ABSTRACT
Background/objective
Bone marrow biopsy, the gold standard for the diagnosis of multiple myeloma (MM), has main limitation of the invasiveness. Here, we explored the diagnostic and prognostic values of circulating miR-1246 in patients with MM.
Material and methods
Ninety MM patients and 30 healthy donors (control group) were recruited in this study. The expression of miR-1246 in the peripheral blood samples was detected using qPCR. The receiver operating characteristic (ROC) curve was used to assess the diagnostic value of miR-1246 in MM. The Kaplan–Meier survival analyze was performed to evaluate the prognostic value of miR-1246.
Results
The expression level of serum miR-1246 from newly diagnosed MM patients was significantly higher than that of the control group. Circulating miR-1246 level was decreased after treatment in remission patients, but remained high levels in relapsed patients (P < 0.05). ROC analysis demonstrated that miR-1246 showed a high diagnostic value in MM with an area under the curve (AUC) of 0.952, the sensitivity of 87%, and the specificity of 95% [95% confidence interval (CI) 0.902-1.007; P < 0.001]. Kaplan–Meier analysis showed that the progression-free survival (PFS) (14.0 months vs. 26.5 months, P = 0.045) and overall survival (OS) (20.5 months vs. 55.5 months, P = 0.014) were significantly shorter in patients with high miR-1246 expression as compared with those in patients with miR-1246 low expression. Multiple Cox regression model analysis showed that circulating miR-1246 was an independent prognostic factor for PFS (HR 2.786, 95% CI: 1.420–5.467, P = 0.003) and OS (HR 2.995, 95% CI: 1.166–7.689, P = 0.023) in MM patients.
Conclusion
This study demonstrates that circulating miR-1246 level is elevated in MM patients, which shows high values in the diagnosis and prognosis prediction in patients with MM.
Introduction
Multiple myeloma (MM) is the second most prevalent hematologic malignancy, accounting for 1.3% of all malignancies and 15% of hematologic neoplasms, with an incidence of 4.5-6 cases per 100,000 people annually [Citation1]. It is characterized by the monoclonal proliferation of plasma cells in bone marrow (BM) microenvironment, triggering the overproduction of nonfunctional intact immunoglobulins or immunoglobulin chains [Citation2,Citation3]. This disease is more common in adults over 40 years old, with a median age at diagnosis of 70 years and the 5-year survival of about 46.6% [Citation4–6]. Bone marrow biopsy is the traditional gold standard for the diagnosis of MM, but it may not be accepted by a number of patients mainly because it is invasive method which can cause pain and thereafter bring psychological stress to the patients. Limitations in accessing fresh bone marrow material from MM prevent the diagnosis and delay the optimal treatment period. Thus, it is an urgent need to find a more sensitive, convenient, and noninvasive biomarker for the early clinical diagnosis of MM.
Liquid biopsy is a comprehensive approach as it is noninvasive, simple, more accessible, less painful and contains all the biologically relevant information about the disease. MicroRNA (miRNA) is a type of small and non-coding RNA molecules with about 19–25 bases in length and high stability in blood [Citation7,Citation8]. miRNAs are implicated in regulating of multiple biological processes, such as cell proliferation, survival, apoptosis, stemness, and cell cycle through inducing the degradation and translational repression of target mRNAs via complementary sequences [Citation9–11]. Masri et al. [Citation12] was the first group who revealed the involvement of miRNAs in MM pathogenesis, that the expression of miR-125b, miR-133a, miR-1, miR-124a, miR-15, and miR-16 were decreased in MM patients and cells. Soon afterwards, many miRNAs were verified to be closely involved in the pathogenesis of MM [Citation13,Citation14]. In addition, increasing evidence has demonstrated the potential diagnosis value of circulatory miRNAs in MM [Citation15,Citation16]. For instance, circulating miR-125b-5p level was elevated in MM patients, and the receiver operating characteristic (ROC) analysis demonstrated that miR-125b-5p displayed a high diagnostic accuracy for MM with an area under the curve (AUC) of 0.954 (P < 0.001) [Citation17]. Also, Gupta et al. [Citation17] reported miR-203 level was decreased in the blood samples of MM patients, which showed a high diagnostic value in MM.
miR-1246 was first identified in human embryonic stem cells through a high throughput sequencing technique [Citation18]. Subsequent studies have mapped the human miR-1246-coding gene, i.e. MIR1246 gene on chromosome 2q31.1 and demonstrated p53 effect on the modulation of its expression [Citation19]. Recently, Ren et al. [Citation20] demonstrated that miR-1246 expression was significantly increased in MM patients, which was independent of age, gender, stage, serum levels of β2-microglobulin (β2-MG), albumin, calcium, creatinine, myeloma protein, hemoglobin, as well as the population of bone marrow plasma cells and chromosome 13 deletion, indicating that miR-1246 may be a useful marker in the diagnosis of MM. In the present study, we aimed to determine the diagnostic and prognostic values of circulating miR-1246 in patients with MM.
