ABSTRACT
Background
Despite conspicuous advances in innovating novel drugs and combination regimens in multiple myeloma (MM) in recent decades, the most appropriate maintenance regimens after inductive therapy are still controversial and opaque.
Objective
We aimed to identify the most effective maintenance treatment for newly diagnosed multiple myeloma (NDMM) patients via network meta-analysis.
Method
We searched PubMed, Embase, Cochrane Library, Scopus, and Google Scholars from inception to April, 2022. Odds ratios (ORs) were generated for dichotomous variants. The primary endpoint was overall survival (OS).
Results
Eventually a total of 19 trials, including 11 treatments and 8337 patients, were included in this analysis. For OS, lenalidomide (OR ranged from 1.61 to 1.99) and daratumumab (OR ranged from 1.83 to 2.41) showed significant efficacy over placebo. Maintenance therapy comprising lenalidomide-carfilzomib (OR ranged from 3.19 to 6.95), lenalidomide-prednisone (OR ranged from 2.62 to 4.44), bortezomib-thalidomide (OR ranged from 2.48 to 3.64), daratumumab (OR ranged from 2.0 to 2.98), lenalidomide (OR ranged from 1.4 to 3.19), ixazomib (OR ranged from 1.36 to 2.05), thalidomide (OR ranged from 1.5 to 1.86) demonstrated significant effects in prolonging PFS compared with placebo; Among the efficient therapies, lenalidomide-carfilzomib was significantly superior to lenalidomide (OR ranged from 2.18 to 2.20), daratumumab (OR ranged from 1.49 to 2.66) and ixazomib (OR ranged from 2.75 to 3.57).
Conclusion
Considering OS and PFS, lenalidomide-carfilzomib should be recommended as the best therapy. In clinical practice, this must be weighed against the increased risk of adverse events and financial burden. However, more head-to-head studies are needed to confirm these findings.
1. Introduction
Multiple myeloma (MM) is the second most common hematological malignancy that originates from plasma cells. Despite advances in novel agents, the relapse and progression of MM were still inevitable after induction and consolidation treatment [Citation1]. Therefore, to conquer this dilemma, literally to prolong the long-term survival of MM patients, maintenance therapies presumably enhancing the response of initial treatment have been proposed and widely investigated [Citation2].
In the 1990s and early 2000s, interferon with glucocorticoids as maintenance therapies demonstrated marginal benefit in OS and PFS, but came along with significant toxicity. Since the toxicity outweighed benefits, the strategies fell out of favor [Citation3].
Then, thalidomide, the first immunomodulatory drug (IMiD), had been studied in multiple phase III RCTs and meta-analyses as maintenance therapy which showed a significant increment in PFS but not OS. However, the unaccepted adverse events relevant to thalidomide led to high discontinuation rate, compromising its efficacy [Citation4].
After that, in 2005, lenalidomide, the second IMiD, was come out resembling the intention of thalidomide. Its efficacy was widely confirmed and it was established as the standard treatment of MM maintenance treatment [Citation5].
Recently, thriving novel agents with multiple mechanisms were innovated, including proteasome inhibitors bortezomib, carfilzomib, and ixazomib, and the monoclonal antibodies daratumumab, elotuzumab.
Certainly, it is blithe to own so many new agents with validated efficacy of extend the life of MM patients. However, simultaneously, it is equivocal for clinicians to choose those agents owing to the paucity of direct comparisons among these drugs.
Given the fact there were meager head-to-head studies on efficacy and safety of novel drugs in maintenance treatment, we conducted a network meta-analysis to materialize indirect comparisons aiming at providing evidence for clinical practice.
2. Method
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systemic Review and Meta-analyses (PRISMA) checklist. ()
2.1 Search strategy
A comprehensive literature search was performed using databases including PubMed, Embase, Cochrane Library, Scopus, and Google Scholars to identify relevant RCTs from inception to April 2022. We also tried to contact the authors of the literature for more published and unpublished studies.
