ABSTRACT
Objectives
Dyskeratosis congenita (DC) is a rare inherited disease characterized by the triad of reticulate hyperpigmentation, nail dystrophy and oral leukoplakia. DC patients are considered vulnerable to external pressure, such as immunochemotherapy. There are very few cases reporting severe therapy-induced toxicities in patients with DC.
Methods
A 27-year-old woman was admitted to our hospital with a 4-month history of pancytopenia and a 7-day history of dyspnea with coughing. She was diagnosed with non-Hodgkin’s lymphoma 5 months ago. She received immunochemotherapy due to non-Hodgkin’s lymphoma but experienced recurrent fever, oral ulcer, pancytopenia, dyspnea and other symptoms during immunochemotherapy. On admission, she experienced an aggravation of respiratory symptoms, recurrent infections and acute heart failure.
Results
Laboratory examination confirmed pancytopenia, and chest computed tomography showed interstitial lung disease (ILD). Genetic analysis results confirmed the presence of DC and a TINF2 gene mutation. With continuous supportive and anti-infection treatment, her condition finally stabilized. She was discharged from the hospital after nearly 2 months.
Discussion
We reviewed similar cases and found common features that could be useful. However, the reported cases are very limited. More cases and studies are needed.
Conclusion
These cases indicate that DC patients seem more vulnerable to therapy toxicities; thus, physicians should be careful when treating these patients with chemotherapy drugs or radiation therapy. Reduced-intensity therapy may be considered.
Introduction
Dyskeratosis congenita (DC) is a rare inheritable disease with an estimated prevalence of 1/100000 in the population and a 13:1 male predominance[Citation1]. DC belongs to a class of diseases referred to as telomere biology disorders (TBDs), in which abnormally short telomere lengths and telomere biological defects act as pathogenic factors [Citation2]. To date, mutations in more than a dozen genes encoding telomere biology proteins have been described to cause TBDs. These mutations can be inherited in an X-linked recessive, autosomal dominant, or autosomal recessive pattern, while de novo and somatic mutations have only rarely been found [Citation1–3].
The classic manifestation of DC is the dermatological triad of reticulate hyperpigmentation, nail dystrophy and oral leukoplakia[Citation1]. Many patients present with a spectrum of clinical complications, including neurological, pulmonary, liver, hematological and immunologic abnormalities [Citation3]. Bone marrow failure (BMF), pulmonary disease and malignancy represent the main causes of mortality in DC patients [Citation4], accounting for 67-70%, 15-18%, and 8-10% of the deaths in DC, respectively, according to Alsabbagh’s literature review [Citation1].
Considering the disturbed telomere biology, DC patients are thought to be more vulnerable to external pressure factors, such as chemotherapy or radiation therapy, and these external pressure factors may cause serious toxicities. Shorter telomere length is associated with prolonged and severe toxicities after chemotherapy [Citation5,Citation6], and chemotherapy could impair telomere length [Citation7]. However, few cases support this hypothesis. Here, we report a patient with DC who developed severe myelosuppression and interstitial lung disease after receiving immunochemotherapy for non-Hodgkin’s lymphoma. We hope this case report will increase knowledge of this rare but complicated disease.
Case presentation
A 27-year-old woman presented to the hospital with a 4-month history of pancytopenia and a 7-day history of dyspnea with coughing. The patient had right lower abdominal pain and an abdominal mass 5 months prior to presentation. She went to the local hospital, underwent laparoscopic right hemicolectomy and was diagnosed with non-Hodgkin’s lymphoma, high-grade B-cell lymphoma, stage IVB. She received 1 cycle of R-EPOCH but had recurrent fever during the rest period, with severe oral ulcer and pancytopenia (WBC: 0.29*109/L, NEUT: 0.01*109/L, HGB: 56 g/L, PLT: 4*109/L). Her symptoms were relieved after a combined anti-infection, blood transfusion and elevation of WBC treatments (WBC: 2.42*109/L, NEUT: 1.99*109/L, HGB: 85 g/L, PLT: 73*109/L, 5 days later). Therefore, her treatment was changed to R-CHOP for the second cycle, and she still had a recurrent fever. Three weeks ago, she received rituximab 600 mg iv. She still had fever and pancytopenia (WBC: 2.9*109/L, NEUT: 2.12*109/L, HGB: 82 g/L, PLT: 51*109/L), and 7 days after receiving rituximab, she began to experience mild dyspnea with coughing. The patient underwent bilateral nasolacrimal duct reconstruction 3 years ago. The personal and family history did not reveal any problems.
