ABSTRACT
Purpose Our aim is to analyze the clinical characteristics and prognostic factors of Epstein-Barr (EB) virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in children. Methods Children with newly diagnosed HLH were retrospectively analyzed. Results Finally, a total of 95 children with HLH were enrolled in this study, including 43 (45.3%) with EBV-HLH and 52 (54.7%) with non-EBV-HLH. Laboratory tests showed that the levels of absolute neutrophil count (ANC) decrease (P = 0.031) and triglycerides (TG) increase (P = 0.036) in the EBV-HLH group were statistically significant compared with those in the non-EBV-HLH group, respectively. We found that the remission rate during induction period in the EBV-HLH group and non-EBV-HLH group was 75.8% and 89.3%, respectively. The correlation analysis showed that the elevated degree of total bilirubin (TBIL) (P = 0.042), triglyceride (TG) (P = 0.009), serum ferritin (SF) (P = 0.008) and interleukin-8 (IL-8) (P = 0.004) were related with the remission rate during induction in the EBV-HLH group. Further univariate analysis showed that the elevated degree of TBIL (P = 0.048) and TG (P = 0.019) were significant risk factors for the remission rate during induction in the EBV-HLH group. In the multivariate analysis, we observed that there was statistical significance for the degree of TG elevation (P = 0.015) between the two groups. The correlation analysis showed that the elevated degree of TBIL (P = 0.030), the elevated degree of SF (P = 0.020) and the elevated degree of interleukin-6 (P = 0.010) were related to the induction mortality of children with EBV-HLH. Conclusion TBIL and TG are valid indicators to assess the efficacy and prognosis of EVBHLH among children in induction period.
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disease characterized by excessive activation of cytotoxic T lymphocytes (CTL), natural killer (NK) cells, and macrophages, leading to hypercytokinemia and immune-mediated impairment of multiple organ functions [Citation1]. Clinical characteristics include persistent fever, peripheral cytopenia, hepatosplenic lymph node enlargement, hepatic impairment, and coagulation abnormalities. The age of onset can be seen in all age groups, but is more common in children [Citation2]. Given the high incidence of EBV-HLH in Asia, it warrants further study. In this study, we intend to retrospectively analyse the clinical and laboratory data of 95 children with HLH in our department from January 2009 to September 2020, and divide them into EBV-HLH and non-EBV-HLH groups according to the causative factors. By comparing and analysing the clinical information and laboratory data, remission rate during the induction period and mortality rate of the two groups, and performing risk factor analysis related to the prognosis of EBV-HLH, we will provide a basis for the clinical diagnosis and treatment of EBV-HLH in children.
2. Patients and methods
2.1. Patients
Children with newly diagnosed HLH and under 15 years old between January 2009 and September 2020 from Children’s Medical Center of Sun Yat-sen Memorial Hospital were recruited.
2.2. Diagnosis of HLH
The diagnosis of HLH is currently based on the diagnostic criteria of HLH-2004 [Citation3]. The diagnosis HLH can be established if one of either 1 or 2 below is fulfilled, of which molecular biological evidence is an important basis for the diagnosis of primary hemophagocytic lymphohistiocytosis (pHLH).
Molecular biology meets the diagnostic criteria, i.e. detection of gene mutation associated with HLH included PRF1, UNC13D, STX11, STXBP2, SH2D1A, BIRC4/XIAP, ITK deficiency, CD27 deficiency, MAGT1, GS-2, CHS-1, HPS-II, CHS1/LYST, Rab27a and AP3β 1.