2.2 Selection and eligibility criteria
Two investigators independently searched and assessed the eligibility of each study by reading the title and abstract or even full-text when necessary.
The inclusion criteria were:
The study subjects were newly diagnosed MM patients;
The study design was RCT;
Different maintenance treatments were compared, with maintenance treatments should include 1 or more novel agents (thalidomide, lenalidomide, bortezomib, carfilzomib, ixazomib and daratumumab) in at least 1 arm;
Sufficient information was provided on PFS and/or OS;
The exclusion criteria were as follows:
Duplicate literature;
Data required for analysis was not reported
Animal studies or in vitro studies or conference proceedings.
2.3 Data extraction and quality assessment
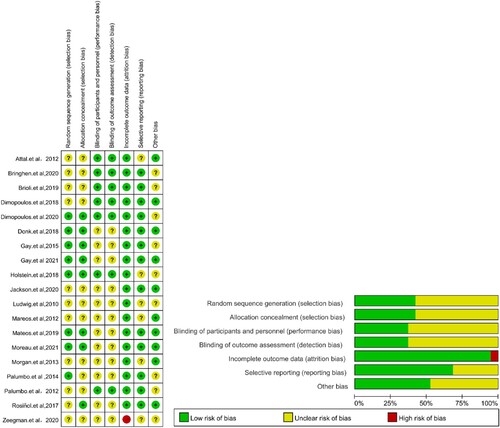
The quality of each randomized controlled trial was evaluated according to Cochrane bias risk tools by Review Manager 5.3. ()
The following data were collected independently by two investigators:
Study characteristics (first authors’ surname, year of publication, sample sizes, status of ASCT, reported outcomes);
Clinical characteristics of participants (number of randomized patients, maintenance therapy, median age)
2.4 Statistical analysis
Odd ratios were generated for dichotomous variants including OS rates and PFS rates at each time node by network meta-analysis based on frequentist theory. The surface under cumulative ranking (SUCRA) curve was calculated and a higher SUCRA value indicated a higher probability of being the best treatment. However, whether the effect size of any pairwise comparison of all the regimen reached significance was judged from net-league table, literally matrix in algebra. The overall and loops inconsistency was examined to justify the robustness of all the results. Small sample effect was explored by network funnel plot. p < 0.05 was considered to be statistically significant. The software implemented was STATA 17.0 MP.
3. Results
3.1. Results of document search
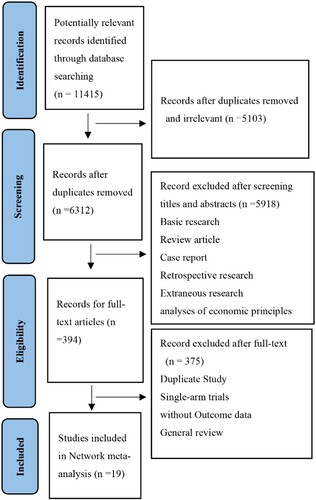
As shown in , a total of 11415 records were identified after the initial search of databases. After reading the title/abstract, 11021 records were excluded via removal of duplicates or irrelevant studies. The remaining 394 records were further analyzed by full-text reading, we identified 19 eligible randomized controlled trials that enrolled 8337 patients were included for this meta-analysis. 11 maintenance regimes were evaluated. The overall quality of the included trials was gratifying. All the included studies reported PFS data. However, five trials did not report OS data. The sample size of study ranged from 19 to 444. the mean time of follow-up ranged from 12.9 months to 4.1 years. Median age of included patients ranged from 55–76 years old. The clinical characteristics of the included studies are summarized in .
Table 1. Baseline characteristics of the studies included in this NMA.