On admission, her temperature was 37.0°C, pulse was 104 bpm, respiratory rate was 20 breaths/min, and blood pressure was 102/68 mmHg. Hyperpigmentation areas were found on her neck, face, abdomen and back. Leukoplakia and cracks were observed on her tongue. Her nails were atrophic. Mild wet rales could be heard in the inferior area of the left lung. After hospitalization, blood analysis confirmed her pancytopenia (WBC: 1.72*109/L, NEUT: 0.85*109/L, HGB: 80 g/L, PLT: 26*109/L) and excluded common rheumatic and immune diseases. There was no evidence of infectious diseases. Positron emission tomography/computed tomography (PET/CT) showed multiple bilateral reticular and patchy opacities, with a maximal standardized value of uptake (SUVmax) of 4.7. Bone marrow biopsy revealed significant bone marrow hypoplasia. Karyotype analysis, immunohistochemistry, flow cytometric assessment and clonal gene rearrangement showed no abnormalities. Her peripheral blood sample was sent out for genetic analysis, which was returned 3 weeks later and revealed a heterozygous mutation: NM_001099274.3: c.844C > T, p.R282C (rs121918545 – CM085745). This mutation was not found in her family members.
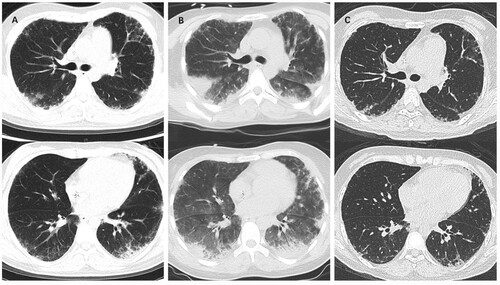
After admission, the patient’s condition deteriorated during treatment with high-grade anti-infection drugs and transfusion of RBCs and/or apheresis of platelets. On the 6th day, the patient experienced hemoptysis, and her hemoptysis progressed even with the use of hemostatics. A consultation was held, and her respiratory symptoms were determined to be acute ILD, drug-related lung injury and alveolar hemorrhage, and methylprednisolone shock therapy (240 mg qd for 2 days and then 160 mg qd for 2 days) was recommended. Her hemoptysis was relieved after methylprednisolone treatment. However, at noon on the 8th day, she suddenly experienced an exacerbation of dyspnea and became drowsy and delirious, and her SpO2 was reduced to 78%. A noninvasive ventilator was applied, which improved her condition. Chest CT on the 9th day showed that her ILD had progressed (shown in ). Laboratory examination revealed elevated NT-proBNP (7668 pg/ml), indicating acute heart failure. The patient was then sent to the respiratory intensive care unit for further monitoring and treatment. Through unremitting efforts, she gradually recovered while she still had agranulocytosis despite using recombinant human granulocyte colony-stimulating factor (rhG-CSF) (WBC: 2.13*109/L, NEUT: 1.54*109/L, HGB: 84 g/L, PLT: 27*109/L). She was transferred back to the general ward on the 25th day of admission. During hospitalization, she also experienced gastrointestinal bleeding (considered secondary to poor coagulative function), upper respiratory tract infection and headache but was relieved with immediate and appropriate treatment. The patient’s condition finally stabilized, but her peripheral blood counts were still low. Danazol was used on the 54th day (WBC: 0.87*109/L, NEUT: 0.16*109/L, HGB: 68 g/L, PLT: 10*109/L). The patient’s peripheral blood counts increased after using danazol for 2 days (WBC: 1.73*109/L, NEUT: 0.76*109/L, HGB: 67 g/L, PLT: 32*109/L, RET: 2.33%), and she was discharged from the hospital on the 56th day.