Materials and methods
Clinical information
A total of 90 serum samples were collected from 90 newly diagnosed MM patients without any treatment in the Department of Hematology, the Fourth Hospital of Hebei Medical University, between July 2013 and October 2020. Inclusion criteria were: [Citation1] patients with a precise diagnosis of primary MM according to the 2015 International Myeloma Working Group guidelines; [Citation2] the age of patients ≥ 18 years; [Citation3] patients with no treatment before blood sample collection; [Citation4] patients signed the informed contents. Exclusion criteria: [Citation1] patients with MM developing into plasma cell leukemia; [Citation2] patients accompanied by other malignancies or organ failures; [Citation3] patients suffered from immune system defects, or other hematologic diseases; [Citation4] patients treated prior to the blood sample collection. Disease remission and relapse were defined according to the International Myeloma Working Group criteria [Citation21]. Another 30 healthy donors with no family history of hematological diseases were included in this study as control. This study got the approval of the Medical Ethics Committee of the Fourth Hospital of Hebei Medical University to use the clinical material for research purposes.
Serum collection and miRNA extraction
Five ml peripheral venous blood samples from 90 MM and 30 healthy controls were placed in EDTA (Ethylene Diamine Tetraacetic Acid) anticoagulation test tubes. RNA was extracted using the miRNeasy serum/plasma separation kit provided by Qiagen (Hilden, Germany) according to the manufacturer’s instructions. Then, the NanoDrop spectrophotometer (ND-2000, Thermo Fisher Scientific, Waltham, MA) was used to assess the concentration and purity of the isolated RNAs. RNA samples were stored at −80°C for further analysis.
microRNA qPCR analysis
The miRcute Plus miRNA first-strand cDNA synthesis kit (Tiangen Co., Beijing, China) was applied to synthesize the cDNA of miR-1246. Then, the PCR was carried out using the using TaqMan miRNA Assay Kits (Applied Biosystems, Foster, CA, USA) to specially quantify mature miR-1246 according to manufacturer’s instructions. The PCR conditions were as follows: pre-denaturation at 95°C for 15 min, and then 45 cycles of 94°C (20 s) and 60°C (34 s) using ABI 9700 real-time PCR system (Applied Biosystems). U6 was used as an internal control. Relative miR-1246 expression levels were determined by the 2−ΔΔ(CT) method [Citation22]. The primers for miR-1246 forward was 5′-AAUGGAUUUUUGGAGCAGG-3′.
Statistical analysis
All statistical analyses were performed using the SPSS 20 software package. The student’s t and one-way ANOVA tests were used to analyze the relationships between clinical characteristics and miR-1246 expression levels. ROC analysis was used to evaluate the diagnostic value of miR-1246 in MM. Patient survival curves were plotted using the Kaplan–Meier method, and comparisons between curves were made using the log-rank test. Multifactorial survival analysis was performed using the Cox proportional risk model. Progression-free survival (PFS) was calculated from the date of diagnosis to disease progression or death, and overall survival (OS) was calculated from the date of diagnosis to death. P < 0.05 or P < 0.01 showed that the differences were statistically significant.
Result
Characteristics of the patients
A total of 90 MM patients and 30 healthy donors (Control group) were included in this study. The control group included 14 males and 16 females, while the MM group included 43 males and 47 females. Forty-eight out of the 90 patients were diagnosed at revised-ISS (R-ISS) stage I/II and 42 cases were at stage III. The clinicopathological features of the included samples were summarized in . The patients were followed up for 3.0–79.2 months, with a median follow-up time of 45.5 months. Eight patients (8.9%) died during the study, among which 2 patients died of primary disease progression, 4 patients died of severe infection, and 2 patients died of multiple organ dysfunction syndrome (MODS). In addition, 2 patients (2.2%) were lost to follow-up.
Table 1. Characteristics of the study subjects.