3.2. Survival
3.2.1. Primary analysis: OS and PFS
3.2.1.1. 6-month overall survival
Compared with placebo, no regimens showed significant efficacy compared with placebo on 6 months OS rate. ()
Table 2. Matrix of pairwise comparisons of regimens on 6 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.1. 12-month overall survival
We did not also observe any significant difference of all the regimens when compared with placebo on 12 months OS rate. ()
Table 3. Matrix of pairwise comparisons of regimens on 12 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.3. 18-month overall survival
Compared with placebo, merely lenalidomide (OR = 1.99, 95%CI: 1.05–3.76) significantly increased the 18 months OS rate. ()
Table 4. Matrix of pairwise comparisons of regimens on 18 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.4. 24-month overall survival
Compared with placebo, only lenalidomide (OR = 1.79, 95%CI: 1.22–2.61) significantly increased the 24 months OS rate. ()
Table 5. Matrix of pairwise comparisons of regimens on 24 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.5. 30-month overall survival
Compared with placebo, barely lenalidomide (OR = 1.61, 95%CI: 1.09–2.36) significantly increased the 30 months OS rate. ()
Table 6. Matrix of pairwise comparisons of regimens on 30 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.6. 36-month overall survival
Compared with placebo, daratumumab (OR = 1.83, 95%CI: 1.31–2.58), lenalidomide (OR = 1.67, 95%CI: 1.23–2.28) significantly increased the 36 months OS rate. And daratumumab (OR = 1.10, 95%CI:0.69–1.74) was equivalent to lenalidomide on 36 months rate. ()
Table 7. Matrix of pairwise comparisons of regimens on 36 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.7. 42-month overall survival
Compared with placebo, daratumumab (OR = 2.07, 95%CI: 1.50–2.86), lenalidomide (OR = 1.67, 95%CI: 1.25–2.23) significantly increased the 42 months OS rate. And daratumumab (OR = 1.24, 95%CI: 0.8–1.91) was comparable with lenalidomide on 36 months OS rate. ()
Table 8. Matrix of pairwise comparisons of regimens on 42 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.8. 48-month overall survival
Compared with placebo, daratumumab (OR = 2.41, 95%CI: 1.76–3.31), lenalidomide (OR = 1.65, 95%CI: 1.16–2.35) significantly increased the 48 months OS rate. And daratumumab (OR = 1.46, 95%CI: 0.91–2.35) was equivalent to lenalidomide on 48 months OS rate. ()
Table 9. Matrix of pairwise comparisons of regimens on 48 months OS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.9. 6-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 3.19, 95%CI: 1.03–9.86), daratumumab (OR = 2.00, 95%CI:1.34–2.98), lenalidomide (OR = 1.4, 95%CI: 1.01–1.95) and ixazomib (OR = 1.36, 95%CI: 1.04–1.77) significantly increased the 6 months PFS rate. Among the efficient interventions, there was no significant difference regarding the 6 months rate. ()
Table 10. Matrix of pairwise comparisons of regimens on 6 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.10. 12-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 3.24, 95%CI: 1.48–7.08), lenalidomide-prednisone (OR = 2.62, 95%CI: 1.56–4.40), lenalidomide (OR = 2.00, 95%CI: 1.54–2.59), daratumumab (OR = 1.79, 95%CI: 1.22–2.63), and ixazomib (OR = 1.48, 95%CI:1.07–2.05) significantly increased the 12 months PFS rate. Pairwise comparison of the five regimens showed no statistically significant difference. ()
