 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective:
Herein, we aimed to determine the clinical efficacy of recombinant human thrombopoietin (rhTPO) combined with glucocorticoids for treating immune thrombocytopenia (ITP).
Methods:
Clinical data of 87 patients with ITP admitted to our hospital were retrospectively analyzed, and patients were divided into two groups according to the treatment employed: 42 patients in the control group (CG) were prescribed glucocorticoids, and 45 patients in the study group (SG) received rhTPO combined with glucocorticoids.
Results:
The total effective treatment rate in the SG (95.56%) was higher than that in the CG (76.19%) (P < 0.05). The SG achieved a platelet (PLT) count > 50 × 109/L faster and required fewer PLT transfusions than the CG (P < 0.05). At 1, 7, and 14 days after treatment, the PLT count increased in both groups and was higher in the SG than in the CG (P < 0.05). After treatment, CD3+, CD4+, and CD4+/CD8+ T cells increased, whereas CD8 + decreased in both groups, with the SG exhibiting a superior improvement to the CG (P < 0.05). Considering prothrombin time, activated partial thromboplastin time, and fibrinogen, differences between the two groups were not statistically significant, both before and after treatment (P > 0.05).
Conclusion:
rhTPO combined with glucocorticoids for treating ITP can effectively enhance the therapeutic effect, regulate the T lymphocyte subpopulation, rapidly increase the PLT level, and induce no significant effect on the coagulation function of patients, with good safety and high clinical promotion value.
Introduction
As a common disorder of the hematological system, idiopathic thrombocytopenic purpura (ITP) is an acquired autoimmune disease caused by thrombocytopenia [Citation1]. At disease onset, most patients with ITP exhibit only a reduction in platelet (PLT) count, and only approximately 5% of patients experience severe bleeding symptoms. The risk of bleeding events increases with disease progression and aging, and predominant clinical manifestations typically include bleeding from the nasal cavity, skin, and gums, as well as internal bleeding in severe cases, which can endanger the patient [Citation2]. The current objective of ITP treatment is to elevate PLT levels to within the normal range and reduce the occurrence of bleeding events. Glucocorticoids remain the first-line drugs for ITP in clinical practice and effectively relieve thrombocytopenia symptoms; however, only 40–50% of patients achieve a persistent response following discontinuation or hormone reduction. Moreover, long-term glucocorticoid therapy is well-known to be associated with adverse effects, thereby impacting therapeutic efficacy [Citation3, Citation4].
In recent years, accumulating data have revealed that thrombopoietin can effectively promote PLT production, and recombinant human thrombopoietin (rhTPO) is a widely employed peptide as a second-line therapeutic agent for ITP in clinical settings [Citation5]. However, the effect of rhTPO alone is unsatisfactory, and its overall efficacy needs to be improved [Citation6]. Combining rhTPO and glucocorticoids for treating ITP could afford synergistic effects and enhance the therapeutic effect [Citation7]. Although the combined use of the two agents has been previously explored, few reports have examined the impact of this combination on immune function and coagulation in patients with ITP [Citation8]. In the present study, we retrospectively analyzed clinical data of 87 patients with ITP and compared the therapeutic effects of different treatment strategies to provide a reference for the clinical formulation of drug treatment protocols for ITP.
Materials and methods
Clinical data
Clinical data of 87 patients with ITP admitted to our hospital between July 2019 and November 2020 were retrospectively analyzed. Patients were divided into two groups according to the different treatment protocols: 42 patients in the control group (CG) and 45 in the study group (SG). Inclusion criteria include patients who met the relevant diagnostic criteria of the Chinese Expert Consensus on the Diagnosis and Treatment of Adult Primary ITP [Citation9], aged between 18 and 60 years, those with a PLT count <20 × 109/L upon admission, and those with active bleeding in the oral, nasal, and urinary tract. Exclusion criteria were allergy to study drugs, acute infections, family history of malignant hematological diseases, serious cardiovascular and cerebrovascular diseases, pregnant or lactating women, and those previously treated with drugs affecting the evaluation of efficacy before enrollment. The study was approved by the ethics committee of the Suqian First People's Hospital affiliated with Nanjing Medical University. All participants provided written informed consent before study participation.
