ABSTRACT
Background
HbA1c is the validated biomarker for glycemic management in diabetic individuals. Here, we report a compound heterozygote for β0-thal and Hb J-Lomeand evaluate its effect on HbA1c measurements.
Methods
A 51-year-old female was suspected of harboring a hemoglobin variant following no value of HbA1c levelby Arkray HA-8180 V (48s HbA1c mode), abnormal hematological data, and abnormalhemoglobin analysison capillary electrophoresis (Capillarys 2 Flex Piercing, Hb program). Sanger sequencing of the α and β genes was subsequently performed on the proband.HbA1c was reanalyzed using D10 (Bio-Rad), Capillarys 2 Flex Piercing (Sebia), and Roche Cobas c501 (Roche Diagnostics).
Results
Sanger sequencing identified a compound heterozygote for β0-thal [β17(A14) Lys > Stop, HBB: c.52A > T] and Hb J-Lome [β59(E3) Lys > Asn, HBB: c.180G > C].HbA1c values determinedby D10, Capillarys 2 Flex Piercing (HbA1c program), and Roche Cobas c501were 2.3%, no HbA1c value, and 5.1 (32 mmol/mol), respectively. During pedigree analysis, the son of the proband was found to have normal blood glucose (5.55 mmol/L), decreased HbA1c (3.6%, 16 mmol/mol)by Arkray HA-8180 V (48s HbA1c mode), an abnormal band on the electrophoretogram of Capillarys2 (Hb program), and the Hb J-Lome mutation in the β globin gene.Subsequently, HbA1c values determinedby D10, Capillarys 2 Flex Piercing (HbA1c program), and Roche Cobas c501 were4.0% (20 mmol/mol), no HbA1c value, and 5.0 (31 mmol/mol), respectively.
Conclusion
Atypically low HbA1c levels or a discrepancy between blood glucose and HbA1c levels should raise concerns about hemoglobin variations.
Hemoglobin variants are a group of prevailing inherited genetic defects caused by a point mutation of globin gene that produces an amino acid substitution [Citation1]. HbA1c, as a monitor for previous 2–3 months glucose levels, plays an important role in assessing glycaemic control and predicting the risk for the development of chronic complications [Citation2,Citation3]. Previous studies have shown that Hb variation may cause HbA1c values to not correspond to blood glucose levels in the same patient, and the degree of interference depends on the analysis method and the specificity of the variation [Citation4,Citation5]. In the present study, we discovered a variant with both Hb J-Lome and β0-thal interfered with the quantification of HbA1c.
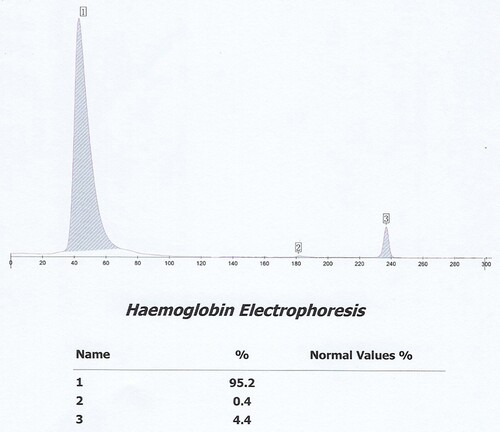
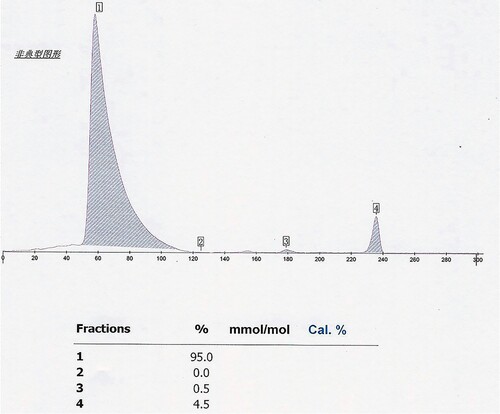
The proband is a 51-year-old female from Yunnan Province in China. She went to the Second Affiliated Hospital of Kunming Medical University for a routine health check. The hematological data of the proband suggested small cell hypochromic anemia. The following data included the red blood cell count (RBC): 5.06 × 1012/L (reference: 3.8-5.1 × 1012/L), Hb: 106 g/L (reference: 115-150 g/L), mean corpuscular volume (MCV): 68fL (reference: 82-100fL) and RBC distribution width: 14.0% (reference: 11.5-14.5%). Her HbA1c level provided no value by Arkray HA-8180 V (48s HbA1c mode, HA-8180 V, Tokyo, Japan). The cation exchange high-performance liquid chromatography (IE -HPLC) chromatogram from HA-8180 showed the usual position of HbA1c without a labeled peak, which indicated ‘abnormal separation’. The 75-g oral glucose tolerance test showed fasting plasma glucose of 5.35 mmol/L (reference: 3.9-6.1 mmol/L) and 2-hour postprandial plasma glucose of 6.63 mmol/L (reference: 3.90-7.80 mmol/L). We further investigated the other blood data included total bilirubin 16.1umol/L (reference: 3.4-20.5umol/L), direct bilirubin 5.1umol/L (reference: 0.0-6.8umol/L), indirect bilirubin 11.0umol/L (reference: 1.7- 13.7umol/L), ferritin (FER): 199 ng/ml (reference: 11-306.8 ng/ml) and transferrin (TRF): 1.77 g/L (reference: 2.02-3.36 g/L). Hemoglobin analysis revealed a significantly abnormal electropherogram assayed by Sebia CapillaryS2 (), comprising 95.2% (Hb X) of the total area in zone 14, 0.4% (Hb F) of the total area in zone F, and 4.4% (Hb A2) of the total area in zone E. So, we suspected that she might suffer from a common erythrocyte disorder-hemoglobinopathy.
