ABSTRACT
Objectives
HemoTypeSCTM is one of the immunoassay methods currently used for the early diagnosis of Sickle Cell Disease (SCD) in newborns. Earlier diagnosis remains the key strategy for early preventive care needs and parents’ education about the child's future well-being throughout his life. Before considering these children as sick and aligning them for regular medical monitoring, it may be valuable to confirm the HemoTypeSC result with a secondary laboratory testing method. In resource-limited settings, where confirmatory methods are not always available, we propose testing the parents to validate the HemoTypeSC result.
Methods
This study explored this approach in the city of Kisangani. It was a prospective diagnostic accuracy study using genotype biological parents to evaluate HemoTypeSC's performance in the newborn.
Results
Fifty-eight children born to 46 known mothers, and 37 known fathers, have been tested. The phenotyping showed that 41 (70.7%) children were SS, whose 37 were born to a couple AS/AS and 4 to a couple AS/xx. Of the 41 SS children, 8 (19.5%) were newborns and 33 (80.4%) were children; 12 (20.6%) children were AS, one of whom was born to a couple SS/AA and 11 to a couple AA/SS; 5 (8.6%) children were AA. In this population, the probability of offspring born to AS/AS parents being SS rather than AS is high (odds, 1.25). No statistical difference was observed between girls and boys. The pedigree of all 58 children has been confirmed.
Conclusion
We demonstrated that testing biological parents with HemoTypeSC is a reliable confirmatory method for newborn screening but it presents some limitations discussed in the present article.
Introduction
Sickle cell disease (SCD) is an inherited autosomal recessive genetically transmitted hemoglobinopathy responsible for significant morbidity and mortality [Citation1,Citation2]. It is characterized by the presence of homozygous hemoglobin S (HbSS), or by the presence of compound heterozygous HbS with a second variant or a β-thalassemia allele. HbSS mainly affects children originating from black populations, particularly in sub-Saharan Africa (SSA) [Citation3–5]. It alone contributes to around 5–16% of the mortality of children under 5 on the African continent [Citation3,Citation5,Citation6]. Approximately 500 children under the age of 5 die daily from SCD due to a lack of rapid diagnosis and appropriate care, yet SCD remains an invisible global health problem [Citation7]. In the Democratic Republic of the Congo (DRC), studies reported approximately 1.5% of homozygotes and 20–40% of heterozygotes in the population [Citation8,Citation9]. Thirty thousand to forty thousand SS newborns are born there each year [Citation8], bringing the neonatal prevalence to 1.4% [Citation8–10].
According to the literature, diagnosis at birth and educational awareness of parents remains an important strategy for getting parents to adhere to medical care as early as possible and to live well with the disease [Citation11–13]. In developed countries with a policy for newborn diagnosis of SCD, the diagnosis is made early at birth or during the prenatal period for couples at risk [Citation14–16]. In Africa, the diagnosis is often made late when the child presents the first signs or complications of the disease [Citation11,Citation14,Citation16]. Also, penicillin prophylaxis between 2 and 3 months of life and vaccination, preferably before the onset of the first symptoms of SCD, remains the key strategy of preventive care [Citation14,Citation16,Citation17].
Technically, neonatal screening of HbSS, in resource-limited settings, requires the use of rapid, valid, easily practicable, and less expensive phenotyping tests, such as HemoTypeSC or isoelectric focusing(IEF), compared to capillary electrophoresis, HPLC, or LC-MS methods [Citation3,Citation4,Citation9,Citation13,Citation14,Citation18]. In Kisangani (DRC), HemoTypeSC has been available for the past two years now. The results of a preliminary study carried out locally have shown that it is a Point of Care (POC) type test, sensitive, specific, less expensive (<2$), easy to perform, and suitable for neonatal screening [Citation8,Citation19]. In the absence of an accurate alternative method, some authors recommend genotype biological parents and match the results [Citation3,Citation6,Citation18,Citation21]. Besides the burden of SCD on the state of health of children, there is, in Africa, another social and psychological burden affecting the genetic responsibility of a father who refuses to accept paternity, suspecting the mother of infidelity, refusing to join the child’s care, and even demanding divorce [Citation12,Citation22]. In this case, the phenotyping of the parents may also help confirm the HbSS pedigree of the child [Citation11]. The sensitivity of the HemoTypeSC assay is higher among adult populations, due to the higher concentration of Sickle hemoglobin [Citation2]. To explore this approach, in this paper, we aimed to combine the HemoTypeSC test of parents and offspring to validate the results and confirm the sickle cell pedigree in a small sample of Kisangani families, allowing us to initiate the prophylactic antibiotic treatment.