Treatment options
The CG was treated with glucocorticoids. Dexamethasone sodium phosphate injection (Jilin Aodong Pharmaceutical Group Yanji Co., Ltd., H22022888) was administered intravenously at 0.6 mg/(kg·day) once daily after 3–5 days of admission. After the PLT count increased to the normal range, patients were treated with prednisone acetate tablets (Jilin Gurette Pharmaceutical Co., Ltd., H22021758) at 1 mg/(kg·day) once daily, and the dosage was gradually reduced according to the patient’s condition. The SG was treated with rhTPO (Shenyang Sansheng Pharmaceutical Co., Ltd., S20050049) based on glucocorticoid treatment and subcutaneously injected with 300 U/(kg·day) once daily for 14 days. During the treatment period, a PLT count of ≤20 × 109/L could present a marked bleeding tendency, and a PLT suspension was transfused. Given that all patients included in this group had a PLT count ≤20 × 109/L, a PLT suspension was administered according to the specific situation. Both groups were treated for 14 days, and a curative effect was observed.
Outcome measurement
Clinical efficacy. Markedly effective: PLT returned to the normal range after treatment and no bleeding symptoms; effective: PLT increased by 30 × 109/L or PLT ≥50 × 109/L after treatment and no bleeding symptoms; ineffective: not meeting the criteria of effective and effective or symptoms worsened. The total effective rate is the sum of the markedly effective and effective rates.
Time to PLT count > 50 × 109/L, number of transfusions of apheresis PLTs, and bleeding score. The time to PLT count > 50 × 109/L and the number of transfusions of apheresis PLTs in both groups were recorded. Bleeding score: According to the standards of literature [Citation10], the bleeding score of patients was calculated according to the severity of skin bleeding, mucosal bleeding, and deep organ bleeding, combined with the age of patients.
Changes in PLT levels before and after treatment. Venous blood was drawn from the upper extremities of patients in both groups before, treatment and 1, 7, and 14 days after treatment, and PLT levels were measured using a COULTER LH 750 automatic blood cell analyzer (Beckman Coulter Trading (China) Co).
T lymphocyte subpopulations. Fasting venous blood (5 mL) was drawn from the two groups before and after treatment, placed in EDTA anticoagulant tubes, mixed with corresponding antibodies and their isotypes, and incubated for 15 min at 4°C in the dark. Red blood cells were lysed with Q-PREP and centrifuged at 1200 rpm for 5 min; subsequently, the supernatant was discarded, and cells were washed in phosphate-buffered saline. The levels of T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+) were measured using a CytoFLEX flow cytometer (Beckman Coulter Commercial Enterprise [China] Co., Ltd).
Coagulation function. Prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (FIB) levels were measured before and after treatment using an SYSMEXCA-550 automatic coagulation analyzer (Shanghai YuYan Scientific Instruments Co.)
Adverse reactions. The occurrence of adverse reactions such as muscle pain, dizziness, nausea and vomiting, and fever during treatment were recorded.
Follow-up. After discharge, all patients were followed up by telephone or outpatient review once every 15 days.
Statistical analysis
Data analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA), and measured data conforming to normal distribution were expressed as Paired and independent t-tests were performed to compare between and within groups, respectively. The count data are expressed as n (%). In addition, the χ2 test was performed. A P value < 0.05 was considered statistically significant.
Results
General information
The differences in sex, age, disease duration, and body mass index between the two groups were not statistically significant (P > 0.05) and presented comparable values ().
Table 1. Comparison of baseline data between two groups (n, ±S).
Clinical efficacy
The total effective treatment rate in the SG (95.56%) was higher than that in the CG (76.19%) (P < 0.05), indicating that rhTPO combined with glucocorticoids for ITP could effectively enhance the treatment efficacy ().
Table 2. Comparison of clinical efficacy (n)%.
Time to PLT > 50*109/L, number of transfusions of apheresis platelets, and bleeding score
The time to achieve a PLT count > 50 × 109/L, number of platelet transfusions, and bleeding score were lower in the SG than those in the CG (P < 0.05), suggesting that rhTPO combined with glucocorticoid could help shorten the time to normalize PLT, reduce platelet transfusions, and improve bleeding symptoms ().
Table 3. Comparison of the time to PLT > 50*109/L, the number of platelet transfusions and bleeding score in both groups (±S).
Changes in PLT count
Before treatment, no significant difference in PLT count was noted between the two groups (P > 0.05); however, PLT count increased in both groups 1, 7, and 14 days after treatment and was higher in the SG than in the CG (P < 0.05), indicating that rhTPO combined with glucocorticoids could increase the PLT count ().
Table 4. Comparison of PLT before and after treatment between the two groups (±S, *109/L).
