ABSTRACT
Objective
Spliced X-Box binding protein 1 (sXBP1) modulates malignant cell activities and enhances the bortezomib sensitivity in multiple myeloma (MM) cells, while its clinical value in MM patients remains elusive. Hence, the current study aimed to explore this issue, particularly the correlation of sXBP1 with treatment outcomes of bortezomib-based therapy in MM patients.
Methods
Totally, 97 newly-diagnosed MM patients undergoing bortezomib-based therapy, 20 disease controls (DCs), and 20 health controls (HCs) were enrolled. Bone marrow plasma cell samples were acquired to determine sXBP1 by RT-qPCR.
Results
sXBP1 was lowest in MM patients, followed by DCs, and highest in HCs (P < 0.001). Beyond that, sXBP1 discriminated MM patients from DCs with area under curve (AUC) of 0.728 (95% confidence interval (CI): 0.610–0.847) and HCs with AUC of 0.855 (95% CI: 0.771–0.939). sXBP1 was negatively associated with t (4; 14) (P = 0.047), Revised International Staging System stage (P = 0.008). There was a trend that sXBP1 was negatively correlated with β2-MG, LDH, and t (14; 16) (without statistical significance). sXBP1 was higher in patients with complete response (CR) compared to those with non-CR (P = 0.017) and higher in patients with objective response rate (ORR) compared to those with non-ORR (P = 0.006). sXBP1 (high vs. low) was linked with longer progression-free survival (PFS) (P = 0.011) and overall survival (P = 0.045) in MM patients. Additionally, sXBP1 (high vs. low) (P = 0.025) independently estimated a longer PFS.
Conclusion
sXBP1 forecasts a favorable treatment response and survival benefit toward bortezomib-based therapy in multiple myeloma patients.
Introduction
Multiple myeloma (MM), featured by clonal and malignant plasma cell accumulation into bone marrow (BM), takes up about 10% of total hematologic malignancies; which is categorized as subtypes of immunoglobulin (Ig) G, IgA, IgD, IgM, etc. according to M component [Citation1–3]. Although several treatment regimens (including bortezomib, thalidomide, lenalidomide, pomalidomide, dexamethasone, and autologous stem cell transplantation) have been applied in MM treatment, a fair proportion of patients are still burdened with refractory or relapsed MM [Citation4–7]. Moreover, the 5-year survival rate of MM patients ranges from 49.6% to 54.0% [Citation8, Citation9]. Therefore, it would be necessary to find out additional effective prognostic biomarkers for monitoring the disease biology of MM patients and further optimizing their treatment and prognostication.
X-Box binding protein 1 (XBP1), located at chromosome 22 in humans, serves as an important transcriptional factor that modifies genes involving the immune system and cellular stress response [Citation10]. Besides, XBP1 is also disclosed to regulate MHC class II gene, plasma cell differentiation, eosinophil differentiation, angiogenesis and viral replication [Citation11]; resulting from those, XBP1 is closely engaged in the development of MM [Citation12, Citation13]. Spliced XBP1 (sXBP1) is a spliced form of XBP1 generated under endoplasmic reticulum stress [Citation14, Citation15]. Interestingly, sXBP1 is reported to involve in tumor development in a series of solid carcinomas [Citation16–18]. In hematological malignancies, elevated sXBP1 links to the sensitivity to bortezomib in B-cell precursor lymphoblastic leukemia [Citation19]. Elevated sXBP1 is correlated with suppressed tumor growth in a diffuse large B-cell lymphoma mice model [Citation20]. In regard to multiple myeloma, one study reports that sXBP1 decreases the drug resistance to bortezomib in MM cells [Citation21]. However, its clinical relevance in MM patients remains elusive. Consequently, the present study aimed to evaluate the linkage of sXBP1 with clinicopathological features, as well as its correlation with treatment outcomes to bortezomib-based therapy in MM patients.