Table 11. Matrix of pairwise comparisons of regimens on 12 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.11. 18-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 6.05, 95%CI: 3.01–12.16), lenalidomide-prednisone (OR = 3.5, 95%CI: 2.23–5.5), lenalidomide (OR = 2.76, 95%CI: 2.21–3.44), bortezomib-thalidomide (OR = 2.48, 95%CI: 1.04–5.91), daratumumab (OR = 1.89, 95%CI: 1.38–2.60), ixazomib (OR = 1.83, 95%CI: 1.36–2.45) and thalidomide (OR = 1.50, 95%CI: 1.01–2.22) significantly increased the 18 months PFS rate. Among the efficient therapies, compared with lenalidomide-carfilzomib, lenalidomide (OR = 2.20, 95%CI: 1.13–4.26), daratumumab (OR = 3.20, 95%CI: 1.49–6.87), ixazomib (OR = 3.31, 95%CI: 1.56–7.05), thalidomide (OR = 4.05, 95%CI: 1.81–9.03) significantly decreased the 18 months PFS rate. lenalidomide-carfilzomib (OR = 1.73, 95%CI:0.80–3.74) was equivalent to lenalidomide-prednisone on 18 months rate. Compared with ixazomib, lenalidomide (OR = 1.51, 95%CI:1.05–2.71) significantly increased the 18 months PFS rate. ()
Table 12. Matrix of pairwise comparisons of regimens on 18 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.12. 24-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 6.34, 95%CI: 3.33–6.47), lenalidomide-prednisone (OR = 4.12, 95%CI: 2.62–6.47), bortezomib-thalidomide (OR = 3.64, 95%CI: 1.56–8.53), lenalidomide (OR = 2.87, 95%CI: 2.30–3.58), bortezomib-prednisone (OR = 2.67, 95%CI: 1.01–7.88), daratumumab (OR = 2.38, 95%CI: 1.74–3.27), ixazomib (OR = 1.8, 95%CI: 1.32–2.44) and thalidomide (OR = 1.86, 95%CI: 1.22–2.83) significantly increased the 24 months PFS rate. Thalidomide-interferon (OR = 2.16, 95%CI: 0.71–6.56) and interferon (OR = 1.14, 95%CI: 0.52–2.54) were comparable with placebo on 24 months rate. Lenalidomide-carfilzomib (OR = 1.54, 95%CI:0.75–3.17) was equivalent to lenalidomide-prednisone on 24 months rate. Compared with ixazomib, lenalidomide (OR = 1.60, 95%CI:1.10–2.33) significantly increased the 24 months PFS rate. Compared with lenalidomide-carfilzomib, lenalidomide (OR = 2.21, 95%CI: 1.21–4.04), daratumumab (OR = 2.66, 95%CI: 1.30–5.45), ixazomib (OR = 3.53, 95%CI: 1.73–7.20) significantly increased the 24 months PFS rate. ()
Table 13. Matrix of pairwise comparisons of regimens on 24 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.13. 30-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 6.95, 95%CI: 3.36–14.38), lenalidomide-prednisone (OR = 4.44, 95%CI: 2.60–7.59), bortezomib-thalidomide (OR = 3.11, 95%CI: 1.20–8.06), lenalidomide (OR = 3.19, 95%CI: 2.42–4.20), daratumumab (OR = 2.91, 95%CI: 1.96–4.31), ixazomib (OR = 1.95, 95%CI: 1.32–2.87) significantly increased the 30 months PFS rate. Thalidomide-interferon, bortezomib-prednisone, thalidomide and interferon showed no efficacy compared with placebo. Lenalidomide-carfilzomib (OR = 1.56, 95%CI:0.69–3.53) was equivalent to lenalidomide-prednisone on 30 months rate. Compared with lenalidomide-carfilzomib, lenalidomide (OR = 2.18, 95%CI: 1.11–4.27), daratumumab (OR = 2.39, 95%CI: 1.05–5.47), ixazomib (OR = 3.57, 95%CI: 1.57–8.13) significantly decreased the 30 months PFS rate. Compared with ixazomib, lenalidomide (OR = 1.64, 95%CI: 1.02–2.63) significantly increased the 30 months PFS rate. ()
Table 14. Matrix of pairwise comparisons of regimens on 30 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.14. 36-month progression-free survival
Compared with placebo, lenalidomide-carfilzomib (OR = 5.57, 95%CI: 2.41–12.87), lenalidomide-prednisone (OR = 3.56, 95%CI: 1.87–6.97), bortezomib-thalidomide (OR = 2.98, 95%CI: 1.01–8.81), lenalidomide (OR = 3.32, 95%CI: 2.31–4.76), daratumumab (OR = 2.98, 95%CI: 1.01–8.81), ixazomib (OR = 2.03, 95%CI: 1.23–3.33) significantly increased the 36 months PFS rate. Compared with ixazomib, lenalidomide-carfilzomib (OR = 2.75, 95%CI:1.04–7.27) significantly increased the 36 months PFS rate. there was no significant difference among the pairwise comparison of other efficient interventions regarding the 36 months rate. ()
Table 15. Matrix of pairwise comparisons of regimens on 36 months PFS rate (shown as odds ratios and 95% confidence intervals).