Diagnostic criteria for HLH fulfilled (five out of the eight criteria below)
① Persistent fever over than seven days (Temperature over than 38.5°C); ② Splenomegaly; ③ Cytopenias (affecting two of three lineages in the peripheral blood): Hb <90 g/L (in infants <4 weeks: Haemoglobin (Hb) <100 g/L), Platelets (PLT) <100 × 109/L, absolute neutrophils counts (ANC) <1 × 109/L; ④ Hypertriglyceridemia and/or hypofibrinogenemia: Fasting triglycerides ≥3.0 mmol/L (i.e. ≥265 mg/dl), Fibrinogen ≤1.5 g/L; ⑤ Hemophagocytosis in bone marrow or spleen or lymph nodes; ⑥ Low or absent NK-cell activity (according to local laboratory reference); ⑦ Ferritin ≥500 mg/L; ⑧ Soluble CD25 (i.e. soluble IL-2 receptor) ≥2400 U/mL.
2.3. Inclusion criteria
New-onset children who met the International Histiocyte Society HLH-2004 diagnostic criteria.
2.4. Exclusion criteria
① Those with missing research data; ② Treated in an outside hospital; ③ Death before treatment; ④ Severe multi-organ dysfunction; ⑤ Patients who withdrew from treatment midway.
2.5. HLH typing criteria
Disease severity was typed according to the CCLG-HLH-2018, as described in Supplementary Table S1 and .
2.6. Data collection
We collect general information such as gender, age, family history and clinical symptoms such as fever, hepatomegaly, splenomegaly, superficial lymph node enlargement, jaundice, skin rash, plasma cavity effusion, central nervous system (CNS) symptoms. As for laboratory findings, we collect blood routine, liver function which included alanine aminotransferase (ALT), glutathione aminotransferase (AST), albumin (ALB), γ-glutamyl transferase (γ-GGT), serum total bilirubin (TBIL), and triglycerides (TG). Coagulation function include prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB). In addition, inflammatory factors which included soluble interleukin 2 receptor (sIL2R/sCD25), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumour necrosis factor-α (TNF-α), serum ferritin (SF), hemophagocytosis, whole blood/plasma EBV-DNA load and gene mutation associated with HLH.
2.7. Therapeutic regimen
Induction and maintenance treatment was performed according to HLH-1994, HLH-2004, and Chinese Children’s Histiocytic Group (CCHG)-HLH-2018 regimen. Due to the small number of cases, all those who were given VP16 treatment were grouped into the VP16-containing group. Treatment was divided into two groups, the VP16-containing group (including HLH-1994 regimen, HLH-2004 regimen, and CCHG-HLH-2018 regimen) and the other group (mainly using glucocorticoids) with anti-infective and supportive therapy (including anti-infective therapy, immunoglobulin therapy, component transfusion, and plasma filtration). Finally, efficacy was assessed by conventional methods [Citation4], and the two groups were compared for induction period. The remission rate and the mortality rate of children during the induction period were compared between the two groups, and the prognosis-related risk factors were analysed.
2.8. Efficacy assessment
In induction therapy, it is recommended that efficacy be evaluated once at week 1, week 2 and week 4. Evaluation indicators include sIL2R, SF, blood routine, TG, transaminases, hemophagocytic phenomenon, mental status (in patients with CNS-HLH).
Complete response (CR) which means all indicators returned to normal.
Partial response (PR), includes ≥2 symptoms/laboratory indicators improved by 25% or more, and partial indicators need to meet the following criteria, ① sCD25 level decreased by more than 1/3, ② SF and TG decreased by more than 25%, ③ Under non-transfusion conditions: ANC <0.5 × 109/L, need to rise by 100% and >0.5 × 109/L. If ANC between 0.5 × 109/L and 2.0 × 109/L, need to increase 100% and return to normal, ④ ALT >400 U/L, need to decrease more than 50%.
No response (NR), which means those whose disease continues to progress, die or fail to achieve PR during induction therapy.