Methods
Subjects
This monocentric study enrolled 97 newly-diagnosed MM patients who received proteasome inhibitor (bortezomib) between May 2017 and March 2021. The recruitment criteria were: (i) newly confirmed as symptomatic MM per International Myeloma Working Group (IMWG) recommendation [Citation22]; (ii) over 18 years old; (iii) underwent induction therapy based on bortezomib; (iv) volunteered to comply with the study protocol. The exclusion criteria were: (i) diagnosis of secondary MM or relapsed MM; (ii) complicated with other malignant tumors; (iii) during pregnancy or breastfeeding. Additionally, this study also included 20 patients with non-malignant hematologic diseases (such as thrombocytopenic purpura, aplastic anemia, and Fanconi anemia) who received BM examinations as disease controls (DCs). Besides, a total of 20 healthy BM donors were included in the study as health controls (HCs). The exclusion criteria for MM patients were suitable for DCs and HCs. Each subject signed the informed consent. The study was permitted by Ethics Committee with the approval number of PJ-KS-KY-2017-117(X).
Collection of data and samples
Clinical characteristics of MM patients were obtained for study use. In addition, the MM patients were staged based on the Durie-Salmon stage, International Staging System (ISS), and Revised International Staging System (R-ISS) [Citation23–25]. BM samples were respectively obtained from MM patients at diagnosis (before treatment), from DCs and HCs at BM examination, which were collected by BM puncture or biopsy and kept in the EDTA anticoagulant tubes for the subsequent examinations. Then, the collected BM samples were submitted to the laboratory, and BM plasma cell samples were isolated using CD138-immunomagnetic beads (Miltenyi Biotec, France) by the instruction.
Examination of samples
BM plasma cell samples were acquired for determining sXBP1 by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Total RNA was extracted by RNeasy Protect Mini Kit (Qiagen, Germany). Reverse transcription was done by PrimeScript™ RT reagent Kit (Takara, China). Besides, qPCR was finished by QuantiNova SYBR Green PCR Kit (Qiagen, Germany). The 18S Ribosomal RNA was chosen for internal reference; meanwhile, the 2−ΔΔCt method was used to determine sXBP1. Beyond that, the primer used herein took a previous study for reference [Citation26].
Treatment
The MM patients received induction therapy with bortezomib for 4 cycles with a 3-week cycle, and the regimen included BD (bortezomib and dexamethasone) and BTD (bortezomib, thalidomide, and dexamethasone). Bortezomib was administrated at 1.3 mg/m2 on the 1st, 4th, 8th, and 11th days by intravenous infusion; dexamethasone was administrated at 40 mg from the 1st day to the 4th day (for 4 cycles), and from 9th day to the 12th day (for 1–2 cycles) by intravenous infusion; thalidomide was administrated orally at the dose of 100 mg per day [Citation27].
Evaluation
Clinical response evaluation of MM patients was conducted after the induction therapy per the IMWG criteria [Citation28], then complete response (CR) rate and objective response rate (ORR) were imputed. Besides, follow-up was carried out every 3 months, and the last follow-up date was February 28, 2022. Then, progression-free survival (PFS) and overall survival (OS) were figured up.
Statistics
Data analyses were carried out using SPSS v.20.0 (IBM Corp., USA). Graphs were made using GraphPad Prism v.7.01 (GraphPad Software Inc., USA). Comparison of sXBP1 among groups was determined using Kruskal–Wallis H rank-sum test, followed by post hoc comparisons with Bonferroni test. The performances of sXBP1 in differentiating subjects were presented using receiver operating characteristic (ROC) curves. Correlation of sXBP1 with ISS stage and R-ISS stage was detected using Spearman's rank correlation test. MM patients were divided into sXBP1 high and sXBP1 low according to the median value of sXBP1 mRNA expression. PFS and OS were shown using Kaplan-Meier curves and determined using log-rank test. Prognostic factors of PFS and OS were assessed using univariate Cox’s proportional hazard regression model analysis, and the potential parameters were included in the backward-stepwise multivariable Cox’s proportional hazard regression model analysis. Patients who died during treatment or without clinical response assessment due to early loss of follow-up were not included in the final analysis. P value <0.05 was considered significant.