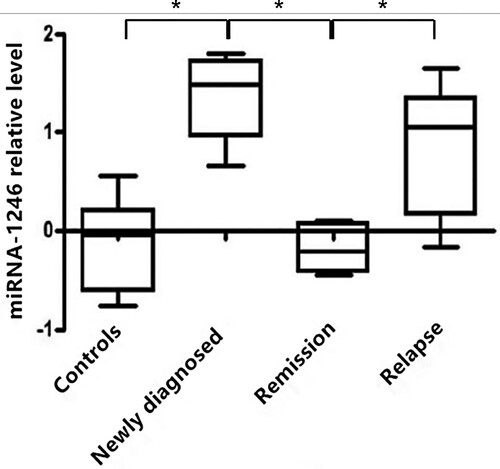
miR-1246 expression levels were increased in the peripheric blood samples of MM patients
To reveal the diagnostic and prognostic value of miR-1246 in MM, we first compared the expression levels of miR-1246 in the peripheral blood from MM patients and healthy donors. As shown in , miR-1246 levels were significantly higher in patients with newly diagnosed MM compared with those of the healthy donors (P < 0.05). In addition, we found that miR-1246 expression levels in MM patients in remission after chemotherapy were decreased as compared with the primary MM samples, which were close to those in normal controls, while miR-1246 levels in relapse samples were elevated which were similar to the levels at the time of new diagnosis (P < 0.05). These results indicated that miR-1246 level was increased in MM patients, which was also associated with the curative effect.
Correlation between miR-1246 expression and the clinical characteristics of MM patients
We then evaluated the correlation between the expression levels of miR-1246 and the clinical characteristics of MM patients. As shown in , miR-1246 level was significantly increased in patients with extramedullary infiltration (P < 0.05), while showed no obvious difference in patients with different ages, gender, R-ISS stages, light chain types, heavy chain types, renal injury, LDH (lactate dehydrogenase) levels, albumin levels, β2-MG levels, and M protein levels (P all >0.05). These results indicated that the blood levels of miR-1246 were significantly increased in MM patients with extramedullary infiltration, and were independent from age, gender, R-ISS stage, light chain type, heavy chain type, renal injury, and the levels of LDH, albumin, β2-MG and M protein.
Table 2. Correlation between miR-1246 expression and clinical characteristics of MM patients.
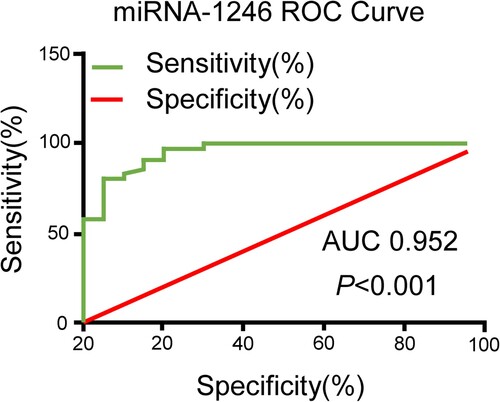
Diagnostic value of peripheral blood miR-1246 in MM
To clarify the diagnostic value of circulating miR-1246 in MM patients, the ROC curve was made. As shown in , miR-1246 could distinguish MM patients from healthy controls with an AUC of 0.952, the sensitivity of 87%, and the specificity of 95% [95% confidence interval (CI), 0.902-1.007; P < 0.001]. These results demonstrated a high value of circulating miR-1246 in the diagnosis of MM.
High expression of miR-1246 in peripheral blood indicated worse prognosis in MM patients
Moreover, we assessed the relationship between miR-1246 expression and the prognosis of MM patients. The univariate analysis results showed that the aged > 60 years, R-ISS stage III, two-drug regimen chemotherapy, and extramedullary infiltration were the impact factors of both PFS and OS (P < 0.05 or P < 0.001, ). Using ROC curve analysis, we defined 1.67 as the threshold value for the miR-1246 expression, 57 MM patients with the expressions of miR-1246 being ≤1.67 were divided into miR-1246 low expression group, and 33 patients with miR-1246 expression greater than 1.67 were divided into miR-1246 high expression group. Both of PFS (14.0 months vs. 26.5 months, P = 0.045) and OS (20.5 months vs. 55.5 months, P = 0.014) were significantly shorter in miR-1246 high expression group as compared with the miR-1246 low expression group (, ).
Figure 3. Positive expression of miR-1246 was associated with adverse prognosis of MM patients. Kaplan-Meier survival analysis revealed that miR-1246 expression was significantly associated with a shorter OS and PFS of MM patients.