Discussion
Telomeres are specialized nucleoprotein structures at the ends of eukaryotic chromosomes that protect the ends of chromosomes and maintain genomic stability[Citation8]. Telomerase, a ribonucleoprotein complex that consists of the enzyme telomerase reverse transcriptase (TERT), scaffolding noncoding human telomerase RNA and associated cofactors, maintains the length of telomeres[Citation2,Citation8,Citation9]. Since progressive telomere shortening occurs in all normal cells that are actively dividing, telomerase is vital in regulating cell proliferation. Human germline mutations in genes that are involved in telomerase function or telomere maintenance result in a range of clinical conditions, including DC[Citation2]. To date, CTC1, ACD, NHP2, DC1, PARN, NOP10, TERC, RTEL1, TINF2, TERT, and WRAP53 genes have been found to contain pathogenic mutations that are known to cause DC[Citation10,Citation6].
Patients with telomere and/or telomerase dysfunction have a significantly increased risk of developing cancer[Citation4]. Head and neck squamous cell carcinoma, acute myeloid leukemia, NHL and anal squamous cell carcinoma are the most common cancer types in DC patients, and these patients are reported to develop cancer at a younger age[Citation4,Citation11]. DC patients who accept chemotherapy or radiation therapy are thought to be predisposed to severe adverse effects due to impaired telomere and/or telomerase biology. For example, a study revealed that a shorter telomere length is associated with prolonged and severe neutropenia after chemotherapy[Citation5], and anecdotal evidence suggests that chemotherapy may increase the risk for pulmonary and hepatic toxicities[Citation6]. We searched the PubMed, Scopus, Medline, CNKI and Wanfang databases for related cases. However, very limited cases were found, and they are reviewed in .
Table 1. The literature review of severe therapy-induced toxicities in DC patients.
These cases are mainly young people and carry TERT and DKC1 mutations. Types of malignancy and therapy strategies are quite different in these cases. The first case had nodular sclerosing classical Hodgkin’s lymphoma and received chemotherapy. The second case had stage IV rectal cancer and received immunochemotherapy with cetuximab and OK-432. The third and fourth cases had breast cancer and tongue cancer, respectively, and they both received radiation therapy. Skin rash or mucositis are reported in most cases, while they were not observed in our case. Pneumonitis and cytopenia are reported in over half of the cases, and they could simultaneously occur, as in Agrusa’s case and our case. The outcome and prognosis of these patients are not optimistic. These cases indicate that physicians should pay close attention to skin or mucosal symptoms when treating DC patients with chemotherapy drugs or radiation therapy. Pneumonitis and cytopenia are common but severe toxicities; therefore, chest CT should be routinely performed and peripheral blood counts should be closely surveilled.
Early recognition of DC is very important; thus, physicians should be more careful when potentially toxic therapies are administered. However, it may be challenging because family and personal history are often inconspicuous[Citation12], and physicians may lack the essential knowledge to identify this rare disease. In China, most DC cases are diagnosed in dermatological departments[Citation16]; thus, we suggest that physicians in other departments request a dermatology consultation after finding skin or mucosal abnormalities in patients with unknown cytopenia. In addition to an increased risk of cancer, patients with DC are predisposed to unreversible manifestations, such as bone marrow failure, pulmonary fibrosis, liver disease and vascular abnormalities. External pressure factors such as chemotherapy or radiation therapy can trigger or accelerate these lethal manifestations. Therefore, a reduced intensity of chemotherapy, immunochemotherapy or radiation therapy was recommended[Citation17].
There are many limitations in this case. For example, we did not obtain telomere length data. The cases we found were so limited that the literature review may be unconvincing. This may be because the incidence of DC is rare, and the number of DC patients who develop malignancy and accept chemotherapy, immunochemotherapy or radiation therapy is even lower; additionally, doctors may ignore the genetic background of the patients and regard the toxicities as usual side effects of therapy. More cases and studies are needed to explore severe therapy-induced toxicities in DC patients.