Material and methods
Conceptual framework
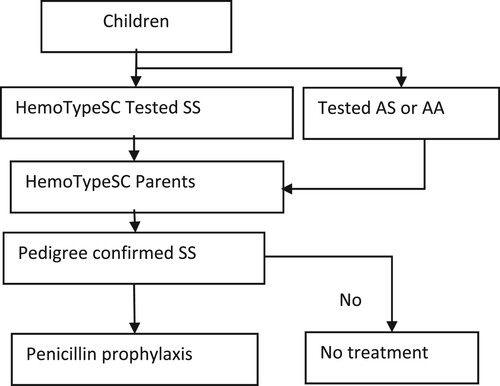
This clinical research was a prospective diagnostic accuracy study using genotype biological parents to evaluate HemoTypeSC's performance in the newborn. The approach of the research protocol was that when the HemoTypeSC test result cannot be validated by confirmatory testing or the pedigree is disputed, the parents can be tested to validate the result before initiating prophylactic antibiotic therapy ().
Study sites
The study took place in Kisangani, the capital of Tshopo province in Northeastern DRC. The DRC is a secular country. It is a multi-ethnic and culturally diverse federation with five traditional churches (Catholic, Protestant, Church of Jesus of Latter-day Saints, Muslim, Kimbanguisme) and several other so-called revivalist churches [Citation23]. All our patients had a belief in God and were affiliated with a church. No cases of atheism were reported. The participants were conveniently recruited in eight Medical Centers (MCs), namely STANLEY, NVDP, CS JAMAA, HGRK, CUKIS, HGRMK, CS IMANI, and CHC. The MCs were selected by convenience as well. They belong to two Health Zones (HZ), namely Kabondo and Makiso-Kisangani, which have an urban-rural configuration and a daily income of less than $2/person [Citation10,Citation11,Citation22]. These MCs have served as sites for the realization of 2 preliminary studies. The first was conducted between March and April 2019, before the advent of the covid-19 pandemic, to validate the HemoTypeSC test and initiate the implementation of neonatal screening [Citation19]. The second, carried out during the first wave of covid-19, aimed to assess the acceptability of the test, as some parents resisted taking the blood of their newborns [Citation8].
Study population sample
The two Health Zones’ population is around 558,786 inhabitants, of which around 22.351 newborns are expected per year, including about 313HbSS types/year (approximately 26 newborns per month) [Citation8]. For convenience and reliability of our method, the population tested included newborns, children with HbSS under treatment, their biological parents, heterozygous descendants born from AA/SS or SS/AA parents, and offspring of AA/AA couples screened before marriage without discrimination of ethnic group or native province of parents. It is important to note that AA/AA parents and their AA offspring are not well represented in this cohort. Most refused to expose themselves unnecessarily to a blood test as their AA result was already known before their marriage, and they were sure not to have affected children. Couples who had not given their consent to testing the children or themselves were excluded from the study. In total, 58 children, born to 46 mothers and 37 fathers, were tested.
Ethical consideration
The conduct of this study respected the ethical principles of the Declaration of Helsinki. We have obtained the authorization of the ethics committee of the University of Kisangani (N°UNIKIS/CER/005/2018). Respect for the principle of confidentiality has been applied. Before including subjects in the study, the written informed consent of both parents was required. Their participation was optional and they were free to withdraw it at any time. Parents had previously benefited from awareness sessions initiated by a team of gynecologists to persuade pregnant women to get tested and have their newborns tested for SCD within 72 h of birth [Citation8]. Outreach staff worked in teams of 4–5 people to target as many parents as possible. The information given to parents did not exceed 20 min [Citation8]. The content of the message consisted in describing the mode of transmission of the disease, in explaining that the condition was compatible with life despite its severity and chronicity, in giving the time to onset of clinical manifestations and complications, the current progress of the care and the possibility of early detection [Citation11].
