?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives: To compare the outcomes of tyrosine kinase inhibitors (TKIs) in combination with reduced-dose chemotherapy with those of standard induction chemotherapy, as well as the outcomes between chemotherapy and transplantation, in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
Methods: We retrospectively reviewed cases of Ph+ ALL treated with TKIs and combination chemotherapy. The patients were allocated to either the TKIs with reduced-dose chemotherapy group or the TKIs with standard chemotherapy group. In additions, patients were further stratified into either the transplant group or the non-transplant group.
Results: The complete remission rate (88.7% vs. 83.9%, p = 0.372), major molecular response (58.9% vs. 56.0%, p = 0.750), molecular complete response (20.5% vs. 22.0%, p = 0.891), and early mortality rate (3.2% vs. 3.5%, p = 0.922) were similar between the TKIs with reduced-dose chemotherapy group and the TKIs with standard chemotherapy group. The proportions of lung infections, bloodstream infections, patients with >21 days of hospitalization, the total costs, transfusion costs, and antimicrobial costs were higher in the standard chemotherapy group than in the TKIs with reduced-dose chemotherapy group. The 3-year overall survival rates (59.0% [95% CI, 46.6–74.7%] vs. 38.4% [95% CI, 29.9–49.4%]) and disease-free survival rates (48.6% [95% CI, 34.2–69.1%] vs. 32.0% [95% CI, 23.5–43.7%]) were significantly better in the transplant group than in the non-transplant group.
Conclusion: An induction regimen combining TKIs with reduced-dose chemotherapy and transplantation during the first complete remission remains a suitable and effective option for patients with Ph+ ALL.
Introduction
Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) is a distinct subtype of acute lymphoblastic leukemia (ALL) associated with a poor prognosis. The incidence of Ph+ ALL increases with age, accounting for 3–5% of childhood ALL, 20–30% of adult ALL, and >50% of ALL cases in patients >50 years of age [Citation1]. Before the advent of tyrosine kinase inhibitors (TKIs), the complete remission (CR) rate of patients with Ph+ ALL treated with standard ALL chemotherapy regimens was at least 10% lower than the CR rate of patients with Ph- ALL, who have a median survival time of approximately 8 months. The use of TKIs has increased the CR rate and reduced the incidence of adverse events, allowing more opportunities for allogeneic hematopoietic stem cell transplantation (allo-HSCT), and significantly prolonging survival in patients who were unable to undergo transplantation [Citation2]. Allo-HSCT may be the only available treatment option for achieving long-term survival after CR. However, the 5-year overall survival (OS) rate of Ph+ ALL is only approximately 50% [Citation3,Citation4]. The survival of patients with Ph+ ALL has improved greatly in recent years, with the 5-year OS rate increasing to 40–70%, and the efficacy of non-transplant treatment gradually approaching that of allo-HSCT with the use of TKIs [Citation5].
Although the clinical efficacy of TKIs combined with chemotherapy has been confirmed, not all patients can tolerate chemotherapy, and high-dose chemotherapy often results in severe myelosuppression or complications, including infection and bleeding, leading to early death. To minimize toxicity, the combination of TKIs with low-dose chemotherapy has shown favorable results [Citation6,Citation7]. Data from the Italian Group for Hematological Diseases in Adults (GIMEMA) suggested that elderly Ph+ ALL patients may benefit from an imatinib-steroids protocol (imatinib 800 mg/day in combination with prednisone), which does not require chemotherapy [Citation8]. Nevertheless, very few data on adult ALL are available from developing countries, and whether intensive chemotherapy is needed at all is still controversial.
In response to this knowledge gap, we conducted a retrospective analysis aimed at exploring an effective and safe induction regimen for Ph+ ALL. We thus compare the effects of TKIs combined with either reduced-dose chemotherapy or standard induction chemotherapy, with or without Allo-HSCT, in patients with Ph+ ALL. We also explored other prognostic factors associated with outcome in our sample.