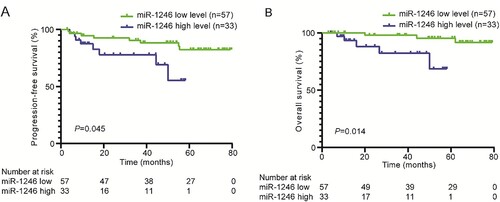
Table 3. Univariate analysis of PFS and OS risk factors in MM patients.
In addition, the multivariate Cox regression model analysis showed that peripheral blood miR-1246 was an independent prognostic factor for PFS (HR 2.786, 95% CI: 1.420–5.467, P = 0.003) and OS (HR 2.995, 95% CI: 1.166–7.689, P = 0.023) in MM patients, as well as R-ISS, extramedullary infiltration also had an independent prognostic impact on PFS and OS (). These above results demonstrated that miR-1246 level was an independent prognostic factor of the prognosis in MM.
Table 4. Multivariate analysis of PFS and OS risk factors in MM patients.
Discussion
Circulating miRNA has been proposed as an attractive diagnostic marker in cancer with high specificity and sensitivity [Citation15,Citation23]. Numerous studies have evaluated the potential of circulating miRNAs as diagnostic biomarkers in various cancers, including breast cancer, gastric cancer, hepatocellular carcinoma, and non-small cell lung cancer [Citation24,Citation25], as well as MM [Citation16]. Ren et al. [Citation20] previously demonstrated that miR-1246 expression was significantly increased in MM patients. The current study further explored its value in the diagnosis of MM and the results revealed a high value of the circulating miR-1246 as a blood marker for the diagnosis of MM.
It has been demonstrated that circulating miR-1246 is a potential marker for the diagnosis of different kinds of cancers. The sensitivity of miR-1246 for the early diagnosis of breast tumors (stage I-IIa) was 80% [Citation26]. Circulating exosomes miR-1246 was also a potential biomarker for the early diagnosis of gastric cancer (GC), which was upregulated in the serum samples of GC patients and could differentiate GC patients with TNM stage I from healthy controls and benign diseases with AUC of 0.843 and 0.811, respectively [Citation27]. Chuma et al. [Citation28] reported that serum miR-1246 was upregulated in hepatocellular carcinoma (HCC) patients with early recurrence, which showed a ROC curve area of 0.762, with a specificity of 77.4% and a sensitivity of 54.1% in discriminating HCC patients with early recurrence from HCC patients without early recurrence. All of the above studies revealed a higher expression pattern of miR-1246 in cancers, but Yang et al. [Citation29] found that miR-1246 level was dramatically decreased in cervical cancer tissues, and miR-1246 level was negatively correlated with clinical stage and HPV16E6 infected status. It cannot be ruled out the difference may be caused by the various sample types. Ren et al. [Citation20] demonstrated that miR-1246 expression was significantly increased in MM patients, which was independent from age, gender, stage, serum levels of β2-MG, albumin, calcium, creatinine, myeloma protein, hemoglobin, and the population of bone marrow plasma cells and chromosome 13 deletion, indicating that miR-1246 may be a useful marker in the diagnosis of MM. Consistently, we found that miR-1246 level was increased in the blood samples of MM as compared with the healthy individuals. In addition, circuiting miR-1246 showed a high value in distinguish MM from healthy individuals (AUC, 0.952; sensitivity, 87%; specificity, 95%).
The Extra-medullary disease (EMD) in MM is associated with poor prognosis and resistance to chemotherapy [Citation30]. Here, we found that miR-1246 expression was significantly increased in MM patients with extramedullary infiltration, indicating a crucial value of miR-1246 in predicting the extramedullary infiltration of MM patients. Evidence has shown that miR-1246 can target CXC motif chemokine receptor type 4 (CXCR4) to inhibit cell migration and invasion in renal cell carcinoma [Citation31] and lung cancer [Citation32]. CXCR4 is a G protein-coupled receptor that also has been verified to be accelerated dissemination from the bone marrow [Citation33], thus we speculate miR-1246 may promote the extramedullary infiltration of MM by targeting CXCR4.