2.9. Statistical analysis
For measurement (quantitative) data that met normal distribution and satisfied the chi-square, independent samples t-test was used for comparison between groups, and mean ± standard deviation (X ± s) was used for descriptive statistics. When normal distribution was satisfied but the variance was not chi-square, corrected test was used for comparison between groups. For count data that were not normally distributed overall or the overall distribution was unknown, Wilcoxon rank sum test was used for comparison between groups, and descriptive statistics were used for median or quartiles. The Pearson χ2 test was used for intergroup comparisons of count (qualitative) data. The corrected formula was used for the total number of cases (n ≥ 40), but the theoretical frequency was less than 5. The Fisher’s cut-off probability method was used for the total number of cases (n < 40), and the theoretical frequency was less than 5. Univariate or multivariate logistic regression analyses were used to analyse the risk factors related to the prognosis of children with HLH and the difference factors between groups. All data were analysed using SPSS 25.0 statistical software developed by IBM, and the results were considered significant at P < .05.
3. Results
3.1. General information
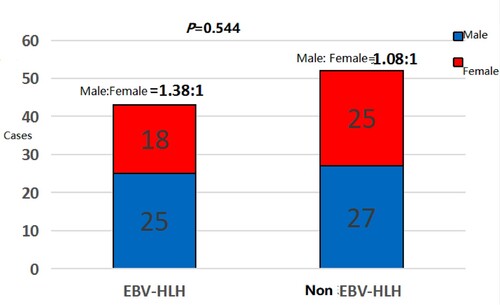
A total of 95 children with HLH were recruited from January 2009 to September 2020, including 43 cases (45.3%) with EBV-HLH and 52 cases (54.7%) with non-EBV-HLH. There were 52 (54.7%) male and 43 (45.3%) female children, the youngest being 1 month old and the oldest being 15 years old, with a median age of onset of three years. None had a family history of HLH. Among non-EBV-HLH group, there were 35 cases (67.3%) of non-EBV infection-related HLH, 9 cases (17.3%) of malignancy-related HLH, 6 cases (11.5%) of macrophage activation syndrome, and 2 cases(3.9%) of drug-related HLH.
3.2. Clinical presentation
Ninety-five cases (100%) had HLH with persistent fever >7 days, temperature between 38.5°C and 42°C, hepatomegaly in 86 cases (90.5%), splenomegaly in 78 cases (82.1%), lymph node enlargement in 45 cases (47.4%), jaundice in 11 cases (11.6%), pleural effusion in 10 cases (10.5%), rash in 9 cases (9.5%), and CNS symptoms in 8 cases (8.4%). Among them, the above clinical presentation was compared between the EBV-HLH and non-EBV-HLH groups (). Comparative analysis of the two groups revealed that the incidence of splenomegaly, jaundice, and CNS symptoms was higher in the EBV-HLH group than in the non-EBV-HLH group, but there was no statistically significant difference (P > .05). Twenty-nine cases (67.4%) were heavier in the EBV-HLH group than in the non-EBV group (53.8%), but there was no statistically significant difference between the two groups (P > .05).
Table 1. Comparison of clinical presentation in the EBV-HLH group and the non-EBV-HLH group.
3.3. Laboratory examination results
Routine blood, liver function, coagulation function, SF, bone marrow routine, and gene mutation associated with HLH were performed on 95 children. The comparison of the experimental results between the two groups () showed that there were differences in the data of several laboratory test results between the two groups, but they were not statistically significant (P > .05). Further logistic regression analysis of the above laboratory test data between the two groups was performed to find the difference factors, and it was found that the differences in the degree of ANC decrease (P = .031) and TG elevation (P = .036) were statistically significant (), suggesting that the degree of ANC decrease and TG elevation were more significant in children with EBV-HLH at the initial diagnosis.
Table 2. Comparison of laboratory data in the EBV-HLH and non-EBV-HLH group.
Table 3. Laboratory results of the EBV-HLH group and non-EBV-HLH about laboratory results.
