ABSTRACT
Objective Follicular helper T cells (Tfh) drive proliferation and differentiation of B cells into plasma cells, leading to antibody production; however, their role in multiple myeloma (MM) is unknown. We aimed to determine the alteration of Tfh subsets and their clinical significance in patients with MM.
Method Forty-nine patients with MM were recruited in this study, including 12 newly diagnosed patients, 10 relapsed patients, and 8 patients who received autologous hematopoietic stem cell transplantation (ASCT) from Zhejiang Provincial People's Hospital. Total CD4 + CXCR5 + CD25lowCD127intermediate-high Tfh cells, CXCR3 + CCR6−Tfh1 cells, CXCR3−CCR6−Tfh2 cells, and CXCR3−CCR6 + Tfh17 cells from the peripheral blood of these patients were analyzed by flow cytometry.
Result Although total Tfh cells were not significantly changed in patients with MM compared to that in healthy controls (HCs), the Tfh17/Tfh ratio was significantly elevated in patients with MM compared to that in HCs (P = 0.0001). Importantly, relapsed patients had higher Tfh17/Tfh ratio than the newly diagnosed patients (P = 0.0077). Moreover, the Tfh17/Tfh ratio was significantly decreased in patients with MM who received ASCT (post-ASCT) when compared to that in HCs and non-ASCT patients (P < 0.0001), but no change was observed between post-ASCT patients and HCs (P = 0.7498).
Conclusion The Tfh17/Tfh ratio was significantly elevated in patients with MM, especially in relapsed patients, indicating that Tfh17 cells may play a critical role in the clinical progression of MM.
KEYWORDS:
Introduction
Multiple myeloma (MM) is characterized by the accumulation of clonal and malignant plasma cells in the bone marrow[Citation1]. Recently, autologous hematopoietic stem cell transplantation (ASCT), B-cell antibodies, and CAR-T therapy have significantly improved the survival rate of patients with MM over the past two decades. However, MM is still incurable and its pathogenesis has not been fully elucidated. Therefore, understanding the pathogenesis of the immune system could be helpful for effective treatment of MM.
Tfh cells are a subset of CD4+ T cells that are characterized by CXCR5, PD-1, and ICOS expression in the germinal centers (GCs)[Citation2, Citation3]. Tfh cells provide co-stimulatory factors, such as ICOS, to promote B-cell proliferation and differentiation. Akkaya et al[Citation4] revealed that B cells without the presence of activated Tfh cells are most likely subject to dysfunction and apoptosis within 24 h. Tfh cells not only facilitate the differentiation of B cells into plasma cells but also play a deleterious role in autoimmune diseases that contribute to excess autoantibodies in the human body[Citation5]. The monoclonal plasma cells of MM, the most long-lived plasma cells residing in the bone marrow, are derived from GC B cells[Citation6]. Thus, as critical partners of B cells, Tfh cells may play an important role in the clinical progression of MM.
In hematological malignancy, Wu et al[Citation7] illustrated that abnormal Tfh populations are associated with chronic lymphocytic leukemia (CLL) development. In addition, the elevated levels of co-stimulatory molecules and cytokines produced by Tfh cells may support CLL proliferation. Furthermore, Zhou et al[Citation8] revealed increased ICOS+CD4 + CXCR5+ and PD-1 + CD4 + CXCR5 + Tfh cell levels in patients with MM compared to that in healthy controls (HCs). However, Zhou et al [Citation8] reported that Tfh cells were not excluded from the CD4 + CXCR5+ Tfh cell population. Together, Tfh cells may play a critical role in the development of malignant lymphoid diseases, especially MM. Thus, in this study, we analyzed total Tfh cells, Tfh1 cells, Tfh2 cells, and Tfh17 cells from the peripheral blood of 49 patients with MM by flow cytometry, as well as the frequency distribution of different Tfh subsets in patients with MM.
Methods
Subject characteristics
A total of 49 patients with MM from Zhejiang Provincial People's Hospital between January 2020 and March 2022 were enrolled in this study, including 12 newly diagnosed patients, 10 relapsed patients, and 8 patients who underwent ASCT. The study was approved by the ethics committee of Zhejiang Provincial People's Hospital. The 49 patients with MM were diagnosed using the IMWG criteria[Citation9]. Additionally, patients with other hematological or autoimmune diseases were excluded.