Procedure of HemoTypeSC phenotyping
Newborn screening was performed using the HemoTypeSC rapid phenotyping test. The kit was kept at room temperature (between 22°C and 33°C in Kisangani) and away from humidity. When opened, the test was used within 30 days. The strict respect for these conditions reassured us about the quality of the test and was proof that the test was done correctly and met the manufacturer’'s instructions [Citation5,Citation16]. A sample of 1.5 µL of whole blood, collected from the newborn’'s heel or the finger of the parent or older child, was taken for testing in situ. During the preliminary study before the Covid-19 pandemic, we also kept the blood drop on blotting paper (Whatman 903) for confirmation of results by LC-MS at the CHU of Liege [Citation13,Citation19]. This was no longer possible because of the COVID-19 pandemic. The various stages of carrying out the test and the principle of reading the result have been described by Mukherjee [Citation2], Olatunya [Citation16], Kasai [Citation19], and Danho [Citation24]. The result was immediately communicated to the parents by the investigator after having clarified to them the concepts poorly understood during the collective lesson. When this result was positive (SS), the investigator persuaded both parents to be tested with HemoTypeSC. Also, parents who received a genetic counseling quickly planned to consider care for their children within three months, depending on the results.
Statistical analyses of data
The sociodemographic and blood data were edited, entered in Excel Windows, then transferred to version SPSS v20 and the Epi info® 7.2.2.6 software (CDC, 2018). Categorical variables were presented in numbers and percentages. We set the significance level at the value of P < 0.05 for the Chi-square test.
Results
Sociodemographic characteristics
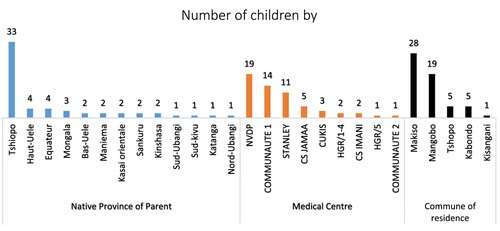
The sample consisted of 58 children born to 46 known mothers with a mean age of 31.4 ± 7.5 (18–45) years, and 37 known fathers. and present the distribution frequencies of sociodemographic characteristics of parents. It appeared that most parents were catholic (22.4%) and Assemblée (25.9%) worshipers, nurses (17.2%) and students (12.1%), of Topoke (25.9%) and Lokele (13.8%) ethnic, with superior education level (31%), natives of Tshopo province (n = 33), residents of Makiso (n = 28), and Mangobo (n = 19) commune. The majority of cases were found in three hospitals.
Table 1. Distribution frequency of parents’ characteristics for 58 children.
Paired profile of child–parent genotypic pedigree
shows the distribution frequency of the parent's genotype in relation to the offspring's genotype AA, AS, and SS. Globally, there were 5(9.6%) AA/AA, 5(9.6%) AA/SS, 1(1.9%) SS/AA, 37(71.1%) AS/AS, and 4(7.6%) AS/xx couples with unknown fathers who could be AS or SS or beta thalassemic or any other hemoglobinopathies carrier. Out of 58 children screened, 41(70.7%) were homozygotes SS, of whom 37(90.2%) were born to parents AS/AS and 4(9.7%) were born to mother AS with an untested father. There were 5(8.6%) AA-children born to AA/AA-parent, 12(20.7%) AS-children born to AA/SS (11), and SS/AA-parent (1). The pedigree of all children has been confirmed; no ambiguous cases were detected.
Table 2. Distribution frequency of parent and child genotype result by child characteristics.
As shown in for SS children, no statistical difference was observed between girls and boys or between age groups. We observed that the educational level of the parents included in this study was significantly associated with couples with a high risk of having SS children. The proportion of HbSS children born to university-level parents is significantly lower than HbSS children born to less educated parents (OR 0.169; p = 0.0049). The profile is likely different comparing the two main medical centers and two main communes of residence.
Table 3. Factors related to the risk of encountering HbSS children than AS or AA.
Discussion
Neonatal screening is the key to the early management of children with SCD (SS, SC) [Citation14,Citation18,Citation21]. For countries with limited resources, World Health Organization (WHO) recommends the use of rapid phenotyping tests based on lateral flow immunoassay devices (i.e. HemoTypeSC) or chromatographic immunoassay approach (i.e. Sickle Scan) [Citation11], to be validated for use in Africa, namely low cost, easy storage, sensitive, specific, capable of diagnosing HbS and HbC [Citation4]. HemoTypeSC allows rapid detection of Hb phenotypes including HbAA, HbSS, HbSC, HbCC, HbAS, and HbAC [Citation1,Citation24]. We currently use HemoTypeSC in Kisangani, a POC type test that has been validated locally [Citation13,Citation19], and in some SSA countries, such as Nigeria [Citation1,Citation16], Ghana [Citation15], and Ivory Coast [Citation24]. It is the only test currently suitable for neonatal screening for SDC [Citation13,Citation15,Citation16]. However, regardless of its performance, the authors require that its result be confirmed, as the same for each POC type test, by a molecular or electrophoresis -type test, HPLC, LC-MS before considering these children as sick and aligning them for regular medical monitoring [Citation3,Citation4,Citation6,Citation14,Citation17,Citation18,Citation24]. It is known that HemoTypeSC does not identify all types of hemoglobinopathies from HbSS and identifies these cases as the HbSS phenotype [Citation2]. However, these hemoglobinopathies are almost non-existent or very rare in DRC [Citation10,Citation13].