Results
Clinical characteristics of MM patients
The mean age was 60.8 ± 8.7 years with 38 (39.2%) females and 59 (60.8%) males (). Regarding the immunoglobulin (Ig) subtype, 46 (47.4%) and 21 (21.6%) patients were with IgG and IgA; 30 (30.9%) patients were with other subtypes. Besides, 64 (66.0%) patients had bone lesion; meanwhile, 40 (41.2%) patients developed renal impairment. Moreover, 10 (10.3%), 2 (2.1%), 6 (6.2%) patients were carrying t (4; 14), t (14; 16), and del (17p), separately. Additionally, 16 (16.5%) and 81 (83.5%) patients were with Durie-Salmon stage II and III separately; for the ISS, the number of patients with stage I, II, and III were 10 (10.3%), 34 (35.1%), and 53 (54.6%), respectively; the numbers at R-ISS stage I, II, and III were 9 (9.3%), 42 (43.3%), and 46 (47.4%), separately. More detailed information was listed in .
Table 1 . Clinical characteristics of MM patients.
sXBP1 in MM patients, HCs, and DCs
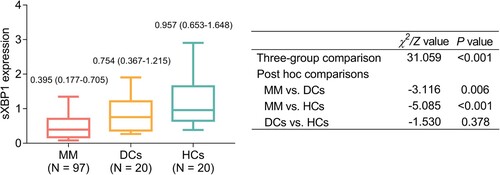
Median sXBP1 was lowest in MM patients [median (interquartile range (IQR): 0.395 (0.177–0.705)], followed by DCs [median (IQR): 0.754 (0.367–1.215)], and highest in HCs [median (IQR): 0.957 (0.653–1.648)] (P < 0.001, ). Besides, post hoc comparisons showed that sXBP1 was lower in MM patients compared with DCs (P = 0.006); meanwhile, sXBP1 was lower in MM patients compared with HCs (P < 0.001). While sXBP1 was of no difference between DCs and HCs (P = 0.378).
Figure 1 . The expression of sXBP1 in MM patients, DCs, and HCs. sXBP1, spliced X-Box binding protein 1; MM, multiple myeloma; DCs, disease controls, HCs, health controls.
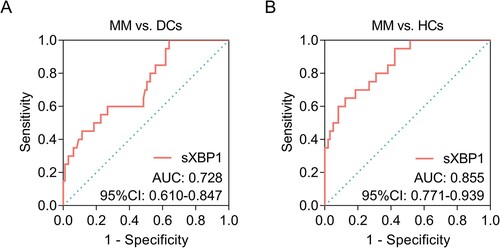
Beyond that, sXBP1 could discriminate MM patients from DCs with area under curve (AUC) of 0.728 (95% confidence interval (CI): 0.610–0.847, A); meanwhile, sXBP1 could also discriminate MM patients from HCs with AUC of 0.855 (95% CI: 0.771–0.939, B).
Figure 2 . ROC curves of sXBP1 in discriminating different subjects. ROC curves of sXBP1 in discriminating MM patients from DCs (A); ROC curve of sXBP1 in discriminating MM patients from HCs (B). ROC, receiver operating characteristic; sXBP1, spliced X-Box binding protein 1; MM, multiple myeloma; DCs, disease controls, HCs, health controls.
Correlation of sXBP1 with clinical characteristics in MM patients
In MM patients, sXBP1 was negatively linked with the occurrence of t (4; 14) (P = 0.047) and R-ISS stage (P = 0.008). Additionally, there was a trend that sXBP1 was negatively correlated with beta-2-microglobulin (β2-MG) (P = 0.061), lactate dehydrogenase (LDH) (P = 0.131), and the occurrence of t (14; 16) (P = 0.131) slightly (but without statistical significance). However, sXBP1 was not correlated with other clinical features such as age, gender, Ig subtype, bone lesion, renal impairment, Hb, calcium, Scr, ALB, del (17p), Durie-Salmon stage, and ISS stage (all P > 0.05).