3.4. Gene mutation associated with HLH
A total of 28 cases were sent for testing the gene mutation associated with HLH in this study from 2017 to 2020, and 17 (60.7%) cases were positive. While 15 cases were detected in the non-EBV-HLH group, and 8 cases were positive, with a positive rate of 53.3%, which were higher than those in the EBV-HLH group (9/13, 69.2%). There were 10 cases (35.7%) of UNC13D missense mutations, the highest mutation frequency in this study, 6 cases (21.4%) of LYST missense mutations, 5 cases (17.9%) of STXBP missense mutations, 4 cases (14.3%) of AP3β1 missense mutations, and 2 cases (7.1%) of XIAP missense mutations. Two cases of pHLH were diagnosed, both of which were EBV-HLH, one of which. The other pHLH case was a pathological mutation of XIAP hemizygous, and the family survey confirmed that the mutation originated from the mother. The remaining cases had genetic missense mutations but were not diagnosed with primary HLH because they were all single nucleotide polymorphism (SNP) loci and the mutations were not pathogenic.
3.5. Flow cytometry assay
NK cell activity was detected in 15 cases, including 10 cases in the EBV-HLH group and 5 cases in the non-EBV-HLH group. NK cell activity was reduced in six cases among the EBV-HLH group, and three cases in the non-EBV-HLH group. Flow cytometry detected ΔCD107a in 13 cases and 3 out of 5 cases detected in the EBV-HLH group were reduced, with a positive rate of 60%. When in the non-EBV-HLH group, one out of four cases detected were reduced, with a positive rate of 25%. Due to the small number of cases, statistical analysis was not performed in this study.
3.6. Efficacy
3.6.1. Comparison of remission rate and mortality during the induction period in the EBV-HLH group and the non-EBV-HLH group
The remission rate in the EBV-HLH group was 75.8%, which was lower than that in the non-EBV-HLH group (89.3%), but there was no statistically significant difference between the remission rates of the two groups (P > .05). The mortality rates during the induction period were 17.2% and 14.3% in the EBV-HLH and non-EBV-HLH groups, respectively, with no statistically significant differences between the two groups (P > .05).
3.6.2. Analysis of factors related to remission rate and mortality during the induction period in the EBV-HLH group
The correlation analysis of experimental results and remission rate during the induction period in the EBV-HLH group revealed that the degree of TBIL elevation (P = .042), TG elevation (P = .009), SF elevation (P = .008), IL-8 elevation (P = .004) were associated factors affecting the induction remission rate in children with EBV-HLH, and the differences were all significant (). Further univariate analysis was performed to find the independent risk factors affecting the induction remission rate of children in the EBV-HLH group, and TBIL elevation (P = .048) and TG elevation (P = .019) were found to be the independent risk factors affecting the induction remission rate of children in the EBV-HLH group, with significant differences (). A multivariate regression analysis of TBIL and TG levels revealed that the difference in the degree of TG elevation (P = .015) was still statistically significant ().
Table 4. Laboratory results and remission rate in 43 EBV-HLH cases.
Table 5. Independent risk factors affecting the rate of induction remission in the EBV-HLH group.
Table 6. Multivariate analysis of independent risk factors affecting the rate of induction phase remission in the EBV-HLH group.
3.6.3. Effect of VP16-containing regimen of EBV-HLH group during the induction period
In 29 patients who used VP16, 22 (75.9%) patients achieve CR with a mortality rate of 17.2% (5/29). While in 14 cases without VP16, 11 (78.5%) patients achieve CR, with a mortality rate of 14.3% (2/14). However, the difference was not statistically significant (P > .05).
3.6.4. Effect of EBV-DNA on remission rate and mortality during the induction period
One of the important bases for determining whether EBV infection is whether the whole blood/plasma EBV-DNA copy number is elevated (≥1.0 × 103 copies/ml was considered positive in this study). Our study showed that the whole blood EBV-DNA was detected in 43 cases, with a positive rate of 100% and the load of EBV-DNA ranged from 1.1 × 103 to 6.7 × 108 copies/ml. In the meantime, plasma EBV-DNA was detected in 38 cases, with a positive rate of 71.0%, and EBV-DNA load was 1.0×103–2.9×106 copies/mL. The remission rate was significantly higher in the EBV-DNA load <106 copies/mL group than in the EBV-DNA load ≥106 copies/mL group.