This study aimed to demonstrate the feasibility of validating the HemoTypeSC result of newborn-SS infants by testing the parents to confirm the pedigree before initiating early care in a context of limited resources. The immediate analysis of the Hb of the parents, also carried out by the HemoTypeSC, made it possible to confirm the result. According to Couque N and De Montalembert M, when confirmation by accuracy tests is not possible, resorting to parental Hb analysis is an alternative to confirm the results and determine the genotype of affected children [Citation25]. Tshilolo’'s series [Citation10] confirmed the result 6 months later, using the IEF as first-line neonatal screening. In our series, we used HemoTypeSC itself in newborn and their parents. Because of the COVID-19 pandemic, 60% of mothers refused any extra blood collection other than the drop for the on-site test. They feared sending blood samples for experiments to set up a COVID-19 vaccine as circulated by fake news on social media [Citation8], and this no longer allowed sending samples for confirmation. With our approach, we found an alternative of confirming the results of SS newborns and initiating their care within three months without wasting time.
Of 8(19.5%) SS newborns having performed the primary screening and 33(80.5%) SS children with status already known using LC-MS at CHU of Liege [Citation13,Citation19] but aligned to validate the HemoTypeSC result, 100% of their parents were carriers of sickle cell trait (SCT). Furthermore, all the descendants of known AA/SS or SS/AA couples, whose phenotype was determined by the LC-MS test [Citation13,Citation19], were diagnosed as heterozygous. In homozygotes, the sensitivity and specificity of HemoTypeSC is 100% [Citation1,Citation15,Citation24]. Genetically, children with SS are always born to parents who each carry at least one sickle cell trait (SCT or SCD). At the same time, descendants of marriage between sick homozygous (SS) and healthy homozygous (AA) should all be carriers of the SCT [Citation11,Citation14].
Furthermore, some key observations were raised from this study. Concerning the study population, our observation showed a high prevalence of the SCT among Topokes (25.9%) and Lokeles (13.8%). In the same city, Batina et al. [Citation26] observed a high prevalence among Mboles (33.3%), Topokes (23.3%), and Lokeles (19.5%). They attributed this to consanguinity following endogamic marriage [Citation26]. However, the demographic weight of these ethnic groups in the city of Kisangani is a factor to be taken into account as well. SS children were more screened at NVDP (32.7%) and Stanley clinic (18.9%), two pediatric structures receiving sick children of all ages, including SCD. In the Batina series, they were more numerous at the Provincial General Hospital (PGH) of Kisangani (30.6%) and at the General Reference Hospital of Makiso-Kisangani (HGR /Mak-Kis) (25.8%) because it was neonatal screening which targeted maternity hospitals [Citation26]. In general, it emerges from this study that SS children come more from families with a low level of education. We assume that this result is most likely related to the composition and size of our sample. However, Kambale-Kombi et al. observed that approximately 85.6% of respondents were unaware of the risk of children developing SCD when both parents are carriers of the SCT [Citation27]. The government must identify effective communication channels to raise awareness among different population segments on the importance of pre-marital testing and the mode of transmission of this disease [Citation8,Citation28]. In our case, parents had previously benefited from awareness sessions initiated by a team of gynecologists to persuade pregnant women to get tested and have their newborns tested for SCD within 72 h of birth. The period immediately following neonatal screening is ideal for the health care provider to begin informing families of children with potential health complications and reproductive considerations [Citation13].
In Africa, mothers are vulnerable because, in many cultures, the transmission of blood diseases is blamed on them. It is mandatory to explain genetic transmission to parents knowing that it is difficult because they will not admit to having transmitted the disease even though they are in good health [Citation21]. In this study, comparing the results of parents with those of their newborns made it possible to establish the responsibilities of two parents through basic notions of genetics. It is recommended to help family members differentiate an AS child from an SS child and show parents and possible newborns’ different implications for family planning. Each case of the SCT detected is an opportunity to educate a family about the possible associated health outcomes and the possibility of having another carrier or sick child [Citation13,Citation28].