Flow cytometry
The EDTA anticoagulant peripheral blood was diluted with phosphate-buffered saline at a ratio of 1:1, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll separation (Ficoll-Paque, Dakewei, China) by centrifugation at 2000 rpm for 20 min. Fluorescent antibodies were then added to the PBMCs for surface marker staining for 30 min. The following fluorescent antibodies were used: BV510-CD4 (Cat:562970), BV421-CXCR5 (Cat:562747), Alexa 647-CD127 (Cat:558598), APC-R700-CD25 (Cat:565106), BB700-PD-1 (Cat:566460), BB515-CCR7 (Cat:565869), PECy7-CXCR3 (Cat:560831), PE-CCR6 (Cat:551773), PE-Cy7-CD45RO (Cat:560608), BB700-CCR7 (Cat:566437), and BB515-ICOS (Cat:564549). All antibodies were purchased from BD Bioscience. After 30 min of incubation, the cells were analyzed using flow cytometry (Navios, Beckman Coulter, Brea, CA, USA). Post-acquisition analysis was performed using the Kaluza software. The following subsets were analyzed: CD4 + CXCR5 + CD25lowCD127intermediate-highTfh, CXCR3 + CCR6−Tfh1, CXCR3−CCR6−Tfh2, and CXCR3−CCR6 + Tfh17 cells.
Statistical analysis
All data were analyzed using GraphPad Prism software (version 5.01, La Jolla, CA, USA). Multiple comparisons between groups were first conducted by one-way analysis of variance. If significant, a Mann–Whitney comparison was performed by a unpaired t-test. All data were expressed as mean ± standard error of measurement. P < 0.05 was considered as statistically significant. *P< 0.05, **P< 0.01, ***P< 0.001.
Results
CXCR3 + CCR6−Tfh17/Tfh ratio was significantly elevated in patients with MM compared to that in HCs
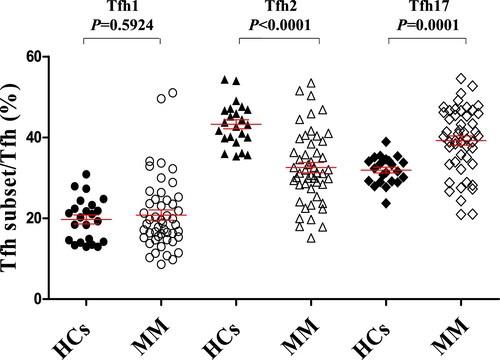
We first assessed the ratio of Tfh/CD4+ T cells in the peripheral blood of HCs and patients with MM. The frequency of Tfh cells was not significantly different between HCs and patients with MM (, P = 0.1762). Tfh cell subsets were subsequently analyzed in HCs and patients with MM to elucidate their possible role in MM clinical progression. As shown in , patients with MM and HCs showed no significant difference in their Tfh1/Tfh ratio (, P = 0.5924), but the Tfh2/Tfh ratio was significantly decreased in patients with MM compared to that in HCs (, HCs vs MM: 43.27 ± 1.11% vs 32.54 ± 1.27%, P < 0.0001). By contrast, the Tfh17/Tfh ratio was significantly elevated in patients with MM compared to that in HCs (, HCs vs MM: 31.92 ± 0.71% vs 39.22 ± 1.17%, P = 0.0001).
Figure 1. Alteration of Tfh/CD4+ T-cell ratio in patients with MM compared to that in HCs. (A-E) Schematic diagram of the flow cytometry analyses of CD4 + CXCR5 + T cell and CD25lowCD127intermediate-highTfh cells. (F) Flow cytometry analyses of CXCR3 + CCR6−Tfh1 cells, CXCR3−CCR6−Tfh2 cells, and CXCR3−CCR6 + Tfh17 cells. (G) Proportion of CD25lowCD127intermediate-highTfh/CD4+ T-cell in the peripheral blood of HCs and patients with MM (HCs vs MM: 90. 95 ± 0.97% vs 86.48 ± 2.19%, P = 0.1762).
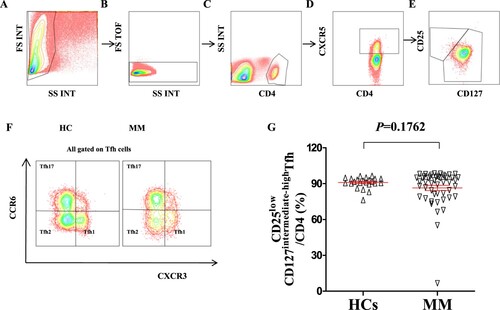
Figure 2. Alteration of Tfh cell subsets in patients with MM compared to that in HCs (A) The proportion of CXCR3 + CCR6−Tfh1/Tfh, CXCR3−CCR6−Tfh2/Tfh, and CXCR3−CCR6 + Tfh17/Tfh in the peripheral blood of HCs and patients with MM. CXCR3 + CCR6−Tfh1/Tfh (HCs vs MM: 19.71 ± 1.10% vs 20.79 ± 1.27%, P = 0.5924); CXCR3−CCR6−Tfh2/Tfh (HCs vs MM: 43.27 ± 1.11% vs 32.54 ± 1.27%, P < 0.0001); CXCR3−CCR6 + Tfh17/Tfh (HCs vs MM: 31.92 ± 0.71% vs 39.22 ± 1.17%, P = 0.0001). HCs: Healthy controls (n = 23); MM: Multiple myeloma (n = 49).