Methods
Study design
This was a retrospective cohort study which analyzed the outcomes of patients aged >14 years with newly diagnosed Ph+ ALL treated at Fujian Union Hospital of Hematology, China, between January 2010 and December 2020. This study was approved by the ethics review board of the Fujian Medical University Union Hospital. The diagnosis of B-lymphoblastic leukemiawas based on the World Health Organization criteria [Citation9]. Patients with known severe cardiac, pulmonary, hepatic, or renal dysfunction; non-primary patients; patients who abandoned the treatment after diagnosis; and pregnant patients were excluded. Written informed consent for research participation was obtained from all patients.
Depending on the induction chemotherapy regimen, patients were non-randomly assigned to either the TKIs with reduced-dose chemotherapy group or the TKIs with standard chemotherapy group, based on the discretion of the treating physician. In cases where further treatment was required, patients were non-randomly assigned to either the transplant group or the TKIs + chemotherapy (non-transplant) group based on their individual preferences.
Chemotherapy regimens
Over a 10-year period, patients with newly diagnosed Ph+ ALLwere treated with diverse protocols. Treatments are shown in . The standard chemotherapy regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) [Citation10] and the protocol of Chinese Acute Lymphoblastic Leukemia Cooperative Group for adult ALL (CALLG2008) [Citation11]. The reduced-dose chemotherapy regimens reduce the use of cyclophosphamide and doxorubicin during induction therapy compared to the protocol of CALLG2008. TKIs (imatinib 400 mg/d, dasatinib 140 mg/d, or nilotinib 400 mg/d) were administered concurrent with chemotherapy once the positive result for BCR-ABL1 was confirmed. Following the achievement of CR, allo-HSCT was recommended to all eligible patients.
Table 1. Chemotherapy regimens.
Minimal residual disease monitoring and response definitions
Patients were monitored for the BCR-ABL1 transcript by reverse-transcription quantitative polymerase chain reaction (qRT-PCR) using bone marrow samples. BCR-ABL1 transcript levels were used for minimal residual disease (MRD) monitoring. Complete hematologic remission (CR) was defined as the absence of primitive cells in the peripheral blood, absence of extramedullary leukemia, blasts 5% in the bone marrow, neutrophil count
1.0×10^9/L, and platelet count
100×10^9/L. Major molecular response (MMR) was definedby a BCR-ABL1/ABL1 ratio
0.1%. MolecularCR (MCR) was definedby a BCR-ABL1/ABL1 ratio
0.01%. Early mortality was defined as death occurring during the induction treatment. Relapse was defined as >5% of leukemia cells in the bone marrow in patients who have achieved CR or had extramedullary disease. OS was defined as the time from the date of diagnosis until death or the final follow-up date. Disease-free survival (DFS) was measured from the date of complete remission to the date of disease relapse, death, or the final follow-up date.
Statistical analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences (version 26.0) and R Studio (version 3.6.2) software packages. Categorical variables were compared between patient groups using a Fisher’sexact test or Chi-squared test. Continuous variables werecompared between patient groups using the non-parametric Mann–Whitney U test. The cumulative incidence of relapse (CIR) was calculated considering death as a competitor and compared between groups using Grey’s test. The role of transplantation was assessed using the time-dependent Mantel–Byar method and visualized using Simon–Makuchplots [Citation12]. Prognostic factors of survival were investigatedusing Cox proportional hazard models. Hazard ratios were obtained with 95% confidence intervals (CIs). A p-value <0.05 was considered statistically significant in all analyses.
Results
Patient characteristics
In this study, 234 patients were newly diagnosed with Ph+ ALL. In total, 29 patients were excluded because they received chemotherapy without TKIs. Therefore, 205 patients with Ph+ ALL enrolled between January 2010 and December 2020 were included in the present analysis. The median follow-up time was 33 months (range, 2–106 months). The median follow-up time was 37 months (range, 3–88 months) in the reduced-dosechemotherapy group and 32 months (range, 2–106 months) in the standard chemotherapy group. The patient characteristics were well-balanced between the standard chemotherapy group and the reduced-dose chemotherapy group. The patients in the non-transplant group were significantly older than patients in the transplant group. No other significant between-group differences in patient characteristics were found. All patients were treated with a combination of TKIs in induction chemotherapy: imatinib (400–600 mg/d), dasatinib (100–140 mg/d), and nilotinib (400–800 mg/d) in 156, 47, and two patients, respectively. The patients who underwent allo-HSCT (n = 55) received a varying number of cycles until a transplant donor was identified. These patients underwent HSCT when their disease was in morphological CR1. The remaining patients were unable to undergo HSCT due to insufficient matching HSCT donors or personal reasons. The patient characteristics are shown in .