There were four deaths in the EBV-DNA load ≥106 copies/mL group, and three deaths in the EBV-DNA load <106 copies/mL group, with a mortality rate of 16.7% and 15.7%, respectively. The difference between the two groups was not significant (P > .05). The remission rate was 70.4% in 27 cases with plasma EBV-DNA load ≥103 copies/mL and 19 cases in remission, and 87.5% in 16 cases with EBV-DNA load <103 copies/mL and 14 cases in remission. The remission rate during the induction period was higher in the group with plasma EBV-DNA load <103 copies/mL than in the group with EBV-DNA load ≥103 copies/mL, but the statistical difference was not significant (P > .05). There were four deaths and 14.8% mortality in those with plasma EBV-DNA load ≥103 copies/mL and three deaths and 18.8% mortality in those with plasma EBV-DNA load <103 copies/mL. The difference between the two groups was not significant (P > .05).
Plasma EBV-DNA load is a key indicator of whether EBV is in a proliferative and lytic state. Although the indicators of clinical staging do not include viral load, the active or inactive EBV may be related to the severity of the disease. The plasma EBV-DNA load was ≥103 copies/mL in 27 cases, of which 5 (18.5%) were mild and 22 (81.5%) were severe. The plasma EBV-DNA load was <103 copies/mL in 16 cases, of which 4 (25.0%) were mild and 12 (75.0%) were severe. The proportion of severe cases was higher in the positive group than in the negative group, but the statistical difference was not significant (P > .05).
4. Discussion
The early diagnosis of HLH is still difficult due to the lack of specific clinical manifestations and laboratory indices. A retrospective analysis of 142 children with EBV-HLH reported that HLH was characterized by fever (100%), hepatosplenomegaly (55–85%), and superficial lymph node enlargement (40–50.0%) as the main clinical manifestations [Citation5]. In this present study, all children with HLH showed persistent fever for more than 1 week and hepatosplenomegaly (82.1–90.5%), while the incidence of superficial lymph node enlargement was lower (47.4%), and this clinical feature is clearly different from infectious mononucleosis and deserves the attention of clinicians.
In the diagnosis and management of HLH, laboratory tests are indicators that clinicians pay close attention to. Although there is a lack of specific indicators in the diagnosis of HLH, progressive haematocrit reduction, elevated liver enzymes, and extremely elevated SF are notable features of HLH. In the current study, we found that the degree of haematocrit, liver injury, and abnormal coagulation in the EBV-HLH group was higher than that in the non-EBV-HLH group. Further regression analysis revealed the degree of ANC reduction at the initial diagnosis and the degree of TG elevation were statistically significant, suggesting that children with a significant degree of ANC decline and TG elevation at initial diagnosis should be alerted to the possibility of EBV-HLH. Allen et al. [Citation6] evaluated 330 patients with SF above 500 µg/L and concluded that SF above 10,000 µg/L had a sensitivity of 90% for HLH and a specificity of 96%. A Japanese single-centre study of 29 children with HLH and 3 adults with HLH suggested that serum IL-6 >300 ng/L reflects poor prognosis in patients with HLH in terms of the effect of cytokines on patient prognosis [Citation7]. In our study, we investigated the EBV-HLH group and found that TBIL, TG, SF, and IL-8 levels at primary treatment were associated with outcome of the children, and TBIL and TG levels were independent risk factors for prognosis, which are consistent with those reported in the previous literature [Citation8,Citation9]. In the analysis of the correlation between the results of experimental examinations and mortality during the induction period in the EBV-HLH group, the degree of elevated TBIL, elevated SF, and elevated IL-6 were significantly different, and our results were consistent with those reported in the literature [Citation10,Citation11]. TG was significant in both univariate and multivariate logistic regression analyses in this study, suggesting that TG is a prognostic correlate with strong clinical value.