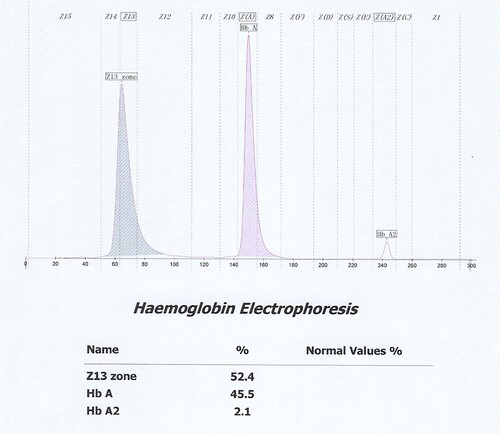
The findings triggered further detection of the proband for gene sequencing. DNA sequencing revealed that the α1 and α2 genes were normal. However, it showed two base substitutions in the β globin gene, an A > T transversion in the exon 1 at nucleotide 102 and a G > C transversion in the exon 2 at nucleotide 88, resulting in the substitution of the lysine at codon 17 by the stop codon () and the substitution of the lysine at codon 59 by asparagine () in the compound heterozygous state. To our knowledge, this compound heterozygous mutation β0-thal [β17(A14) Lys > Stop, HBB: c.52A > T] and Hb J-Lome [β59(E3) Lys > Asn, HBB: c.180G > C] has not been reported. Since this proband expresses only Hb J-Lome and not HbA, the glycated hemoglobin level of the proband should be discussed with Hb J-Lome1c. During pedigree analysis, the son of the proband was found to have normal blood glucose (5.55 mmol/L), decreased HbA1c (3.6%, 16 mmol/mol), and an abnormal band on the electrophoretogram () of Capillarys2 (Hb program) and the Hb J-Lome mutation in the β globin gene.Signs of iron-deficiency anemia were found in her son's hematological data(RBC 5.62 × 1012/L, Hb 116 g/L, MCV 70.6fL, FER 2.30 ng/ml, TRF 3.51 g/L).
Figure 1(B). DNA sequencing analysis showing the nonsense mutation [β17(A14) Lys > Stop, HBB: c .52A > T].
![Figure 1(B). DNA sequencing analysis showing the nonsense mutation [β17(A14) Lys > Stop, HBB: c .52A > T].](/cms/asset/a1bf3df5-8ffb-48c2-9071-c62be64b84e1/yhem_a_2114672_f0001b_oc.jpg)
Figure 1(C). DNA sequencing analysis showing the missense mutation [β59(E3) Lys > Asn, HBB: c.180G > C].
![Figure 1(C). DNA sequencing analysis showing the missense mutation [β59(E3) Lys > Asn, HBB: c.180G > C].](/cms/asset/e32933ac-60ca-4117-b8f5-10e766e08e4e/yhem_a_2114672_f0001c_oc.jpg)
In the follow-up analysis, the glycated hemoglobin level of the proband and her son was measured by alternative methods based on different analytical principles, including IE-HPLC on D10 (Bio-Rad, Hercules, CA, USA), capillary electrophoresis (CE) on CapillaryS 2 Flex Piercing (C2FP) (HbA1c program; Sebia, Lisses, France), and turbidimetric inhibition immunoassay (TINIA) on Roche Cobas c501 (Roche Diagnostics, Basel, Switzerland), respectively (). The proband's HbA1c measurements were 2.3% (D10) and 5.1%, 32 mmol/mol (Roche) and her sons were 4.0%, 20 mmol/mol (D10) and 5.0%, 31 mmol/mol (Roche). C2FP failed to detect HbA1c levels in the proband and her son. The immunodetection was valuable for measuring the precise determination of HbA1c because the HbA1c-specific antibodies were boundto the glycated synthetic peptides located at the N-terminus of the β chain and the amino acid substitution was far from the N-terminal portion of the β chain. So, Roche c501 was used as a comparative method to calculate the accuracy of the HbA1c result. According to the latest NGSP criterion of the accepted bias (within ±5.0%), the Hb variant generated unacceptable bias for D10 and C2FP.