Table 2. Patient characteristics.
Response to tyrosine kinase inhibitors with reduced-dose chemotherapy versus tyrosine kinase inhibitors with standard chemotherapy
Response and early mortality
summarizes the initial response to treatment in our patient sample. The use of standardchemotherapy versus reduced-dose chemotherapy was not associated withhigher rates of early mortality (3.5% vs. 3.2%, p = 0.922) or hematologic CR (83.9% vs. 88.7%, p = 0.372) after induction. Among the 198 patients alive and in CR at the end-of-induction, 139 (67.8%) patients were tested for BCR-ABL. The MMR rate was similar in both groups (56.0% in the standardchemotherapy group and 58.9% in the reduced-dose chemotherapy group, p = 0.750). In total, 30 (21.6%) patients achieved molecular CR at the end-of-induction. The reduced-dosechemotherapy group (20.5%) was non-inferior to thestandard chemotherapy group (22.0%) *(p = 0.891). Seven patients wereconsidered refractory because of treatment failure at the end of the induction period. Infectionwas the major cause of death (5/7, 71%).
Table 3. Response at the end-of-induction.
Complications
The reduced-dose chemotherapy group had a lower incidence of pulmonary infection (OR, 1.853 [95%CI,1.003–3.422]) and blood-based infection (OR, 2.449 [95% CI,1.108–5.412]) after the induction phase (Appendix Table A1). There was no significant difference in the use of granulocyte colony-stimulating factor during the induction phase between the standard chemotherapy group (29.4%) and the reduced-dose chemotherapy group (32.3%) (p = 0.679). The duration of neutropenia (neutrophill count 0.5
109/L) was significantly shorter in the reduced-dose chemotherapy group (12 days, range 7–22 days) compared to the standardchemotherapy group (18 days, range 14–31 days) (p <0.001).
Healthcare utilization
The mean duration of in-hospital care was 23 ± 7 days in 27 ± 8 days the reduced-dose chemotherapy group and the standard chemotherapy group. In the reduced-dose chemotherapy group (n = 62), 33 (52.4%) patients were hospitalized for >21 days. In the standard chemotherapy group (n = 143), 115 (80.4%) patients were hospitalized for >21 days. This difference between the two groups was significant (p <0.001).
The reduced-dose chemotherapy group had lower red blood cell counts and platelet transfusion burden during the induction phase, which are associated with lower hematologic toxicity. Expectedly, the total cost, transfusion cost, and antimicrobial cost were significantly lower in the reduced-dose chemotherapy group compared to the standard chemotherapy group (Appendix Table A2).
Antibiotic use in induction chemotherapy
In total, 14.6% (30/205) of patients received fluoroquinolone prophylaxis during the induction phase, which occurred mainly during the latter 3 years of the study. There was no significant difference in antibiotic use between the reduced-dose chemotherapy group (12.6%) and the standard chemotherapy group (19.3%) (p = 0.208).
Most patients were prescribed antibiotics for infections. The types and dosages of antibiotics used to control infections during hospitalization are recorded (Appendix Table A3). There was a significant difference in the number of patients who received more than four antibiotics between the reduced-dose chemotherapy group (14/62, 22.6%) and the standard chemotherapy group (64/143, 44.8%) (p = 0.003). The use of antifungal drugs, carbapenems, and the restricted class of anti-gram-positive drugs (tigecycline, vancomycin, and linezolid)were similar between the two groups. However, the rates of use of restricted tigecycline and/or polymyxin were significantly higher in the standard chemotherapy group compared to the reduced-dose chemotherapy group (p = 0.031).