CXCR3 + CCR6−Tfh17/Tfh ratio was significantly elevated in relapsed patients compare to that in newly diagnosed patients with MM
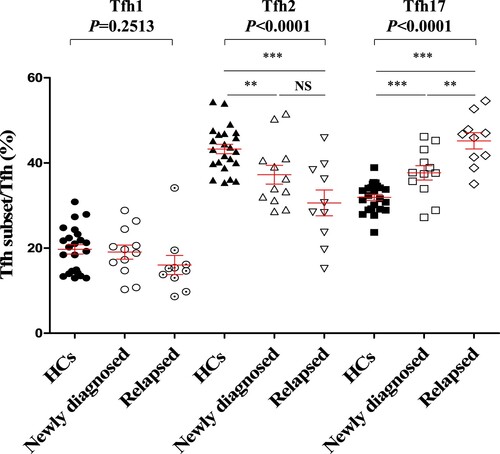
The elevated Tfh17/Tfh ratio in the peripheral blood of patients with MM indicates an important role in the clinical progression of MM. Thus, patients with MM were further divided into a newly diagnosed group and a relapsed group (). More importantly, the Tfh17/Tfh ratio was higher in the relapsed group than in the newly diagnosed group (newly diagnosed vs relapsed: 37.68 ± 1.68% vs 45.19 ± 1.91%, P = 0.0077), further supporting the critical role of Tfh17 cells in the clinical progression of MM.
Figure 3. Alteration of Tfh cell subsets in newly diagnosed patients and relapsed patients with MM compared to that in HCs (A) CXCR3 + CCR6−Tfh1/Tfh median percentage (HCs vs Newly diagnosed vs Relapsed: 19.71 ± 1.10% vs 19.07 ± 1.64% vs 16.03 ± 2.24%, P = 0.2513); CXCR3−CCR6−Tfh2/Tfh (HCs vs Newly diagnosed vs Relapsed: 43.27 ± 1.11% vs 37.24 ± 2.20% vs 30.61 ± 3.03%, P < 0.0001); CXCR3−CCR6 + Tfh17/Tfh (HCs vs Newly diagnosed vs Relapsed: 31.92 ± 0.71% vs 37.68 ± 1.68% vs 45.19 ± 1.91%, P = 0.0001). HCs: Healthy controls (n = 23); Newly diagnosed MM (n = 12); Relapsed patients with MM (n = 10).
CXCR3 + CCR6−Tfh17/Tfh ratio was significantly increased in non-ASCT patients compared to that in post-ASCT patients with MM
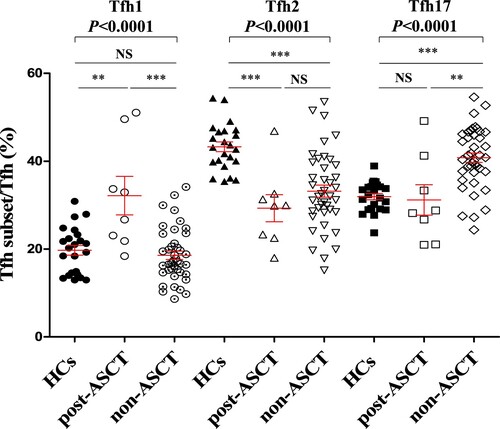
ASCT is the standard treatment for patients with MM and can achieve a complete response and prolong progression-free survival[Citation10]. Thus, 8 patients with MM who received ASCT treatment (post-ASCT) and 41 who did not receive ASCT (non-ASCT) were compared. The results revealed that the Tfh1/Tfh ratio in post-ASCT patients was significantly higher than that in HCs and non-ASCT patients (, HCs vs post-ASCT vs non-ASCT: 19.71 ± 1.10% vs 32.15 ± 4.37% vs 18.58 ± 0.95%, P < 0.0001). By contrast, the Tfh17/Tfh ratio was significantly elevated in non-ASCT patients compared to that in HCs and post-ASCT patients (, HCs vs post-ASCT vs non-ASCT: 31.92 ± 0.71% vs 31.17 ± 3.46% vs 40.79 ± 1.08%, P < 0.0001), but the Tfh17/Tfh ratio showed no significant difference between the post-ASCT patients and HCs (, HCs vs post-ASCT: 31.92 ± 0.71% vs 31.17 ± 3.46%, P = 0.7498). Thus, these results further support the notion that Tfh17 cells may be associated with the treatment response of patients with MM.