3.2.1.15. 42-month progression-free survival
Compared with placebo, lenalidomide-prednisone (OR = 3.62, 95%CI: 1.72–7.63), lenalidomide (OR = 2.94, 95%CI: 1.93–4.5), ixazomib (OR = 2.05, 95%CI: 1.14–3.68) significantly increased the 42 months PFS rate. Pairwise comparison of the three regimens showed no statistically significant differences. ()
Table 16. Matrix of pairwise comparisons of regimens on 42 months PFS rate (shown as odds ratios and 95% confidence intervals).
No overall nor loops inconsistency existed in the analysis of each endpoint.
The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo regarding on 6-, 18-, 24- months OS and 6-, 12-,18-,24- months PFS. Small sample study effects did exist in the comparison between ixazomib and placebo on 30 months OS and 30 months PFS. Comparison between bortezomib-thalidomide and thalidomide showed small sample effects on 12-, 36-, 42- months OS. Small sample study effects also existed in the studies of comparison between thalidomide-interferon vs interferon on 36-, 42- months PFS. ()
Figure 3. A. Network plot of pairwise comparisons of regimens on 6,12,18 months OS. B. Network plot of pairwise comparisons of regimens on 24, 30 months OS. C. Network plot of pairwise comparisons of regimens on 36,42,48 months OS. D. Network plot of pairwise comparisons of regimens on 6, 12, 18, 24, 30, 36 months PFS. E. Network plot of pairwise comparisons of regimens on 42 months PFS. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
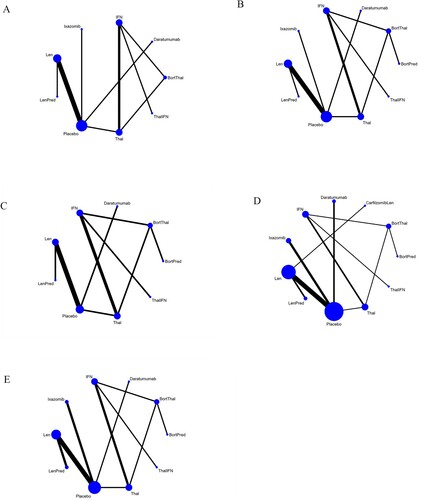
Figure 4. The network funnel plots of pairwise comparisons of regimens on 6 months OS rate. Abbreviation: A = BortThal, B = Daratumumab, C = IFN, D = Ixazomib, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
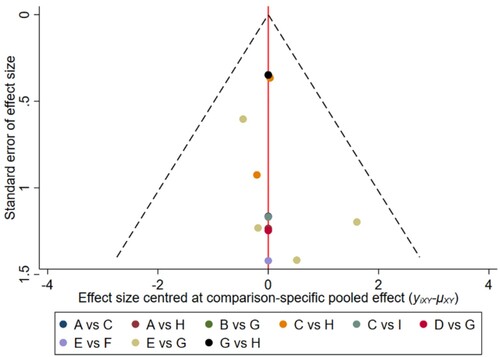