In addition, miR-1246 has been reported to be linked to the drug resistance of cancers. High metastatic tumors induce drug resistance of tumor endothelial cells by transporting miR-1246 [Citation34]. Targeting the miR-1246-AXIN2/GSK-3β-Wnt/β-catenin axis may help overcome chemotherapy resistance in patients with relapsed refractory leukemia [Citation35]. Also, the expression of cerebrospinal fluid exosome miR-1246 after glioma surgery correlates with relapse rate [Citation36]. In this study, we found that the expression level of miR-1246 in the remission group after chemotherapy was close to that of the normal control group, while increased to the level at the time of new diagnosis when recurrence. This finding suggested that the high expression of miR-1246 in MM patients may be related to chemotherapy resistance and relapse, indicating that the circulating miR-1246 level may be used to predict disease recurrence.
Also, circulating miR-1246 level is a prognosis marker of cancers. High levels of miR-1246 predicted adverse event-free survival (EFS) in patients with early breast cancer and poor PFS in patients with metastases [Citation37]. The increased expression of miR-1246 in peripheral blood samples was associated with shorter PFS in patients with MM [Citation20]. In this study, both of univariate and multivariate analysis showed that higher level of miR-1246 was an adverse prognostic factor for both PFS and OS.
Several limitations should be clarified in this study. As mentioned above, serum miR-1246 level was increased in several kinds of cancers and diseases, including MM, challenging only miR-1246 level to distinguish different kinds of cancers. A model should be constructed with combination of miR-1246 and clinical manifestation/information to distinguish different kinds of cancers. In addition, we didn’t compare the expression levels of miR-1246 in MM and its precursor conditions, undetermined significance (MGUS) and smoldering multiple myeloma (SMM). This is meaningful and we intend to include patients with MGUS and SMM for further identification. Moreover, we didn’t reveal the relationship between extramedullary disease and the amount of plasma cells, as well as the role and underlying mechanisms, such as the target genes (e.g. CXCR4), of miR-1246 in the pathogenesis of MM. Further studies focus on these issues are required in future.
Conclusion
Collectively, this study demonstrates that circulating miR-1246 level is elevated in MM patients, which can be used as a biomarker for MM diagnosis and prognosis prediction. Overall, miR-1246 is a potential liquid biopsy marker to identify MM patients with poor prognosis and help to select better combination therapy in time.
Ethics statement
The study involving human participants was approved by the Ethic Committee of the Forth Hospital of Hebei Medical University. The patients/participants provided their written informed consent to participate in this study. This research was conducted in accordance with the principles expressed in the Declaration of Helsinki.
Author contributions
Research concept and design: Guimin Zhao; Collection and/or assembly of data: Guimin Zhao, Xiaotong Jing, Zheng Li; Data analysis and interpretation: Guimin Zhao, Xiaolin Wu, Zhe Gao, Ruijuan Ma; Writing the article: Guimin Zhao; Critical revision of the article: all; Final approval of the article: all.
Acknowledgments
The authors are grateful to patients and healthy donors for donating samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25–35. Epub 2011/04/22.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. Epub 2011/03/18.
- Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–598. Epub 2007/07/25.
- Alexander DD, Mink PJ, Adami HO, et al. Multiple myeloma: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):40–61. Epub 2007/04/04.
- Brigle K, Rogers B. Pathobiology and diagnosis of multiple myeloma. Semin Oncol Nurs. 2017;33(3):225–236. Epub 2017/07/10.
- Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684–1692. Epub 2011/11/03.
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. Epub 2008/07/30.
- Wang H, Peng R, Wang J, et al. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics. 2018;10:59, Epub 2018/05/02.
- Handa H, Murakami Y, Ishihara R, et al. The role and function of microRNA in the pathogenesis of Multiple myeloma. Cancers (Basel). 2019;11(11):1738.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. Epub 2004/01/28.
- Pourhanifeh MH, Mahjoubin-Tehran M, Shafiee A, et al. MicroRNAs and exosomes: small molecules with big actions in multiple myeloma pathogenesis. IUBMB Life. 2020;72(3):314–333. Epub 2019/12/13.
- Masri AA, Price-Troska T, Chesi M, et al. MicroRNA expression analysis in multiple myeloma. Blood. 2005;106(11):1554.
- Soliman AM, Lin TS, Mahakkanukrauh P, et al. Role of microRNAs in diagnosis, prognosis and management of multiple myeloma. Int J Mol Sci. 2020;21(20):7539. Epub 2020/10/18.
- Pula A, Robak P, Robak T. MicroRNA in Multiple myeloma – a Role in pathogenesis and prognostic significance. Curr Med Chem. 2021;28(33):6753–6772. Epub 2021/05/06.