Correlation of sXBP1 with treatment response in MM patients
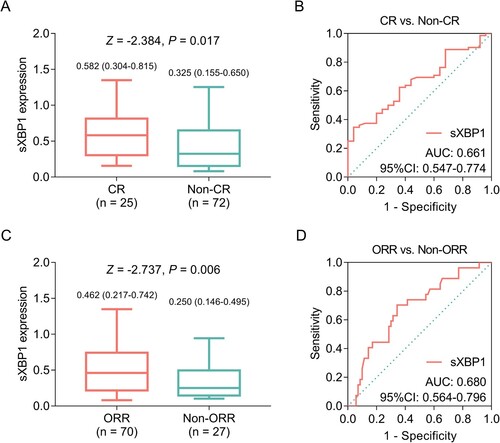
sXBP1 was positively correlated with the achievement of CR; in detail, median (IQR) sXBP1 was 0.582 (0.304–0.815) and 0.325 (0.155–0.650) in patients with CR and non-CR, respectively (A). Meanwhile, sXBP1 could distinguish patients with CR from those with non-CR with AUC of 0.661 (95% CI: 0.547–0.774, B). Additionally, sXBP1 was positively correlated with the achievement of ORR; in detail, median (IQR) sXBP1 was 0.462 (0.217–0.742) and 0.250 (0.146–0.495) in patients with ORR and non-ORR, respectively (C). Meanwhile, sXBP1 could distinguish patients with ORR from those with non-ORR with AUC of 0.680 (95% CI: 0.564–0.796, D).
Figure 3 . Elevated sXBP1 was linked with better treatment response to bortezomib in MM patients. sXBP1 in MM patients with CR and non-CR (A); ROC curve of sXBP1 in discriminating CR patients from non-CR patients (B); sXBP1 in MM patients with ORR and non-ORR (C); ROC curve of sXBP1 in discriminating ORR patients from non-ORR patients (D). sXBP1, spliced X-Box binding protein 1; MM, multiple myeloma; CR, complete response; ROC, receiver operating characteristic; ORR, objective response rate.
Correlation of sXBP1 with survival profile
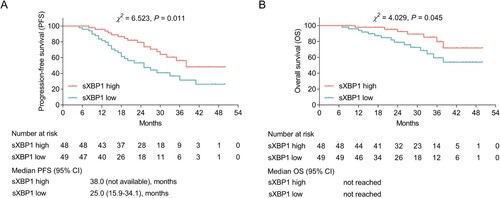
sXBP1 high was linked with longer PFS in MM patients (P = 0.011); in detail, the median PFS (95% CI) was 38 (not available) months and 25 (15.9–34.1) months in MM patients with sXBP1 high and low, separately (A). Beyond that, sXBP1 high was linked with longer OS in MM patients (P = 0.045), whereas the median OS was not reached in patients with sXBP1 high or those with sXBP1 low (B).
Figure 4 . sXBP1 high was linked with longer PFS and OS. Association of sXBP1 (high vs. low) with PFS (A) and OS (B) in MM patients. MM patients were divided into sXBP1 high and sXBP1 low according to the median value of sXBP1 mRNA expression. sXBP1, spliced X-Box binding protein 1; PFS, progression-free survival; OS, overall survival; MM, multiple myeloma.
Additionally, in univariate cox’s proportional hazards regression model, a comparative value of sXBP1 with other classical biological indexes in estimating the PFS was observed; in detail, sXBP1 expression (high vs. low) (P = 0.013), Hb (>100 g/L vs. ≤100 g/L) (P = 0.009), and ALB (>35 g/L vs. ≤35 g/L) were associated with prolonged PFS, while renal impairment (yes vs. no) (P = 0.011), β2-MG (>5.5 mg/L vs. ≤5.5 mg/L) (P = 0.003), LDH (>220 U/L vs. ≤220 U/L) (P = 0.030), t (4; 14) (yes vs. no) (P = 0.002), t (14; 16) (yes vs. no) (P = 0.002), higher ISS (P = 0.001), and higher R-ISS (P = 0.001) were correlated with shortened PFS (). Multivariate backward-stepwise Cox’s analysis for PFS showed that sXBP1 (high vs. low) (HR = 0.481, 95% CI: 0.254–0.911, P = 0.025) was linked with longer PFS; meanwhile, renal impairment (yes vs. no) (HR = 2.787, 95% CI: 1.177–6.598, P = 0.020), β2-MG (>5.5 mg/L vs. ≤5.5 mg/L) (HR = 2.812, 95% CI: 1.255–6.302, P = 0.012), t (4; 14) (yes vs. no) (HR = 3.095, 95% CI: 1.433–6.684, P = 0.004), and t (14; 16) (yes vs. no) (HR = 17.998, 95% CI: 3.298–98.230, P = 0.001) were independently correlated with shorter PFS ().