Figure 4. Alteration of Tfh cell subsets in post-ASCT and non-ASCT patients with MM compared to that in HCs. CXCR3 + CCR6−Tfh1/Tfh median percentage (HCs vs post-ASCT vs non-ASCT: 19.71 ± 1.10% vs 32.15 ± 4.37% vs 18.58 ± 0.95%, P < 0.0001); CXCR3−CCR6−Tfh2/Tfh median percentage (HCs vs post-ASCT vs non-ASCT: 43.27 ± 1.11% vs 29.29 ± 3.09% vs 33.18 ± 1.39%, P < 0.0001); CXCR3−CCR6 + Tfh17/Tfh median percentage (HCs vs post-ASCT vs non-ASCT: 31.92 ± 0.71% vs 31.17 ± 3.46% vs 40.79 ± 1.08%, P < 0.0001). HCs: Healthy controls (n = 23); post-ASCT (n = 8); non-ASCT (n = 41).
Discussion
Over the past few years, significant advances have been made in the understanding of immune dysfunction in patients, and various immunotherapeutic approaches have been developed to improve survival. However, it is still challenging to eliminate malignant plasma cells and cure MM in patients. Therefore, clarifying the interplay between malignant plasma cells and the immune system is of increasing importance[Citation11]. In recent years, aberrant responses and uncontrolled expansion of circulating Tfh cells, a subset of CD4+ T cells, have been observed in various systemic autoimmune diseases[Citation3, Citation12]. Moreover, Morita[Citation5] revealed that Tfh2 and Tfh17 cells could induce naïve B cells to produce immunoglobulins (Ig), while Tfh2 cells promote IgG and IgE secretion, and Tfh17 cells promote IgA and IgG secretion.
Although the function of Tfh cells in autoimmune diseases and immunodeficiency diseases has been well recognized, evidence regarding the role of Tfh cells in hematological diseases is relatively lacking[Citation7, Citation8, Citation13, Citation14]. Wu et al[Citation7] found that the frequencies of CD4+ T cells expressing Tfh1 markers and IL-21 were positively correlated with lymphocyte counts and RAI stage in patients with CLL, suggesting that accumulation of abnormal Tfh cells is concomitant with expansion of the leukemic B-cell clone. Moreover, evidence from co-culturing of Tfh and CLL cells from 28 patients with CLL showed that Tfh cells were significantly expanded and targeted the interaction of Tfh cells with proliferating CLL cells [Citation13], which could open new avenues for therapeutic targeting of CLL.
In 2017, Zhou et al[Citation8] revealed a significant reduction in peripheral ICOS+CD4 + CXCR5+ and PD-1 + CD4 + CXCR5 + Tfh cell levels in patients with MM who achieved effective treatment at the end of the second, fourth, and sixth treatment courses compared to those without treatment. However, their study did not exclude Tfh cells from the CD4 + CXCR5 + Tfh cell population, and the Tfh subsets associated with the clinical progression of MM were also lacking. Thus, our study revealed that the Tfh17/Tfh ratio was significantly elevated in newly diagnosed patients and even higher in relapsed patients with MM. Moreover, the Tfh17/Tfh ratio was higher in non-ASCT patients than in HCs and post-ASCT patients. Taken together, Tfh17 cells may play a critical role in the clinical progression of MM. However, whether Tfh17 cells could directly expand malignant plasma cells or normal B cells in patients with MM requires further co-culture studies.
Previously, Ma et al[Citation15] illustrated that Th17 cell levels were higher in newly diagnosed patients with MM than in HCs. They also found that Th17 cell levels were increased further in the partial remission stage, decreased to normal in the complete remission stage, and increased again in the relapsed stage. Moreover, Prabhala et al reported that elevated IL-17 levels produced by Th17 cells promoted myeloma cell growth and inhibited immune function in patients with MM[Citation16, Citation17]. Similarly, both Th17 cells and Tfh17 cells are characterized by the CCR6 chemokine receptor, which is the sole receptor of chemokine CCL20[Citation18]. It has been reported that CCR6 + tumor cells could migrate to CCL20 enriched site and promote tumor metastasis in various cancers[Citation19, Citation20]. Notably, Palma et al[Citation21] showed that the bone marrow CCL20–CCR6 axis plays an important role in osteolysis in patients with MM. However, it is still unclear whether Tfh17 cells are a subset of Th17 cells. Furthermore, the effect of Tfh cells in promoting MM progression is unknown. Therefore, further in vitro functional studies are warranted.