The plasma/whole blood EBV-DNA load assay is a reliable basis for determining whether EBV infection is active or not, as EBV activity implies the amplification of viral load, and when the amplification reaches a certain level, the infected cells will undergo lysis and release recombinant EBV. Therefore, it is theoretically believed that the rise in EBV copy number in whole blood is earlier than in plasma, reflecting a higher sensitivity on EBV infection, and plasma EBV load is more important in reflecting whether the virus is active or not. Our study showed that the positivity rate of whole blood EBV-DNA and plasma EBV-DNA in the EBV-HLH group were 100% and 71.0%, respectively. Our present research also demonstrated that the remission rate during the induction phase was higher in the whole blood EBV-DNA load <106 copies/mL group than in the EBV-DNA load ≥106 copies/mL group without any significant difference due to the insufficient number of cases. Furthermore, no significant difference was in mortality between the two groups, which may be related to our aggressive antiviral therapy and timely use of chemotherapy containing VP16 regimen.
Positivity for gene mutation associated with HLH is the gold standard for the diagnosis of pHLH, which is highly prevalent in young children, and previous literature reported that 90% of pHLH develops within two years of age [Citation12-14]. Pulliye et al. [Citation15] reported that a 12-year-old boy with a final diagnosis of pHLH, and Clementi et al. [Citation16] reported two adult cases over 20 years of age, which were eventually confirmed by genetic screening to be pHLH due to mutations in the PRF gene. Therefore, genetic testing should be actively performed in children with early onset of pHLH, but pHLH should not be ruled out in teenager and adult patients, and genetic testing should be performed as much as possible. In this study, 28 cases of gene mutation associated with HLH were examined, and the positive rate in the EBV-HLH group was 69.2% compared with 53.3% in the non-EBV-HLH group, which was considered to be related to the fact that some of the patients with EBV-HLH were pHLH, suggesting that we should attach great importance to the genetic testing and the detection of HLH-related protein expression in children with EBV-HLH, so as to clarify the exact mechanism of phagocytosis and guide further treatment.
The HLH-2004 protocol study pointed out that NK cell activity was consistently reduced in EBV-HLH patients, especially in remission, and the diagnostic pHLH compliance rate was 94.4% [Citation17,Citation18], suggesting that NK cell activity assay has important clinical value for the diagnosis of HLH. The ΔCD107a assay is an important tool for rapid screening of pHLH associated with degranulation pathways (FHL-3 to FHL-5, CHS and GS-2) and can target a subset of pHLH patients early. Although the number of cases in this study was too small to draw definite conclusions, preliminary observations showed that the decrease in NK cell activity and the rate of ΔCD107a positivity were higher in the EBV-HLH group than in the non-EBV-HLH group, suggesting an underlying genetic background of EBV-HLH and that some of the EBV-HLH belong to pHLH, and this group of children has a poor prognosis and needs to undergo allo-HSCT. Because early identification of pHLH is important to save the life of the child, these tests should be performed as early as possible in children considered for EBV-HLH.
The early application of VP16 is important in the treatment of EBV-HLH, and Imashuku et al. [Citation19,Citation20] suggested that VP16 inhibits the synthesis of EBV core antigenic determinants in addition to efficiently suppressing excessive inflammatory responses and thus has a concurrent anti-EBV effect. The overall induction remission rate in our study was 76.8%, and the induction remission rate in the EBV-HLH group was 79.1%, which achieved a better outcome, probably due to the timely application of VP16 in severe children with confirmed EBV-HLH in our centre after diagnosis. Further studies showed that the induction remission rate in the EBV-HLH group with the VP16 regimen was lower than that in the non-EBV-HLH group, suggesting that EBV-HLH has a relatively poor prognosis, consistent with the literature [Citation21] ().