There are three limitations of this study: The first is the small sample size which did not allow the application of a rigorous statistic to affirm or deny our conclusion or extrapolate our results to the whole region. A longitudinal study is essential.
The second limitation is that in the DRC inter-ethnic marriages are so frequent that a child can belong to 3 or more tribes at the same time. This is a major limitation of this study and suggests that results related to the distribution of SCD by ethnicity should be interpreted with caution.
The third limitation is that one of the planned analyses (LC-MS), confirmation of all POC test results by definitive testing, was not able to be performed due to the challenges of the COVID-19 pandemic. This confirmation would have been performed to determine the status of heterozygous children [Citation20].
Conclusion
The finding provides a proof-of-concept for implementing HemoTypeSC, test as a first-line test without resorting to accuracy confirmatory tests. The test helps also to facilitate fathers’ understanding of their responsibility in transmitting this disease. The association of the analysis of the parents’ Hb is a novelty here because that is not done in current practice. With the advent of a free POC type test, this has become possible.
Acknowledgements
The authors are grateful to the Academy of Research and Higher Education of the Belgian Development Cooperation for supporting the PRD-DREPAKIS project and the Synergie Save Drepachild project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Oluwole EO, Adeyemo TA, Osanyin GE, et al. Feasibility and acceptability of early infant screening for sickle cell disease in Lagos, Nigeria – a pilot study. PLoS ONE. 2020;15(12):e0242861. DOI:10.1371/journal.pone.0242861
- Mukherjee MB, Colah RB, Mehta PR, et al. Multicenter evaluation of HemoTypeSC as a point-of-care sickle cell disease rapid diagnostic test for newborns and adults across India. Am J Clin Pathol. 2020;153(1):82–87. DOI: 10.1093/ajcp/aqz108
- McGann PT, Schaefer BA, Paniagua M, et al. Characteristics of a rapid, point-of-care lateral flow immunoassay for the diagnosis of sickle cell disease. Am J Hematol. 2016;91:205–210. DOI:10.1002/ajh.24232
- Mulumba LL, Wilson L. Sickle cell disease among children in Africa: an integrative literature review and global recommendations. Int J Africa Nurs Sci. 2015;3:56–64. DOI:10.1016/j.ijans.2015.08.002
- Nnodu OE, Sopekan A, Nnebe-Agumadu U, et al. Implementing newborn screening for sickle cell disease as part of immunization programmes in Nigeria: a feasibility study. Lancet Haematol. 2020;7:e534–e540.
- Hsu L, Nnodu OE, Brown BJ, et al. White paper: pathways to progress in newborn screening for sickle cell disease in sub-Saharan Africa. J Trop Dis. 2018;6:260. DOI:10.4172/2329-891X.1000260
- McGann PT. Time to invest in sickle cell anemia as a global health priority. Pediatrics. 2016;137(6):e20160348. DOI:10.1542/peds.2016-0348
- Kasaï ET, Opara JPA, Agasa SB, et al. Acceptabilité du Dépistage néonatal de la drépanocytose au cours de la Pandémie au Covid-19 à Kisangani, en République Démocratique du Congo. Pan African J Med. 2020;37:299. DOI:10.11604/pamj.2020.37.299.26654
- Aloni MN, Kadima BT, Ekulu PM, et al. Acute crises and complications of sickle cell anemia among patients attending a pediatric tertiary unit in Kinshasa, Democratic Republic of Congo. Hematol Rep. 2017;9(2):6952. DOI:10.4081/hr.2017.6952
- Tshilolo L, Aissi LM, Lukusa D, et al. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: experience from a pioneer project on 31 204 newborns. J Clin Pathol. 2009;62:35–38. DOI:10.1136/jcp.2008.058958
- Balédent F. Génétique et biologie de la drépanocytose. France. Santé et Développement, n°. 2006;182. [Cited 2022 January 3]. Available from: https://devsante.org/articles/genetique-et-biologie-de-la-drepanocytose
- Ojodu J, Hulihan MM, Pope SN, et al. Incidence of sickle cell trait – United States, 2010. Morb Mortal Wkly Rep. 2014;63(49):1155–1158.