Survival outcomes in the transplant group versus the non-transplant group
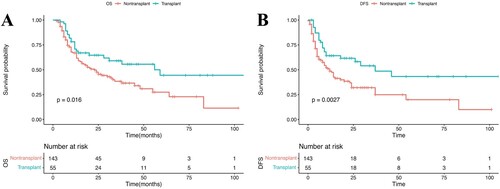
Among the 175 patients in CR, 55 (31%) were transplanted in CR1, including 28 sibling donors, nine unrelateddonors, and 18 cord blood transplantations (Appendix Table A4). No patient receivedan autograft. As illustrated in A–B, the 3-yearDFS in the allo-HSCT group (48.6% [95% CI, 34.2–69.1%]) wassignificantly higher than that in the non-HSCT group (32.0% [95% CI, 23.5–43.7%]). The 3-yearOS in the allo-HSCT group (59.0% [95% CI, 46.6–74.7%]) wassignificantly higher than that in the non-HSCT group (38.4% [95% CI, 29.9–49.4%]).
Relapses and deaths
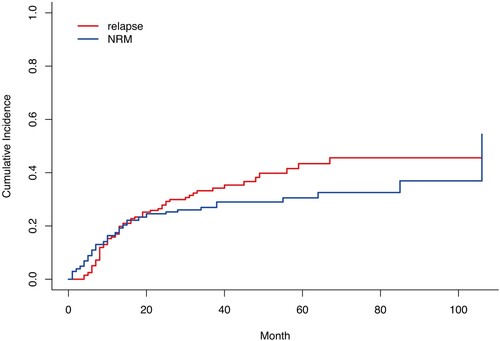
Of the 205 (85%) patients, 175 achieved CR. Thirty patientsfailed to obtain CR; 23 were refractory and obtained CR after salvagetherapy, and seven died during the induction phase. Among the 175 patients in CR after induction, 46 (26.3%) relapsed. Thirteen patients obtained a second CR (28%), six (46.1%) of whom had a secondary relapse event. In total, 120 (58.5%) patients died, including 65 deaths in those with relapse or refractory disease. Non-relapse related mortality (NRM) was reported in 55 patients. There was no significant difference in NRM between the reduced-dose chemotherapy group (30.6% [95% CI, 19.9–43.8%]) and the standard chemotherapy group (25.2% [95% CI, 18.5–33.2%]) (p = 0.417). In considering non-relapse mortality as a competitor event, the 5-year CIR was 43.4% (95% CI, 36.6–50.5%), while the 5-year non-relapse mortality was 30.7% (95% CI, 24.8–37.6%) ().
Prognostic factors
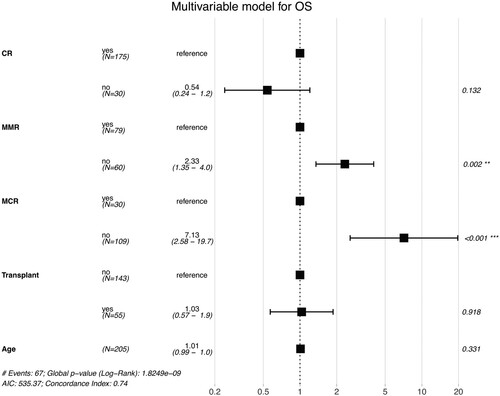
In a univariable analysis, age, hematologic CR, MMR, MCR and transplantation were significant predictors of OS. Gender, white blood cell (WBC) count, the presence of additional cytogenetic alterations (ACAs), and BCRABL1 transcript were not significantly associated with OS. In a multivariate analysis modelling the effects of age, hematologic CR, MMR, MCR, and transplantation, only MMR (HR, 2.33 [95% CI, 1.35–4.0; p = 0.002] and MCR (HR, 7.13 [95% CI, 2.58–19.7; p <0.001] remained significant predictors of OS during the induction phase ().