Changes in T lymphocyte subpopulations
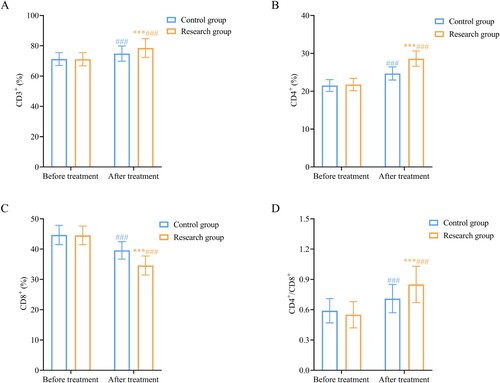
Before treatment, the difference in T lymphocyte subsets between the two groups was not statistically significant (P > 0.05). After treatment, CD3 +, CD4 +, and CD4 +/CD8 + T cells increased, while CD8 + decreased in the two groups, and the SG showed superior improvement to the CG (P < 0.05), indicating that rhTPO combined with glucocorticoids could help improve immune function in patients ().
Figure 1. Comparison of changes in T lymphocyte subpopulations between the two groups.
shows that rhTPO combined with glucocorticoid treatment significantly increases (A) CD3+, (B) CD4+, (D) CD4+/CD8+ levels, and decreases (C) CD8 + levels, suggesting that rhTPO combined with glucocorticoids could improve the immune function of patients. Note: Compared with the control group, ***P < 0.001; compared within the same group before treatment, ###P < 0.001. rhTPO, recombinant human thrombopoietin.
Changes in coagulation function
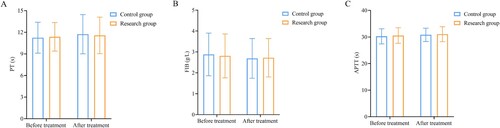
Before and after treatment, we noted no statistically significant differences in PT, FIB, and APTT between the two groups (P > 0.05), indicating that rhTPO combined with glucocorticoids did not impact the coagulation function of patients ().
Figure 2. Comparison of the changes in coagulation function between the two groups.
shows that the differences in (A) PT, (B) FIB, and (C) APTT between the two groups are not statistically significant before and after rhTPO combined with glucocorticoid therapy, suggesting that rhTPO combined with glucocorticoids did not affect the coagulation function of patients. APTT, activated partial thromboplastin time; FB, fibrinogen; PT, prothrombin time; rhTPO, recombinant human thrombopoietin.
Adverse reactions
Comparing the incidence of adverse reactions in the two groups (4.76% vs. 8.89%), the difference was not statistically significant (P > 0.05), suggesting that rhTPO combined with glucocorticoids does not significantly increase adverse reactions and has a good safety profile ().
Table 5. Comparison of adverse reactions between two groups (n)%.
Follow-up
In the SG, 45 patients were followed up for more than 3 months, and treatment effectiveness persisted in 35 patients (77.78%) three months after treatment. In the CG, 42 patients were followed up for more than 3 months, and treatment effectiveness persisted in 25 patients (59.52%) three months after treatment. The long-term response rate in the SG was slightly higher than that in the CG; however, the difference was not statistically significant (χ2 = 3.382, P > 0.05).
Discussion
ITP is a relatively common hemorrhagic, acquired autoimmune disease with complex pathogenesis that seriously affects the quality of life of patients. Currently, there is no clinically effective method to completely cure ITP; however, the main therapeutic objective is to control bleeding and improve the PLT level of patients. Rapid elevation of PLT levels over a short duration is critical to improving the prognosis of patients with severe symptoms [Citation11, Citation12].
Herein, rhTPO combined with glucocorticoids was used for ITP therapy. The results revealed that the total effective rate of treatment in the SG (95.56%) was higher than that in the CG (76.19%), whereas the time to PLT > 50 × 109/L and the number of platelet transfusions were lower than those in the CG. The PLT count increased in both groups at 1, 7, and 14 days after treatment and was higher in the SG than in the CG, suggesting that rhTPO combined with glucocorticoids can effectively enhance treatment efficacy and rapidly increase PLT levels. These findings could be attributed to the use of glucocorticoids as the current drug of choice for treating ITP, which can inhibit macrophage phagocytosis by suppressing autoantibody production [Citation13]. Revilla et al. [Citation14] have found that glucocorticoids significantly increased PLT production in patients. However, the treatment of ITP with glucocorticoids alone was ineffective in some patients, and efficacy was not maintained in most patients after dosage reduction or discontinuation of therapy [Citation15]. Therefore, combined therapy with other drugs needs to be established. As an important physiological factor regulating PLT production, thrombopoietin can promote PLT production by binding to its receptors and activating pathways, such as ATAT and JAK, to promote megakaryocyte maturation [Citation16]. Moreover, thrombopoietin is an endogenous growth factor for megakaryocytes, which play a role in regulating PLT counts by binding to thrombopoietin receptors and PLT on the surface of macrophages [Citation17]. In contrast, rhTPO is a glycoprotein molecule produced by genetic recombination technology, which acts similarly to endogenous thrombopoietin, can effectively promote the proliferation and differentiation of megakaryocytes, and stimulate platelet production [Citation18, Citation19]. Reportedly, thrombopoietin may exhibit immunomodulatory effects, and some patients with ITP can maintain PLT levels within the normal range even after discontinuing rhTPO [Citation20]. Zhang et al. [Citation21] have reported that the efficiency of rhTPO combined with glucocorticoids during ITP treatment was 88.33%, which was significantly higher than that of glucocorticoid treatment alone (71.67%); these findings are consistent with the results of the present study.
