most common form of reactive lymphocytes found in COVID-19 patients, with a higher percentage in those suffering from cancer rather than in those COVID-19 patients without cancer. These data are in agreement with many recent studies from COVID-19 pandemic countries which reported the prevalence of reactive lymphocytes in SARS-COV-2 patients [Citation20,Citation28–31], especially the plasmacytoid lymphocytes which is unusually been detected in the virally infected patients. Hence these abnormal lymphocytes might be considered specific for COVID-19 infection [Citation14,Citation15,Citation28,Citation32]. Moreover, Merino et al, demonstrated that COVID-19 patients who showed atypical or reactive lymphocytes in their blood had a better prognosis and a more favorable outcome rather than those who did not have these cells. They explained this finding as a more effective immune response against SARS-COV-2, especially since the immunophenotyping of these cells revealed a subset of effector memory CD4 and CD8 cells [Citation16]. Additionally, monocytoid and ballerina lymphocytes were significantly present in COVID-19 cancer patients (54.8%), while they were absent in COVID-19 non-cancer patients, and they were found in only 6.7% of the control group. These data are consistent with El Jamal et al. [Citation33], who reported that the classic Downey II-like cells, are less frequently detected in COVID-19 patients, although it is more commonly associated with viral infections [Citation33]. These data indicate the unique effect of the COVID-19 virus on the immunological and morphological features of the patients’ blood cells, especially in those who were suffering from accompanying malignant diseases.
Moreover, there were other morphological abnormalities detected in the blood cells including neutrophils with toxic granulations, Pseudo Pelger-Heut deformity, neutrophils shifting left, monocytes with vacuolations, and aggressiveness. These data are consistent with many recent studies that reported the presence of those morphological changes in the peripheral blood of SARS-COV-2 patients [Citation13–15,Citation20,Citation33–35]. However, our study revealed that neutrophils with abnormal toxic granulations and Pseudo Pelger-Heut abnormality were significantly found in the COVID-19 cancer patients, compared to the COVID-19 non-cancer patients and the control group. Therefore, cancer could aggravate the presence of these cell abnormalities when accompanied by the SARS-COV-2 infection.
There was also an increased number of giant platelets in cancer and non-cancer COVID-19 patients in comparison to the control group. These data are consistent with the other previous studies that reported an increased incidence of giant platelets in COVID-19 patients [Citation15,Citation17,Citation35–38]. This finding could be explained by either the effect of the virus itself or due to lung affection by the virus, which is considered as another place of residence of megakaryocytes [Citation39].
The current data demonstrated that there were neutrophilia, lymphopenia, decreased basophils and eosinophils count in COVID-19 patients compared to the control group. These data are in agreement with many studies that reported increased incidence of lymphopenia and absolute neutrophilia in COVID-19 patients [Citation29,Citation40–44]. This lymphopenia could be due to the lysis of the lymphocytes by SARS-CoV-2 virus since lymphocytes have ACE2 receptors on their surface [Citation45]. Moreover, Anemia, elevated CRP, LDH and serum ferritin levels were also significantly observed in assessed cancer and non-cancer COVID-19 patients [Citation22,Citation46–49].
Though there were many reports that illustrated the morphological features in the blood cells that are associated with COVID-19 infection, only, a few of them described these morphological changes in cancer patients with COVID-19 infection. Our data demonstrated that cancer patients with COVID-19 showed morphological changes in the blood cells that differed from what occurred in COVID-19 non-cancer patients. As cancer patients had deficient immune systems due to the administrated chemotherapy and radiotherapy, in addition to the effect of cancer itself on the blood cells [Citation11,Citation22].
Furthermore, the current study introduced a different type of atypical lymphocytes (Covicytes) that were detected significantly in the peripheral blood of COVID-19 cancer and non-cancer patients. Therefore, these cells could be used as a useful marker to support the diagnosis of SARS-COV-2 infection. Also, combining the presence of the Covicytes with the other laboratory parameters including CRP, LDH absolute lymphocytes count, and serum ferritin could bring out a prognostic algorithm that confers a good prognosis in COVID-19 infection in cancer patients. This algorithm could be adopted for COVID-19 non-cancer patients also.
The limitation of this study was the small number of the included patients. Also, the size of the groups was not balanced, as data were obtained from a single institute. However, we introduce these data as a preliminary observation, and further studies are required on a larger number of patients to validate these results, with more characterization of these detected Covicytes.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of the National Cancer Insitiute, according to the 2011 declaration of Helsinki (No. 201920008.2). Signed informed consent was obtained from all participants in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All required data and materials are available upon request, except for the personal data of the patients.
Additional information
Funding
References
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401–402.