Discussion
Over the last decade,TKIs have revolutionized the treatment and prognosis of patients with Ph+ ALL. TKIs combined with chemotherapy have become the first-line induction chemotherapy regimen for patients newly diagnosed with Ph+ ALL, improving the CR rate and OS and DFS rates, while reducing treatment-related mortality [Citation13,Citation14]. Theintolerance to intensive chemotherapy for older patientshas led to consideration of whether this approach is still needed, especially in older patients who are at greater risk of early death by infections after intensive chemotherapy. Hence, several international clinical studies of TKIs with reduced-dose chemotherapy have been conducted.
The European Working Group on Adult ALL examined the clinical efficacy of dasatinib combined with low-dose chemotherapy in 71 elderly patients with CR, CMR, and MMRrates of 96%, 24%, and 65%, respectively. Moreover, only 10% of patients underwent allo-HSCT, with 5-year relapse-free survival and OS rates of 28% and 36%, respectively [Citation6]. Similar results were obtained in other clinical trials [Citation15–17]. In the present study, patients with reduced-dose chemotherapy had CR, MMR, and CMR rates of 88.7%, 58.9%, and 23.0%, respectively. We did not find differencesin achievement of molecular remission when we de-escalated chemotherapy. Rather, the reduced intensity of chemotherapy allowed patients to achieve high rates of CR while significantly reducing treatment-related toxic side-effects. Our sample had a lower CR rate than samples from studies conducted in developed countries [Citation15–17], but a significantly higher CR rate compared to a study conducted in Brazil, which is also a developing country, with a CR rate of 77.3%–84.6% [Citation18]. Second- and third-generation TKIs combined with chemotherapyare associated with a decreased MRD [Citation19]. Most (76%) of the patients enrolled in this study were treated with the first-generation TKI imatinib, which could explain the worse outcomes found in our sample. A recent randomizedclinical trial performed in pediatric patients with Ph+ ALL showed that dasatinib in the frontline treatment of pediatric Ph+ ALL is associated with better survival and fewer central nervous system (CNS) relapses [Citation20].
Ph+ ALL is a hematological malignancy and a chronic disease that requires repeated hospitalization, resulting in high hospitalization costs and serious threats to the quality of life of affected patients. Wefound that the financial burden faced by Chinese families with a patient with ALL was tremendous, as it is one of the most significant factors affecting compliance, as well as a direct impact on the willingness of the patient to be treated. However, research determiningthe costs associated with ALL in developing countries is rare. This study provideda breakdown of the direct medical costs and anin-depth understanding of financial burden to families. A cost-analysis shows that the mean total treatment cost of childhood ALL per patient was 17,647.5 United States dollars (USD). The important drivers of overall costs were hospital admissions (36.2%) and drug expenditures (31.4%), especially expenditure on antibiotics/antifungals [Citation21], which were the main contributors to high medical cost [Citation22–26]. In the low-intensity chemotherapy group, due to the reduced intensity of chemotherapy, the degree and duration of bone marrow suppression was reduced. In turn, life-threatening infections were also reduced, as evidenced by the reduction of serious infections and the use of fewer types and lower intensity of antibiotics. The number of hospital days was thus shortened, and the cost of antibiotics and total healthcare costs reduced. Therefore, the use of low-intensity chemotherapy in induction may be one of the important ways to lower the treatment costs of Ph+ ALL. Currently, there is a paucity of literature on the comprehensive cost analysis of ALL treatment. Most studies to date have examined the cost-effectiveness of different treatment methods; however, few have analyzed the impact of this financial burden on patient survival.
Infections are a major cause of mortality andmorbidity in patients with ALL. In our study, induction mortality(7/205, 3.4%) was mainly accounted for by infections. The major cause of treatment-related mortality in patients with Ph+ ALL is infections, especially occurring during the induction period [Citation27,Citation28]. Although some studies have shown a reduction in the incidence of infection, bacterial resistance often hampers treatment implementation in clinical settings [Citation29,Citation30]. The role of antibacterial prophylaxis during inductionchemotherapy is still controversial. Inthis study, only 14.6% (30/205 patients) received fluoroquinolone prophylaxis during induction chemotherapy. Most patients received antibiotics while developing feverand/or infections, so the efficacy and safety of antibacterial prophylaxis could not be confirmed. Large randomized controlled trials areneeded to confirm the validity of the approach to induction chemotherapy for ALL described in the present study.