Conclusion
In conclusion, we report a rare case of DC. The patient had severe immunochemotherapy-induced toxicities after receiving immunochemotherapy for her lymphoma. We reviewed similar cases and found some common features that could be useful. These cases indicate that DC patients seem more vulnerable to therapy toxicities; thus, physicians should be careful when treating these patients with chemotherapy or radiation therapy, and reduced-intensity therapy may be considered.
Statements
Statement of ethics
Study approval statement: Informed consent for the collection of the patient’s medical history and blood samples was obtained in compliance with the Declaration of Helsinki and approved by the local ethical committee.
Consent to publish statement
Written informed consent was obtained from the patient and her relatives for publication of her case and any accompanying images. The copies of the written consent are available for review by the Editor of this journal.
Acknowledgement
We thank Xiaoyan Ke, Wei Zhao and Lina Sun for their contributions in diagnosing and treating the patient.
Data Availability Statement
The data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AlSabbagh MM. Dyskeratosis congenita: a literature review. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2020;18:943–967. doi: 10.1111/ddg.14268.
- Grill S, Nandakumar J. Molecular mechanisms of telomere biology disorders. J Biol Chem. 2020;296:100064), PMID: 33482595. doi: 10.1074/jbc.REV120.014017
- Niewisch MR, Savage SA. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2019;12:1037–1052. PMID: 31478401 doi: 10.1080/17474086.2019.1662720
- Alter BP, Giri N, Savage SA, et al. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103:30–39. PMID: 29051281 doi: 10.3324/haematol.2017.178111
- Gerbing RB, Alonzo TA, Sung L, et al. Shorter Remission Telomere Length Predicts Delayed Neutrophil Recovery After Acute Myeloid Leukemia Therapy: A Report From the Children’s Oncology Group. J Clin Oncol. 2016;34:3766–3772. PMID: 27354474 doi: 10.1200/JCO.2016.66.9622
- Savage SA. Dyskeratosis Congenita. University of Washington, Seattle Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK22301/.
- Lee J-J, Nam C-E, Cho S-H, et al. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;82:492–495. doi: 10.1007/s00277-003-0691-4.
- Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1.
- Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol. 2020;21:384–397. PMID: 32242127 doi: 10.1038/s41580-020-0234-z
- Dokal I, Vulliamy T, Mason P, et al. Clinical utility gene card for: Dyskeratosis congenita – update 2015. Eur J Hum Genet. 2015;23:558–558. doi:10.1038/ejhg.2014.170
- Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. PMID: 19282459 doi: 10.1182/blood-2008-12-192880
- Agrusa JE, Bertuch AA, DiNardo CD, et al. Severe therapy-related toxicities after treatment for Hodgkin lymphoma due to a pathogenic TERT variant and shortened telomeres. Pediatr Blood Cancer. 2019;66:e27779. PMID: 31050187 DOI: 10.1002/pbc.27779
- Nakamura K, Terai S, Ohbatake Y, et al. [A Case of Stage ⅣRectal Cancer with Dyskeratosis Congenita]. Gan To Kagaku Ryoho. 2019;46:515–517.
- Walsh MF, Sacca R, Wildman T, et al. Pathogenic Loss-of-Function Germline TERT Mutations in Patients With Solid Tumors. JCO Precision Oncology. 2019;3:1–5. doi:10.1200/PO.19.00230
- Sadamoto A, Hyodo M, Hinohira Y, et al. A Case of Dyskeratosis Congenita Complicated by Tongue Cancer. Pract Otorhinolaryngol (Basel). 1999;92(3):253–257.
- Li F, Li W, Qiao X, et al. Clinical features of dyskeratosis congenita in mainland China: case reports and literature review. Int J Hematol. 2019;109:328–335. doi: 10.1007/s12185-018-02582-x.
- Olson TS, Myers KC. Dyskeratosis congenita and other telomere biology disorders - UpToDate. Dyskeratosis congenita and other telomere biology disorders. Available from: https://www.uptodate.com/contents/dyskeratosis-congenita-and-other-telomere-biology-disorders#H2790077520.