Table 2 . Cox’s proportional hazards regression analysis for PFS.
Apart from that, in univariate cox’s proportional hazards regression model, sXBP1 also indicated a similar role in estimating the PFS compared with other classical biological indexes; in detail, sXBP1 expression (high vs. low) was associated with prolonged OS (P = 0.053), while β2-MG (>5.5 mg/L vs. ≤5.5 mg/L) (P = 0.014), t (4; 14) (yes vs. no) (P = 0.039), higher ISS (P = 0.013), and higher R-ISS (P = 0.045) were correlated with shortened OS (). Multivariate backward-stepwise Cox’s analysis for OS disclosed that only β2-MG (>5.5 mg/L vs. ≤5.5 mg/L) was independently linked with shorter OS (HR = 6.312, 95% CI: 1.993–19.986, P = 0.002) ().
Table 3 . Cox’s proportional hazards regression analysis for OS.
Discussion
sXBP1 is produced under endoplasmic reticulum stress and exhibits a regulatory role in a series of hematology malignancies [Citation29–32]. It is reported that enhanced sXBP1 would modulate the apoptosis in B-acute lymphoblastic leukemia cells and MM cells [Citation29, Citation30]. However, the clinical relevance of sXBP1 in MM patients still needs further investigation.
In the present study, sXBP1 was declined in MM patients compared with DCs and HCs, besides, sXBP1 was negatively correlated with the occurrence of t (4; 14) and R-ISS stage in MM patients. In addition, there was a trend that sXBP1 was negatively linked with β2-MG, LDH, and the occurrence of t (14; 16), while there was no statistical significance. Possible explanations could be that: 1) In MM patients, the inositol-requiring enzyme-1 alpha/XBP1 signaling, which dysregulates the expression of sXBP1, is involved in the development of MM; thereby sXBP1 is dysregulated in MM patients [Citation33]. 2) A previous study discloses that dysregulated sXBP1 is linked with renal injury in chronic kidney disease patients, thus dysregulated sXBP1 would be partially linked with elevated β2-MG, and higher LDH in MM patients [Citation34]. 3) As mentioned above, sXBP1 was negatively associated or partially associated with t (4; 14) or t (14; 16), β2-MG, and LDH in MM patients, separately. Moreover, all of these indexes and cytogenetic abnormalities are linked with higher risk according to R-ISS components. Thus, sXBP1 is negatively correlated with a higher R-ISS stage [Citation25]. In terms of del(17p), a risk factor in MM indicating the poor prognosis, its correlation with sXBP1 and the survival of MM patients were also determined in our study, which showed that del(17p) was neither correlated with sXBP1 nor with the PFS or OS of MM patients. The potential explanation might be that the number of patients with del(17p) was only 6 cases, therefore, the small sample size might cause an insufficient statistical power, therefore, the del(17p) was neither correlated with sXBP1 nor with the PFS or OS of MM patients.
In addition to the information mentioned above, two in vitro studies illuminate that sXBP1 relates to bortezomib susceptibility or resistance; for instance, one study indicates that the sXBP1 could predict sensitivity towards proteasome inhibitors in pediatric precursors B-ALL cells; another study indicates that low sXBP1 level is correlated with bortezomib resistance in MM cell line [Citation19, Citation21]. Whereas no clinical study investigates this issue. In the current study, elevated sXBP1 was correlated with the achievement of CR and objective response to bortezomib in MM patients. Possible explanations for this could be that: 1) sXBP1 would increase the cytotoxicity of bortezomib via interacting with the endoplasmic reticulum to nucleus signaling 1, therefore, elevated sXBP1 is associated with more promising treatment responsiveness to bortezomib in MM patients [Citation21, Citation30]. 2) As mentioned above, sXBP1 is negatively linked with β2-MG, LDH, and the occurrence of t (4; 14), t (14; 16) in MM patients, thus, sXBP1 would be associated with favorable treatment response to bortezomib herein indirectly. Based on results from the previous in vitro studies, this study provided the clinical significance of sXBP1 level in predicting bortezomib response in MM patients.