ASCT is the standard treatment for MM. After ASCT, patients can achieve a complete response and prolong progression-free survival[Citation10]. In this study, non-ASCT patients had significantly elevated Tfh17/Tfh ratios, whereas they were sharply reduced in post-ASCT patients, further supporting that Tfh17 cells may be associated with the treatment response in patients with MM.
In conclusion, we found that the Tfh17/Tfh ratio was significantly elevated in patients with MM, especially in relapsed patients, compared to that in HCs. In addition, the Tfh17/Tfh ratio was reduced in post-ASCT patients compared to that in non-ASCT patients. Our results indicate that Tfh17 cells may play a critical role in the clinical progression of MM.
Consent from all authors
All authors reviewed this manuscript and agreed to submit this manuscipt.
Ethical approal
This study has approved by the ethical committee of Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, China.
Funding information:
Yanxia Chen was supported by the Zhejiang Provincial Natural Science Fund (No: LY20H100008). Zhenni Wang was supported by General research program of Zhejiang Provincial Department of health (2018KY249). Financing entity had no role in the study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Patient consent statement
This study did not involve the personal information, only report the laboratory data. Then the patient consent was waived, and this waive was approved by the ethical committee of Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, China.
Acknowlegement
The authors would like to thank the encouragement and support of the members in the Department of Clinical Laboratory, Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bergstrom DJ, Kotb R, Louzada ML, et al. Consensus guidelines on the diagnosis of multiple myeloma and related disorders: recommendations of the myeloma Canada research network consensus guideline consortium. Clin Lymphoma Myeloma Leuk. Jul 2020;20(7):e352–e367.
- Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. Dec 4 2000;192(11):1545–1552.
- Crotty S. T follicular helper cell biology: A decade of discovery and diseases. Immunity. 2019;50(5):1132–1148.
- Akkaya M, Traba J, Roesler AS, et al. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol. Aug 2018;19(8):871–884.
- Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. Jan 28 2011;34(1):108–121.
- Utley A, Lipchick B, Lee KP, et al. Targeting multiple myeloma through the biology of long-lived plasma cells. Cancers (Basel). 2020;12(8.
- Wu X, Fajardo-Despaigne JE, Zhang C, et al. Altered T follicular helper cell subsets and function in chronic lymphocytic leukemia. Front Oncol. 2021;11:674492.
- Zhou DM, Xu YX, Zhang LY, et al. The role of follicular T helper cells in patients with malignant lymphoid disease. Hematology. 2017;22(7):412–418.
- Kyle RA, Rajkumar SV. Spotlight review series on multiple myeloma. Leukemia. 2009;23(1):R1–R2.
- Minnie SA, Kuns RD, Gartlan KH, et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood, J Am Soc Hematol. Oct 18 2018;132(16):1675–1688.
- Nakamura K, Smyth MJ, Martinet L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood. Dec 10 2020;136(24):2731–2740.
- Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. Sep 18 2009;31(3):457–468.
- Vaca AM, Ioannou N, Sivina M, et al. Activation and expansion of T-follicular helper cells in chronic lymphocytic leukemia nurselike cell co-cultures. Leukemia. Feb 11 2022.
- Dai L, He L, Wang Z, et al. Altered circulating T follicular helper cells in patients with chronic immune thrombocytopenia. Exp Ther Med. Sep 2018;16(3):2471–2477.
- Ma T, Zhang Y, Zhou X, et al. A unique role of T helper 17 cells in different treatment stages of multiple myeloma. Clin Lymphoma Myeloma Leukemia. 2020;20(3):190–197.
- Prabhala RH, Pelluru D, Fulciniti M, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. Jul 1 2010;115(26):5385–5392.
- Prabhala RH, Fulciniti M, Pelluru D, et al. Targeting IL-17A in multiple myeloma: a potential novel therapeutic approach in myeloma. Leukemia. 2016;30(2):379–389.
- Lee AYS, Körner H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology. 2019;224(3):449–454.
- Kadomoto S, Izumi K, Mizokami A. The CCL20-CCR6 axis in cancer progression. Int J Mol Sci. Jul 22 2020;21(15.
- Korbecki J, Grochans S, Gutowska I, et al. CC chemokines in a tumor: A review of Pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. Oct 15 2020;21(20.
- Giuliani N, Lisignoli G, Colla S, et al. CC-chemokine ligand 20/macrophage inflammatory protein-3α and CC-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res. Aug 15 2008;68(16):6840–6850.