Patients with Ph+ ALL are mostly older, have a high WBC count, are at risk of CNS involvement, and are more likely to relapse with chemotherapy alone [Citation31]. Therefore, allo-HSCT is recommended for patients with Ph+ ALL who have achieved CR when possible. Although the advent of TKIs has changed the management of patients with Ph+ ALL, chemotherapy combined with TKIs has been widely used to treat these patients, improving the CR and long-term survival time and reducing the risk of relapse [Citation32], bringing into question the status of allo-HSCT in the treatment of Ph+ ALL. However, several national and international studies still demonstrate the survival benefit of allo-HSCT compared to combination chemotherapy in both the pre- and post-TKIs eras [Citation33,Citation34]. In an analysis of 145 patients (median age, 37; range, 14–65 years) with Ph+ ALL at the Peking University People’s Hospital treated with imatinib in combination with chemotherapy during the induction phase, 57.9% (n = 77) of patients underwent allo-HSCT after remission, and the 4-year cumulative relapse, DFS, and OS rates in the transplant groups were 29.4%, 60.9%, and 69.2%, respectively, a significant advantage over the non-transplant group, especially in those with persistent minimal residual disease level [Citation35]. In our study, OS and DFS were significantly better in the transplant group than in the non-transplant group. Only 27.8% of patients underwent allo-HSCT, of whom six (10.9%)died of relapse, further suggesting that allo-HSCTshould be the treatment of choice.
This study had some limitations. According to Anderson et al. [Citation7,Citation36,Citation37], more potent TKIs, for example, nilotinib and ponatinib, used as frontline therapy together with chemotherapy to reduce minimal residual disease, significantly prolong patient survival. However, considering the small sample size of our study, the roles of second- and third-generation TKIs in combination chemotherapy were not analyzed. Moreover, patients in the transplant group were younger than patients in the non-transplant group, and the non-transplant group included more patients with advanced disease, including those with poor systemic status or refractory relapses, who were often lost to transplantation, and may have masked the efficacy of some TKIs combined with chemotherapy regimens.
Taken together, TKIs combined with reduced-dose chemotherapy is a practical treatment option for patients with Ph+ ALL, since it reduces chemotherapy-related adverse effects, lowers hospital costs, reduces the financial burden of patients, and improves patient compliance, all withoutaffecting the effectiveness of the therapy. Moreover, this study showed that HSCT in CR1 remains a good treatment option for patients with Ph+ ALL. These findings need to be further validated by multi-center, prospective clinical studies with larger sample sizes. Additionally, the treatment of Ph+ ALL continues to face serious problems of disease relapse and drug resistance, and further studies of novel biologic agents and antileukemic regimens, including immunotherapy, will improve the outcome and prognosis of patients with Ph+ ALL.
Acknowledgements
The authors report there are no competing interests to declare.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data and materials support the published claims and comply with field standards. All the content has not been published elsewhere, nor is it under consideration for publication anywhere else.
Additional information
Funding
References
- Ottmann OG. Management of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2013;2011(2):231–237.
- Fielding AK, Zakout GA. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2013;2011(2):231–237.
- Fielding AK, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the international ALL trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–4496. doi:10.1182/blood-2009-01-199380
- Ravandi F, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Advances. 2016;1(3):250–259. doi:10.1182/bloodadvances.2016001495
- Salami K, et al. Hematopoietic stem cell transplant versus chemotherapy plus tyrosine kinase inhibitor in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL). Hematol Oncol Stem Cell Ther. 2013;6(1):34–41. doi:10.1016/j.hemonc.2013.03.001
- Rousselot P, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774–782. doi:10.1182/blood-2016-02-700153
- Chalandon Y, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. doi:10.1182/blood-2015-02-627935
- Vignetti M, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the gruppo italiano malattie ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–3678. doi:10.1182/blood-2006-10-052746
- Swerdlow SH, et al. WHO classification of tumours of haematopoietic and lymphoid tissues (Vol. 2). International agency for research on cancer Lyon; 2008.