- Zhu B, Ju S, Chu H, et al. The potential function of microRNAs as biomarkers and therapeutic targets in multiple myeloma. Oncol Lett. 2018;15(5):6094–6106. Epub 2018/05/08.
- Tiruneh T, Melku M. Circulating MicroRNAs in multiple myeloma: a literature review. Clin Lab. 2020;66(12). Epub 2020/12/19.
- Gupta N, Kumar R, Seth T, et al. Clinical significance of circulatory microRNA-203 in serum as novel potential diagnostic marker for multiple myeloma. J Cancer Res Clin Oncol. 2019;145(6):1601–1611. Epub 2019/03/21.
- Morin RD, O’Connor MD, Griffith M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18(4):610–621. Epub 2008/02/21.
- Zhang Y, Liao JM, Zeng SX, et al. P53 downregulates down syndrome-associated DYRK1A through miR-1246. EMBO Rep. 2011;12(8):811–817. Epub 2011/06/04.
- Ren Y, Li X, Wang W, et al. Expression of peripheral blood miRNA-720 and miRNA-1246 can be used as a predictor for outcome in multiple myeloma patients. Clin Lymphoma Myeloma Leuk. 2017;17(7):415–423. Epub 2017/06/12.
- Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–ee46. Epub 2016/08/12.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. Epub 2002/02/16.
- Condrat CE, Thompson DC, Barbu MG, et al. miRNAs as biomarkers in disease: latest findings regarding their Role in diagnosis and prognosis. Cells. 2020;9(2):276. Epub 2020/01/26.
- Filipow S, Laczmanski L. Blood circulating miRNAs as cancer biomarkers for diagnosis and surgical treatment response. Front Genet. 2019;10:169, Epub 2019/03/28.
- Oura K, Fujita K, Morishita A, et al. Serum microRNA-125a-5p as a potential biomarker of HCV-associated hepatocellular carcinoma. Oncol Lett. 2019;18(1):882–890. Epub 2019/07/11.
- Zhang Y, Zhang X, Situ B, et al. Rapid electrochemical biosensor for sensitive profiling of exosomal microRNA based on multifunctional DNA tetrahedron assisted catalytic hairpin assembly. Biosens Bioelectron. 2021;183:113205, Epub 2021/04/05.
- Shi Y, Wang Z, Zhu X, et al. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int J Clin Oncol. 2020;25(1):89–99. Epub 2019/09/12.
- Chuma M, Toyoda H, Matsuzaki J, et al. Circulating microRNA-1246 as a possible biomarker for early tumor recurrence of hepatocellular carcinoma. Hepatology Research: Off J Japan Soc Hepatol. 2019;49(7):810–822. Epub 2019/03/29.
- Yang Y, Xie YJ, Xu Q, et al. Down-regulation of miR-1246 in cervical cancer tissues and its clinical significance. Gynecol Oncol. 2015;138(3):683–688. Epub 2015/06/16.
- Kawaguchi N, Zhang TT, Nakanishi T. Involvement of CXCR4 in normal and abnormal development. Cells. 2019;8(2):185. Epub 2019/02/23.
- Liu HT, Fan WX. MiRNA-1246 suppresses the proliferation and migration of renal cell carcinoma through targeting CXCR4. Eur Rev Med Pharmacol Sci. 2020;24(11):5979–5987. Epub 2020/06/24.
- Xu X, Cao L, Zhang Y, et al. MicroRNA-1246 inhibits cell invasion and epithelial mesenchymal transition process by targeting CXCR4 in lung cancer cells. Cancer Biomarkers: Section A of Disease Markers. 2018;21(2):251–260. Epub 2017/11/25.
- Roccaro AM, Mishima Y, Sacco A, et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 2015;12(4):622–635. Epub 2015/07/21.
- Torii C, Maishi N, Kawamoto T, et al. miRNA-1246 in extracellular vesicles secreted from metastatic tumor induces drug resistance in tumor endothelial cells. Sci Rep. 2021;11(1):13502. Epub 2021/07/07.
- Xie B, Li L, Zhang Z, et al. MicroRNA-1246 by targeting AXIN2 and GSK-3beta overcomes drug resistance and induces apoptosis in chemo-resistant leukemia cells. J Cancer. 2021;12(14):4196–4208. Epub 2021/06/08.
- Qiu W, Guo X, Li B, et al. Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells. Mol Ther; 2021: 3449–3464. Epub 2021/07/05.
- Zhang Z, Zhang L, Yu G, et al. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer Chemother Pharmacol. 2020;86(6):761–772. Epub 2020/10/18.