One study reports that dysregulated sXBP1 is correlated with longer disease-free survival and overall survival in acute myeloid leukemia patients [Citation35]. However, the prognostic value of sXBP1 in MM patients has not been explored. Herein, sXBP1 high was linked with longer PFS and OS in MM patients with bortezomib-based therapy. However, multivariate backward-stepwise Cox’s analysis showed that sXBP1 only independently correlates with longer PFS but not OS. A possible explanation could be that compared with MM patients with no response to bortezomib, those MM patients with the treatment response to bortezomib-based therapy had an increased sXBP1; which implied that MM patients with increased sXBP1 might be easier to achieve treatment response to bortezomib-based therapy, which led to fewer progression events; therefore, sXBP1 was correlated to a more favorable PFS in MM patients.
There existed some limitations in the current study: 1) The current study was a single-centered study; thus, a patient selection bias might exist. 2) The sample size was relatively small in the present study; hence the statistical power might be reduced. 3) sXBP1 might be changing along with the treatment, thus the forthcoming study would detect sXBP1 at multiple timepoints during the therapy to further evaluate its prognostic value. 4) The median OS was not reached; thus, a longer follow-up should be performed in the forthcoming study.
In conclusion, sXBP1 forecasts a favorable treatment response and survival benefit toward bortezomib-based therapy in multiple myeloma patients.
Author’s contribution
Lingli Zhang and Jichang Gong collected and analyzed the data and drafted the manuscript. Lingli Zhang and Yaqiong Li designed the study, provided valuable suggestions, and reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Medical Masterclass c, Firth J. Haematology: multiple myeloma. Clin Med (Lond). 2019;19(1):58–60. doi:10.7861/clinmedicine.19-1-58
- Joshua DE, Bryant C, Dix C, et al. Biology and therapy of multiple myeloma. Med J Aust. 2019;210(8):375–380. doi:10.5694/mja2.50129
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Lussier T, Schoebe N, Mai S. Risk stratification and treatment in smoldering multiple myeloma. Cells. 2022;11(1. doi:10.3390/cells11010130
- Podar K, Leleu X. Relapsed/refractory multiple myeloma in 2020/2021 and beyond. Cancers (Basel). 2021;13(20. doi:10.3390/cancers13205154
- Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021;32(3):309–322. doi:10.1016/j.annonc.2020.11.014
- Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the international myeloma working group. Blood. 2016;127(24):2955–2962. doi:10.1182/blood-2016-01-631200
- Hemminki K, Forsti A, Hansson M. Incidence, mortality and survival in multiple myeloma compared to other hematopoietic neoplasms in Sweden up to year 2016. Sci Rep. 2021;11(1):17272. doi:10.1038/s41598-021-96804-8
- Yang FY, Wang HF, Xia LH, et al. A propensity score matching study of autologous hematopoietic stem cell transplantation and New drug chemotherapy for newly diagnosed multiple myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30(1):158–165.
- Yoshida H, Nadanaka S, Sato R, et al. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct. 2006;31(2):117–125. doi:10.1247/csf.06016
- Xu W, Wang C, Hua J. X-box binding protein 1 (XBP1) function in diseases. Cell Biol. Int. 2021;45(4):731–739. doi:10.1002/cbin.11533
- Zhang D, De Veirman K, Fan R, et al. ER stress arm XBP1s plays a pivotal role in proteasome inhibition-induced bone formation. Stem Cell Res Ther. 2020;11(1):516. doi:10.1186/s13287-020-02037-3
- Bujisic B, De Gassart A, Tallant R, et al. Impairment of both IRE1 expression and XBP1 activation is a hallmark of GCB DLBCL and contributes to tumor growth. Blood. 2017;129(17):2420–2428. doi:10.1182/blood-2016-09-741348
- Luo X, Alfason L, Wei M, et al. Spliced or unspliced, that Is the question: The biological roles of XBP1 isoforms in pathophysiology. Int J Mol Sci. 2022;23(5).