- Thomas DA, et al. Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi:10.1182/blood-2003-08-2958
- Lou Y, et al. Efficacy and prognostic factors of imatinib plus CALLG2008 protocol in adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Front Med. 2017;11(2):229–238. doi:10.1007/s11684-017-0506-y
- Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi:10.1002/sim.4780030106
- Fielding AK, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. doi:10.1182/blood-2013-09-529008
- Fielding AK. How I treat Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood. 2010;116(18):3409–3417. doi:10.1182/blood-2010-01-242750
- Foà R, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. doi:10.1182/blood-2011-05-351403
- Chiaretti S, et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101(12):1544–1552. doi:10.3324/haematol.2016.144535
- Martinelli G, et al. First report of the gimema LAL1811 phase II prospective study of the combination of steroids with ponatinib as frontline therapy of elderly or unfit patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2017;130(Supplement 1):99–99. doi:10.1182/blood.V130.Suppl_1.99.99
- Silva WF, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. doi:10.1016/j.leukres.2021.106666
- Kim DY, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746–756. doi:10.1182/blood-2015-03-636548
- Shen S, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia. JAMA Oncol. 2020;6(3):358–366. doi:10.1001/jamaoncol.2019.5868
- Ehsani M, et al. Cost-analysis of treatment of pediatrics acute lymphoblastic leukemia based on ALL-BFM protocol. Pediatr Blood Cancer. 2018;65:S113–S113.
- Rae C, et al. Economic evaluation of treatment for acute lymphoblastic leukaemia in childhood. Eur J Cancer Care (Engl). 2014;23(6):779–785. doi:10.1111/ecc.12173
- Ghatak N, Trehan A, Bansal D. Financial burden of therapy in families with a child with acute lymphoblastic leukemia: report from north India. Support Care Cancer. 2016;24(1):103–108. doi:10.1007/s00520-015-2757-y
- Tong WH, et al. Cost-analysis of treatment of childhood acute lymphoblastic leukemia with asparaginase preparations: the impact of expensive chemotherapy. Haematologica. 2013;98(5).
- Kaul S, et al. A retrospective analysis of treatment-related hospitalization costs of pediatric, adolescent, and young adult acute lymphoblastic leukemia. Cancer Med. 2016;5(2):221–229. doi:10.1002/cam4.583
- DiNofia AM, et al. Cost comparison by treatment arm and center-level variations in cost and inpatient days on the phase III high-risk B acute lymphoblastic leukemia trial AALL0232. Cancer Med. 2018;7(1):3–12. doi:10.1002/cam4.1206
- O'Connor D, et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood. 2014;124(7):1056–1061. doi:10.1182/blood-2014-03-560847
- Marwaha RK, et al. Pattern of mortality in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32(5):366–369. doi:10.1097/MPH.0b013e3181e0d036
- Sulis ML, et al. Effectiveness of antibacterial prophylaxis during induction chemotherapy in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65(5):e26952. doi:10.1002/pbc.26952
- Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Cancer Network. 2012;10(11):1412–1445. doi:10.6004/jnccn.2012.0146
- Faiz M, Iqbal QJ, Qureshi A. High prevalence of BCR-ABL fusion transcripts with poor prognostic impact among adult ALL patients: report from pakistan. Asia Pac J Clin Oncol. 2011;7(1):47–55. doi:10.1111/j.1743-7563.2010.01370.x
- Malagola M, Papayannidis C, Baccarani M. Tyrosine kinase inhibitors in Ph+ acute lymphoblastic leukaemia: facts and perspectives. Ann Hematol. 2016;95(5):681–693. doi:10.1007/s00277-016-2617-y
- Chalandon Y, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. doi:10.1182/blood-2015-02-627935
- F., et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Advances. 2016;1(3):250–259. doi:10.1182/bloodadvances.2016001495
- Wang J, et al. Allogeneic stem cell transplantation versus tyrosine kinase inhibitors combined with chemotherapy in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2018;24(4):741–750. doi:10.1016/j.bbmt.2017.12.777
- Ravandi F, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158–4164. doi:10.1002/cncr.29646
- Ravandi F, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214–1221. doi:10.1182/blood-2012-11-466482