Table 1. Comparison of HbA1c results by different methods.
Hb J-Lome (HBB: c.[180G > C or 180G > T]) is a fast hemoglobin discovered in 1977 by Wajcman et al. in Lome, Togo, Africa, and was later reported in China, Japan, and Vietnam [Citation6].According to the HbVar database, heterozygous HbJ-Lome hasnormal stability, oxygen-carrying capability, and clinical presentation [Citation6]. Therefore, we consider that the anemia in the son of the proband is due to iron deficiency.The proband showed mild anemia due to β0-thal. To some extent, the proband can be understood as a homozygous Hb J-Lome patient. Previous studies showed Hb J-Lome interfered with HbA1c measurement by HPLC [Citation7]. However, the interference of Hb J-Lome on HbA1c has not been determined by CE and immunoassayin previous studies. In the current study, a systematic analysis was performed to determine the HbA1c result of both variants.
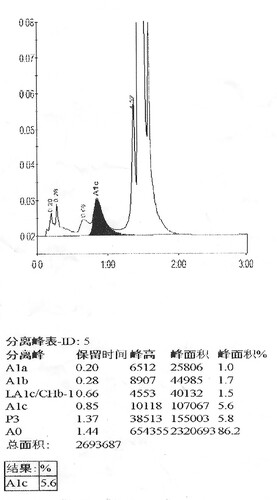
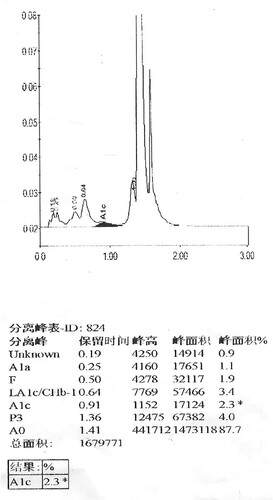
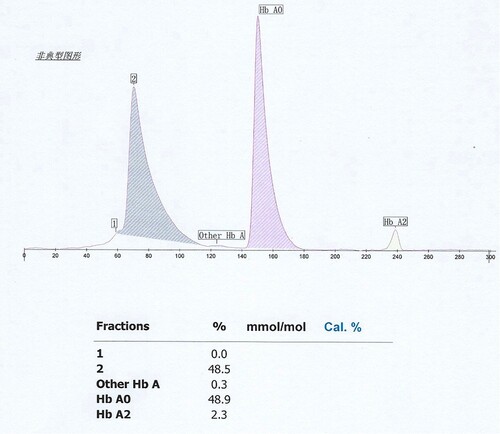
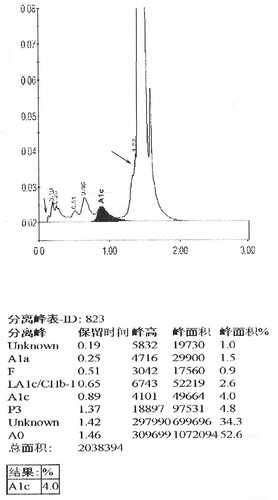
In this case, both IE-HPLC methods produced unacceptable deviations. Hb molecules are separated by IE-HPLC based on charge differences.Therefore, when the charged amount of the variant changes, it interferes with the IE-HPLC separation of the analyte from other hemoglobin fractions.For the IE-HPLC method, the A1c percentage is calculated as the A1c peak area divided by the area sum of A1a, A1b, LA1c, A1c, A0, and HbX(both HbX1c and unmodified HbX) are excludedfrom the calculation. When the peak of HbA1c cannot be completely separated from HbX, part of HbX is calculated into HbA1c, which leads to falsely elevated HbA1c values. Conversely, when HbX is calculated into the denominator of the formula, a spuriously reduced HbA1c value is produced.The Lysine residue at β59 is normally located in the Hb molecule peripheral and protrudes outside the Hb molecule [Citation8]. Amino substitution at this position may result in changes in the charge and mass of Hb. We requested the HPLC chromatogram of her son on D10 (). Compared to the normal chromatogramon D10 (), two abnormal peaks (HbJ-Lom1c and HbJ-Lome) were observed. We considered that the bias of the HbA1c level in her son was due to the incomplete separation of Hb J-Lome and HbA. When we add the Hb J-Lome1c peak and HbA1c peak area, the glycated hemoglobin level is the same as the result measured by the immunoassay, which proves our hypothesis. However, there is only Hb J-Lome in the proband, and the physicochemical properties of each component of Hb have changed, and the elution time has also changed in HPLC (), so the glycation level of this patient cannot be interpreted according to the conventional chromatograms. The HbA1c level of her son was assayed using C2FP (HbA1c program) with no HbA1c value and an abnormal electropherogram, Hb J-Lome could not be separated from HbA1c and Hb J-Lome1c (). As describe above, overlapping elution of HbJ-Lome1c and HbJ-Lome interfered with the proband's detection of glycated hemoglobin on C2FP, as seen in . Additionally, this explains why the electropherogram of glycated hemoglobin () in the proband and the electropherogram of hemoglobin () are so similar.