- Park SM, Kang TI, So JS. Roles of XBP1s in transcriptional regulation of target genes. Biomedicines. 2021;9(7).
- Song M, Sandoval TA, Chae CS, et al. IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423–428. doi:10.1038/s41586-018-0597-x
- Ji H, Huang C, Wu S, et al. XBP1-s promotes colorectal cancer cell proliferation by inhibiting TAp73 transcriptional activity. Biochem Biophys Res Commun. 2019;508(1):203–209. doi:10.1016/j.bbrc.2018.11.112
- Wu S, Du R, Gao C, et al. The role of XBP1s in the metastasis and prognosis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;500(3):530–537. doi:10.1016/j.bbrc.2018.04.033
- Besse L, Besse A, Kraus M, et al. High immunoproteasome activity and sXBP1 in pediatric precursor B-ALL predicts sensitivity towards proteasome inhibitors. Cells. 2021;10(11). doi:10.3390/cells10112853
- Cui Y, Xu H, Yang Y, et al. The regulation of miR-320a/XBP1 axis through LINC00963 for endoplasmic reticulum stress and autophagy in diffuse large B-cell lymphoma. Cancer Cell Int. 2021;21(1):305. doi:10.1186/s12935-021-01992-y
- Borjan B, Kern J, Steiner N, et al. Spliced XBP1 levels determine sensitivity of multiple myeloma cells to proteasome inhibitor bortezomib independent of the unfolded protein response mediator GRP78. Front Oncol. 2020;9:1530. doi:10.3389/fonc.2019.01530
- Ludwig H, Miguel JS, Dimopoulos MA, et al. International myeloma working group recommendations for global myeloma care. Leukemia. 2014;28(5):981–992. doi:10.1038/leu.2013.293
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi:10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi:10.1200/JCO.2005.04.242
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267
- Wang Y, Osakue D, Yang E, et al. Endoplasmic reticulum stress response of trabecular meshwork stem cells and trabecular meshwork cells and protective effects of activated PERK pathway. Invest Ophthalmol Vis Sci. 2019;60(1):265–273. doi:10.1167/iovs.18-25477
- Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–5758. quiz 5982. doi:10.1182/blood-2011-05-355081
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691–4695. doi:10.1182/blood-2010-10-299487
- Bortolozzi R, Viola G, Porcu E, et al. A novel copper(I) complex induces ER-stress-mediated apoptosis and sensitizes B-acute lymphoblastic leukemia cells to chemotherapeutic agents. Oncotarget. 2014;5(15):5978–5991. doi:10.18632/oncotarget.2027
- Bae J, Hideshima T, Zhang GL, et al. Identification and characterization of HLA-A24-specific XBP1, CD138 (Syndecan-1) and CS1 (SLAMF7) peptides inducing antigens-specific memory cytotoxic T lymphocytes targeting multiple myeloma. Leukemia. 2018;32(3):752–764. doi:10.1038/leu.2017.316
- Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–307. doi:10.1038/35085509
- Todd DJ, McHeyzer-Williams LJ, Kowal C, et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med. 2009;206(10):2151–2159. doi:10.1084/jem.20090738
- Chen L, Li Q, She T, et al. IRE1α-XBP1 signaling pathway, a potential therapeutic target in multiple myeloma. Leuk Res. 2016;49:7–12. doi:10.1016/j.leukres.2016.07.006
- Tavernier Q, Mami I, Rabant M, et al. Urinary angiogenin reflects the magnitude of kidney injury at the infrahistologic level. J Am Soc Nephrol. 2017;28(2):678–690. doi:10.1681/ASN.2016020218
- Schardt JA, Weber D, Eyholzer M, et al. Activation of the unfolded protein response is associated with favorable prognosis in acute myeloid leukemia. Clin Cancer Res. 2009;15(11):3834–3841. doi:10.1158/1078-0432.CCR-08-2870