The pathogenesis of ITP is complex and is primarily associated with abnormal immune function, which affects megakaryocyte function, inhibits platelet production and release in the bone marrow, and causes excessive destruction of platelets mediated by cellular and humoral immunity [Citation22, Citation23]. PLTs reportedly contain a large subpopulation of T lymphocytes on their surface; among these, CD3 + reflects the levels of T lymphocytes, and reduced levels indicate a decrease in the immune function, further aggravating the patient's condition [Citation24]. CD4 + cells are part of the immune system and are a type of white blood cell. A reduction in CD4 + levels results in continuous B lymphocyte proliferation, inducing the production of a large number of antibodies and PLT destruction. CD8 + is a cytotoxic T cell exhibiting functions such as immune response suppression, which can play a negative regulatory role by suppressing CD4+; an increase in this level indicates a disordered immune function. The results of the present study revealed that treatment increased CD3+, CD4+, CD4+/CD8 + levels and decreased CD8 + in both groups, and the improvement in these indicators was better in the SG than that in the CG; these findings are consistent with those of Wu et al. [Citation25]. This indicates that rhTPO combined with glucocorticoids can effectively promote immune function recovery.
Moreover, we detected no significant changes in PT, FIB, and APTT before and after treatment in the two groups, and the incidence of adverse reactions in the two groups was comparable. These findings suggest that rhTPO combined with glucocorticoids did not markedly impact the coagulation function of patients and did not significantly increase adverse reactions. However, the present study is a retrospective analysis, and limitations such as the small sample size and single-source data need to be considered. Moreover, the long-term efficacy and recurrence associated with rhTPO combined with glucocorticoid during ITP therapy were not examined, which should be examined in the future.
In conclusion, rhTPO combined with glucocorticoids in the treatment of ITP can effectively enhance the therapeutic effect, regulate the T lymphocyte subpopulation, rapidly increase the PLT level, and spare the coagulation function of patients while affording good safety and high clinical promotion value.
Authors’ contributions
Jing-Xin Zhou, Ling Gao, and Wen-tong Ma conceived the project and designed the experiments. Jing-Xin Zhou, Ling Gao, Nan Hu, Zhi-Ling Yan, Chun-Ying Tian, Jing Su, Ji-Jin Qi, Jun-Shuai Yue, and Wen-Tong Ma performed the experiments and analyzed the data. Jing-Xin Zhou and Wen-Tong Ma wrote and revised the manuscript.
Acknowledgments
This work was supported by the Natural Science Foundation of Suqian-Youth Technology personnel project (grant number K201905).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Qu M, Liu XN, Liu XG, et al. Cytokine changes in response to TPO receptor agonist treatment in primary immune thrombocytopenia. Cytokine. 2017;92:110–117.
- Elalfy MS, Eltonbary K, El Ghamry IR, et al. Intracranial hemorrhage in primary immune thrombocytopenia (ITP): 20 years’ experience in pediatrics. Eur J Pediatr 2021;180:1545–1552.
- Kim HY, Park SW, Kim JH, et al. Romiplostim-related Myelofibrosis in Refractory Primary Immune Thrombocytopenia: A Case Report. Medicine (Baltimore). 2019;98:e15882.
- Lin J, Zhang X, Li X, et al. Cost of bleeding-related episodes in adult patients With primary immune thrombocytopenia: A population-based retrospective cohort study of administrative claims data for commercial payers in the United States. Clin Ther. 2017;39:603–609.e1.
- Yang ZG, Wen RT, Zhang YM, et al. Thalidomide induce response in patients with corticosteroid-resistant or relapsed ITP by upregulating neuropilin-1 expression. Int Immunopharmacol 2019;72:437–444.