Table 7. Related laboratory results and induction period mortality in 43 EBV-HLH cases.
Ethics approval and consent to participate
The study protocol and informed consent were approved by the Ethics Committee of Sun Yat-sen Memorial Hospital. All patients, or the patients’ parents/guardians, provided written informed consent.
Supplemental Material
Download MS Word (40.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are available from the corresponding author for the reasonable reason.
Additional information
Funding
References
- Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol Mech Dis. 2018;13:1.1–1.23.
- Zaher K, Otrock, Daver N, et al. Diagnostic challenges of hemophagocytic lymphohistiocytosis. Clin Lymphoma Myeloma and Leuk. 2017;17:105–110.
- Henter JI, Horne AC, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131.
- Imashuku S, Teramura T, Tauchi H, et al. Longitudinal follow up of patients with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Haematologica. 2004;89(2):183–188.
- Kogawa K, Sato H, Asano T, et al. Prognostic factors of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in children: report of the Japan histiocytosis study group. Pediatr Blood Cancer. 2014;61(7):1257–1262.
- Allen CE, Yu X, Kozinetz CA, et al. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50:1227–1235.
- Fujiwara F, Imashuku S. Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15(1):92–98.
- Khare N, Jinkala SR, Kanungo S. Performance of HScore in reactive hemophagocytic lymphohistiocytosis. Indian J Hematol Blood Transfus. 2021;37(2):256–263.
- Smits BM, van Montfrans J, Merrill SA, et al. A minimal parameter set facilitating early decision-making in the diagnosis of hemophagocytic lymphohistiocytosis. J Clin Immunol. 2021;41(6):1219–1228.
- Zhao XX, Lian HY, Zhang L, et al. Significance of serum ferritin level in hemophagocytic lymphohistiocytosis diagnosis. Int J Lab Hematol. 2019;41(4):503–508.
- Shi J, Chu C, Yu M, et al. Clinical warning of hemophagocytic syndrome caused by Epstein-Barr virus. Ital J Pediatr. 2021;47(1):3.
- Nixon A, Roddick E, Moore K, et al. A qualitative investigation into the impact of hemophagocytic lymphohistiocytosis on children and their caregivers. Orphanet J Rare Dis. 2021;16(1):205. DOI:10.1186/s13023-021-01832-2. PMID: 33957935; PMCID: PMC8101208.
- Merli P, Algeri M, Gaspari S, et al. Novel therapeutic approaches to familial HLH (Emapalumab in FHL). Front Immunol. 2020;11:608492.
- Chang BS F, Morales CE A, et al. Secondary hemophagocytic lymphohistiocytosis in a young hispanic adult. Cureus. 2021;13(2):e13084.
- Puliyel MM, Rose W, Kumar S, et al. Prolonged neurologic course of familial hemophagocytic lymphohistiocytosis. Pediatr Neurol. 2009;41:207–216.
- Clementi R, Emmi L, Maccario R, et al. Adult onset and atypical presentation of hemophagocytic lymphohistiocytosis in siblings carrying PRF1 mutations. Blood. 2002;100:2266–2274.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019 Mar 15;7:71. doi:10.3389/fped.2019.00071.
- Popko K, Górska E, Wołowiec M, et al. Disturbances in NK cells in various types of hemophagocytic lymphohistiocytosis in a population of Polish children. J Pediatr Hematol Oncol. 2019;41(5):e277–e283.
- Imashuku S. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH): update 2010. Pediatr Hematol Oncol. 2011;33(1):35–39.
- Imashuku S, Kuriyama K, Teramura T, et al. Requirement for etoposide in the treatment of Epstein Barr virus associated hemophagocytic lymphohistiocytosis. J Clin Oncol. 2001;19(10):2665–2673.
- Zeng X, Wei N, et al. Treatment outcomes and prognostic analysis of 61 Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Zhong hua xue ye xue za zhi. 2015;36(6):507–510.