- John Hopkins University. Coronavirus resource center; 2021. Available from: https://coronavirus.jhu.edu/
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Bloomgarden ZT. Diabetes and COVID-19. J Diabetes. 2020;12(4):347–348.
- Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513.
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547.
- Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089.
- He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645.
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926.
- Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941.
- Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131–1134.
- Zhang D, Guo R, Lei L, et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109(1):13–22.
- Nazarullah A, Liang C, Villarreal A, et al. Peripheral blood examination findings in SARS-CoV-2 infection. Am J Clin Pathol. 2020;154(3):319–329.
- Kaur G, Sandeep F, Olayinka O, et al. Morphologic changes in circulating blood cells of COVID-19 patients. Cureus. 2021;13(2):e13416.
- Merino A, Vlagea A, Molina A, et al. Atypical lymphoid cells circulating in blood in COVID-19 infection: morphology, immunophenotype and prognosis value. J Clin Pathol. 2020;75(2):104–111.
- Pezeshki A, Vaezi A, Nematollahi P. Blood cell morphology and COVID-19 clinical course, severity, and outcome. J Hematop. 2021;14(3):221–228.
- Merino A, Vlagea A, Molina A, et al. J Clin Pathol. 2022;75:104–111.
- Pozdnyakova O, Connell NT, Battinelli EM, et al. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID-19 infection. Am J Clin Pathol. 2021;155(3):364–375.
- Bahadur S, Kalonia T, Kamini K, et al. Changes in peripheral blood in SARS CoV-2 patients and its clinico-pathological correlation: a prospective cross-sectional study. Int J Lab Hematol. 2021;43(6):1334–1340.
- Rüthrich MM, Giessen-Jung C, Borgmann S, et al. COVID-19 in cancer patients: clinical characteristics and outcome – an analysis of the LEOSS registry. Ann Hematol. 2021;100(2):383–393.
- ElGohary GM, Hashmi S, Styczynski J, et al. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2020;30:S1658-3876(20)30122-9.
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 2020 Mar 13 (No. WHO/2019-nCoV/clinical/2020.4). World Health Organization; 2020.
- Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr Blood Cancer. 2021;68(3):e28843.
- World Health Organization. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected: interim guidance (No. WHO/MERS/Clinical/15.1 Revision 1). World Health Organization; 2019.
- Leach M, Bain BJ. Haematology: from the image to the diagnosis. NK/T-cell lymphoma in leukaemic phase. 1st ed. Hoboken, NJ: John Wiley & Sons; 2022.
- Zambello R, Semenzato G. Large granular lymphocyte disorders: new etiopathogenetic clues as a rationale for innovative therapeutic approaches. Haematologica. 2009;94(10):1341–1345.
- Foldes D, Hinton R, Arami S, et al. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19). Am J Hematol. 2020;95(7):861–862. DOI:10.1002/ajh.25834.
- Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769.
- Gérard D, Henry S, Thomas B. SARS-CoV-2: a new aetiology for atypical lymphocytes. Br J Haematol. 2020;189(5):845.
- Weinberg SE, Behdad A, Ji P. Atypical lymphocytes in peripheral blood of patients with COVID-19. Br J Haematol. 2020;190(1):36–39.
- Chong VCL, Lim KGE, Fan BE, et al. Reactive lymphocytes in patients with Covid-19. Br J Haematol. 2020. DOI:10.1111/bjh.16690.
- El Jamal SM, Salib C, Stock A, et al. Atypical lymphocyte morphology in SARS-CoV-2 infection. Pathol Res Pract. 2020;216(9):153063.
- Zini G, Bellesi S, Ramundo F, et al. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol. 2020;95(7):870–872.
- Singh A, Sood N, Narang V, et al. Morphology of COVID-19-affected cells in peripheral blood film. BMJ Case Rep. 2020;13(5):e236117.
- Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95(8):999–1000.
- Sadigh S, Massoth LR, Christensen BB, et al. Peripheral blood morphologic findings in patients with COVID-19. Int J Lab Hematol. 2020;42(6):e248–e251.
- Ahnach M, Ousti F, Nejjari S, et al. Peripheral blood smear findings in COVID-19. Turk J Haematol. 2020;37(4):310–302.
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848.
- Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180.
- Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113(7):474–481.
- Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory Coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73(11):e4208–e4213.
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847.
- Joharatnam-Hogan N, Khan K. COVID-19 cancer conundrum – evidence driving decisions or the lack of it? BMC Med. 2020;18(1):182.
- Nooh HA, Abdellateif MS, Refaat L, et al. The role of inflammatory indices in the outcome of COVID-19 cancer patients. Med Oncol. 2022;39(1):6.
- Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901.
- Liu YP, Liu Y, Huang QC, et al. Morphological changes of lymphocytes in peripheral blood smears of patients with COVID-19. Ann Palliat Med. 2020;9(6):4420–4422.