- Xu Y, Pan Y, Zhou Z. Recombinant human thrombopoietin combined with interleukin-2 improves the effects of chemosensitivity and thrombocytopenia on a basic gemcitabine and carboplatin combination therapy for non-small cell lung cancer in a nude mouse model. J Thorac Dis. 2019;11:4671–4681.
- Liu Y, Wang R, Han P, et al. Effect of recombinant human thrombopoietin on immune thrombocytopenia in pregnancy in a murine model. Int Immunopharmacol 2019;67:287–293.
- Liu WB, Li S, Yu XL, et al. Research progress on Chinese medicine immunomodulatory intervention for chronic primary immune thrombocytopenia: targeting cellular immunity. Chin J Integr Med. 2019;25:483–489.
- Hemostasis and Thrombosis Group of the Chinese Medical Association Hematology Branch. Chinese expert consensus on the diagnosis and treatment of primary immune thrombocytopenia in adults (2016 edition). Chin J Hematol. 2016;37:89–93.
- Sun L, Wang J, Shao L, et al. Dexamethasone plus oseltamivir versus dexamethasone in treatment-naive primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2021;8:e289–e98.
- Cui D, Lv Y, Yuan X, et al. Increased expressions of OX40 and OX40 ligand in patients with primary immune thrombocytopenia. J Immunol Res. 2019;2019:6804806.
- Kado R, McCune WJ. Treatment of primary and secondary immune thrombocytopenia. Curr Opin Rheumatol. 2019;31:213–222.
- Ptushkin VV, Vinogradova OY, Pankrashkina MM, et al. Thrombopoietin receptor agonists in the treatment of chronic resistant primary immune thrombocytopenia: efficacy and safety data in real. Clinical Practice Ter. Arkh. 2018;90:70–76.
- Revilla N, Corral J, Miñano A, et al. Multirefractory primary immune thrombocytopenia; targeting the decreased sialic acid content. Platelets. 2019;30:743–751.
- Hernández-Sánchez JM, Bastida JM, Alonso-López D, et al. Transcriptomic analysis of patients with immune thrombocytopenia treated with eltrombopag. Platelets. 2020;31:993–1000.
- Yu Y, Wang M, Hou Y, et al. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: A prospective, multicenter, randomized trial. Am J Hematol 2020;95:1542–1552.
- Sun HP, Hu Q, You JH, et al. Efficacy and safety of recombinant human thrombopoietin in adult patients with primary immune thrombocytopenia during the perioperative period. Zhonghua Xue Ye Xue Za Zhi. 2019;40:191–194.
- Luo HQ, Zhong YG, Feng WY. Effect of rhTPO on the immune function of T,B lymphocytes in patients with primary immune thrombocytopenic purpura. Chin J Exp Hematol. 2019;27:2089–2091.
- Cai HC, Wang SJ, Fu L, et al. A prospective study of the efficacy and safety of maintenance therapy with recombinant human thrombopoietin in patients with primary immune thrombocytopenia: a multicenter study. Zhonghua Xue Ye Xue Za Zhi. 2017;38:379–383.
- Sun H, Hu Q, You J, et al. Efficacy and safety of recombinant human thrombopoietin in adult patients with primary immune thrombocytopenia during the perioperative period. Zhonghua xue ye xue za zhi = Zhonghua Xueyexue Zazhi. 2019;40:191–194.
- Zhang YF, Zhou L, Yao GL, et al. Clinical trial of recombinant human thrombopoietin injection combined with dexamethasone injection and prednisone tablets in the treatment of severe immune thrombocytopenia. Chin J Clin Pharmacol. 2018;11:50–52.
- Zhou Z, Feng T, Xie Y, et al. The effect of recombinant human thrombopoietin (rhTPO) on sepsis patients with acute severe thrombocytopenia: a study protocol for a multicentre randomised controlled trial (RESCUE trial). BMC Infect Dis 2019;19:780.
- Deng J, Hu H, Huang F, et al. Comparative efficacy and safety of thrombopoietin receptor agonists in adults With thrombocytopenia: A systematic review and network meta-analysis of randomized controlled trial front. Pharmacol. 2021;12:704093.
- Zhang H, Pan X, Chen Z, et al. Changes in platelet surface CD41, CD62P and T lymphocyte subpopulation levels in patients with primary immune thrombocytopenia. Shandong Med J. 2014;54:60–62.
- Wu K, Zhao Y, Qu Z, et al. Efficacy of recombinant human thrombopoietin injection combined with hormone in the treatment of severe immune thrombocytopenia and the effect on T lymphocyte subsets in patients. Chin Pharm. 2019;22:1660–1662.