- Kasai ET, Opara JPA, Kadima JN, et al. Overview of current progress and challenges in diagnosis, and management of pediatric sickle cell disease in democratic republic of the Congo. Hematology. 2022;27(1):132–140. DOI:10.1080/16078454.2021.2023399
- Olatunya OS, Babatola AO, Ogundare EO, et al. Perceptions and practice of early diagnosis of sickle cell disease by parents and physicians in a southwestern state of Nigeria. Scientific World J. 2020; ID 4801087, 7 pages. DOI:10.1155/2020/4801087
- Steele C, Sinski A, Asibey J, et al. Point-of-care screening for sickle-cell disease in low-resource settings: a multi-center evaluation of HemoTypeSC, a Novel Rapid Test. Am J Hematol. 2018: 1–7. DOI:10.1002/ajh.25305
- Olatunya OS, Albuquerque DM, Fagbamigbe AF, et al. Diagnostic accuracy of HemoTypeSC as a point-of-care testing device for sickle cell disease: findings from a southwestern state in Nigeria and implications for patient care in resource poor settings of sub-Saharan Africa. Glob Pediatr Health. 2021;8:1–10. DOI:10.1177/2333794X211016789
- Tshilolo L, Kafando E, Sawadogo M, et al. Neonatal screening and clinical care programmes for sickle cell disorders in sub-Saharan Africa: lesson for pilot studies. Public Health. 2008;122(9):933–941. DOI:10.1016/j.puhe.2007.12.005
- Bond M, Hunt B, Flynn B, et al. Towards a point-of-care strip test to diagnose sickle cell anemia. PLoS ONE. 2017;12(5):e0177732. [Cited 2022 January 11]. Available from: doi:10.1371/journal.pone.0177732
- Kasaï ET, Boemer F, Djang’eing’a RM, et al. Systematic screening of neo- natal sickle cell disease with HemoTypeSC Kit-Test: case study and literature review. Open J Blood Dis. 2020;10:12–21. DOI:10.4236/2Fojbd.2020.101002
- Abdala AK, Shongo MYP, Tshilolo LMM, et al. Place de l’HemoTypeSC dans le dépistage de la drépanocytose à Kindu, République Démocratique du Congo. Revue de l’Infirmier Congolais. 2021;5(1):51–55.
- Mbiya BM. Suivi clinique, hématologique et identification de la fréquence des indications d’un traitement par hydroxy urée chez les patients drépanocytaires dans une ville reculée d’un pays à faibles ressources [Thèse]. Bruxelles: Université Libre de Bruxelles-Faculté de Médecine; 2021.
- Luboya E, Tshilonda JCB, Ekila MB, et al. Répercussions psychosociales de la drépanocytose sur les parents d’enfants vivant à Kinshasa, République Démocratique du Congo: une étude qualitative. Pan Afr Med J. 2014;19(5). DOI:10.11604/pamj.2014.19.5.2830
- Wikimédia Commons. Religion en république démocratique du Congo, 2021. [cited 2022 January 20]. Available from: https://fr.wikipedia.org/wiki/Religion_en_r%C3%A9publique_d%C3%A9mocratique_du_Congo
- Danho JBK, Atiméré YN, Koné D, et al. Feasibility study of the ‘HemoTypeSC’ test for the rapid screening of sickle cell disease in Côte D’Ivoire. Adv Hematol. 2021;2021:17. DOI:10.1155/2021/8862039
- Couque N, De Montalembert M. Diagnostic d’une hémoglobinopathie. Hématologie, Hémoglobinopathie; Feuillets de Biologie. 2013;54(311):5–18.
- Agasa B, Bosunga K, Opara A, et al. Prevalence of sickle cell disease in a northeastern region of the Democratic Republic of Congo: what impact on transfusion policy? British Blood Transfusion Society, Transfusion Medicine. 2010;20:62–65. DOI:10.1111/j.1365-3148.2009.00943.x
- Kambale-Kombi P, Djang’eing’a RM, Opara JPA, et al. Students’ knowledge on sickle cell disease in Kisangani, Democratic Republic of the Congo. Hematology. 2020;25(1):91–94. DOI:10.1080/16078454.2020.1727174
- Mandu K, Tusuubira SK, Mwambi B, et al. To test or not: occurrence of sickle cell trait and assessment of the awareness toward its screening among patients attending Magale health center iV, namisindwa District, eastern Uganda. J Blood Med. 2018;9:219–225. DOI:10.2147/JBM.S177203