Figure 2(B). HbA1c analysis of her son using D10, arrow indicates the Hb J-Lome1c peak and Hb J-Lome.
In the presented study, the interference produced by both mutants resulted in false low values. As such, patients with abnormally low HbA1c levels can suspect Hb variations, or HbA1c results may differ dramatically from other metabolic control measurements. The IE-HPLC is the most common detection method for HbA1c, and it is also susceptible to interference from Hb variants [Citation9]. From another perspective, IE-HPLC has more advantages than other methods because it is easier to detect abnormal Hb. Therefore, it is particularly important for the laboratory to routinely inspect the chromatogram before issuing the report. In this case, repeat analysis should ideally be performed using methods based on different analytical principles because the effect of a specific Hb variant on HbA1c results is often method dependent. The interference generated from Hb variants depends not only on the analysis method but also on the peculiarity of the variant-such as hemoglobin lifetime. In the follow-up study, the negative results of the Heinz body test both indicated that Hb J-Lome is a stable variant. Negative results of the Heinz body test and hemolysis test suggest that the interference caused by this variant is not related to changes in red blood cell lifespan. For any situation that can cause an abnormal lifespan of red blood cells, the American Diabetes Association (ADA) only recommends the use of glucose criteria for the diagnosis of diabetes [Citation10]. When the interpretation of HbA1c is confused by factors that affect the lifespan of red blood cells, alternative non-Hb-based tests, such as glycosylated serum albumin (GSA), should be performed to assess long-term blood glucose levels.
Author contributions
Zixin Chen wrote the manuscript, researched data and interpreted the laboratory results. Limei Shao and Bingjie Ma contributed to the discussion. Mingfeng Jiang and Xuejiao Ba researched data and contributed to discussion. Run Ma contributed to review the manuscript. Hongfen Ping and Zuo Wang contributed to provide experimental data.
Acknowledgment
Thanks to Hongjin Shi (department of Urology, Kunming Medical University, Kunming, China) for valuable comments on manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Thom CS, Dickson CF, Gell DA, et al. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harbor Perspect Med. 2013 Mar 1;3(3):a011858.
- Nuttall FQ. Comparison of percent total GHb with percent HbA1c in people with and without known diabetes. Diabetes care. 1998 Sep;21(9):1475–80.
- Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Sep 30;329(14):977–86.
- Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008 Aug;54(8):1277–82.
- Dessi M, Pieri M, Pignalosa S, et al. Performances of capillary electrophoresis and HPLC methods in HbA1c determination: diagnostic accuracy in HbS and HbD-Iran variants’ presence. J Clin Lab Anal. 2015 Jan;29(1):57–60.
- Giardine B, Borg J, Viennas E, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014 Jan;42(Database issue):D1063–9.
- Oshima Y, Ideguchi H, Takao M, et al. A patient with a hemoglobin variant (Hb JLome) unexpectedly detected by HPLC for glycated hemoglobin (Hb A1c). Int J Hematol. 1998 Oct;68(3):317–21.
- Sack JS, Andrews LC, Magnus KA, et al. Location of amino acid residues in human deoxy hemoglobin. Hemoglobin. 1978;2(2):153–69.
- Bots M, Stroobants AK, Delzenne B, et al. Two novel haemoglobin variants that affect haemoglobin A1c measurement by ion-exchange chromatography. Clin Chem Lab Med. 2015 Aug;53(9):1465–71.
- Standards of medical care in diabetes–2013. Diabetes care. 2013 Jan;36 Suppl 1(Suppl 1):S11–66.