Figure 5. The network funnel plots of pairwise comparisons of regimens on 12 months OS rate. Abbreviation: A = BortThal, B = Daratumumab, C = IFN, D = Ixazomib, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of bortezomib-thalidomide comparing thalidomide. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
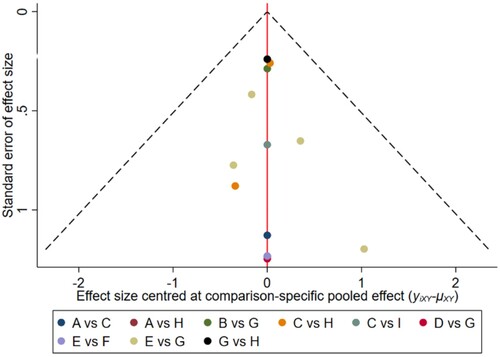
Figure 6. The network funnel plots of pairwise comparisons of regimens on 18 months OS rate. Abbreviation: A = BortThal, B = Daratumumab, C = IFN, D = Ixazomib, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
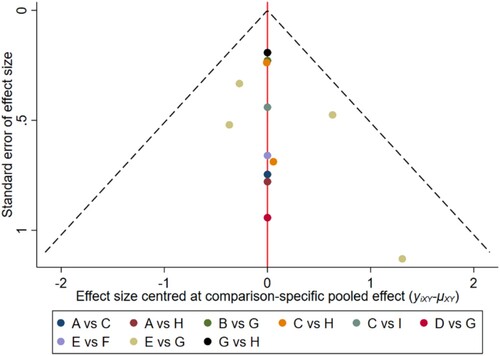
Figure 7. The network funnel plots of pairwise comparisons of regimens on 24 months OS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Ixazomib, F = Len, G = LenPred, H = Placebo, I = Thal, J = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
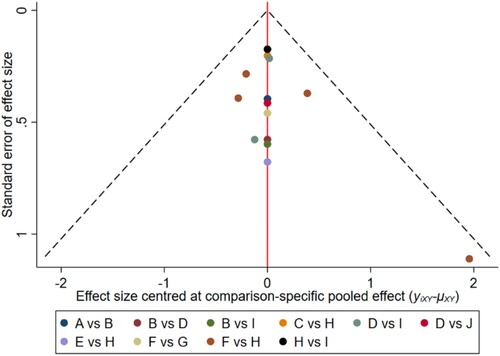
Figure 8. The network funnel plots of pairwise comparisons of regimens on 30 months OS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Ixazomib, F = Len, G = LenPred, H = Placebo, I = Thal, J = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of ixazomib comparing placebo. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
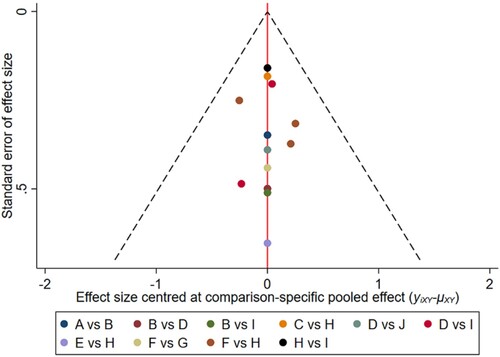
Figure 9. The network funnel plots of pairwise comparisons of regimens on 36 months OS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of bortezomib-thalidomide comparing thalidomide. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
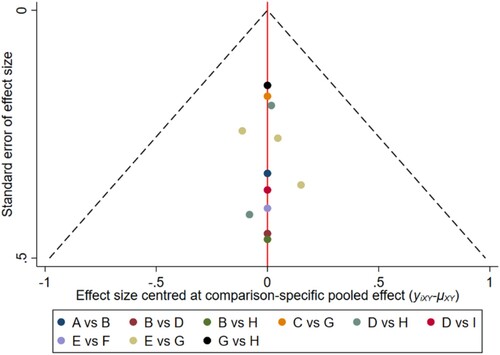
Figure 10. The network funnel plots of pairwise comparisons of regimens on 42 months OS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of bortezomib-thalidomide comparing thalidomide. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
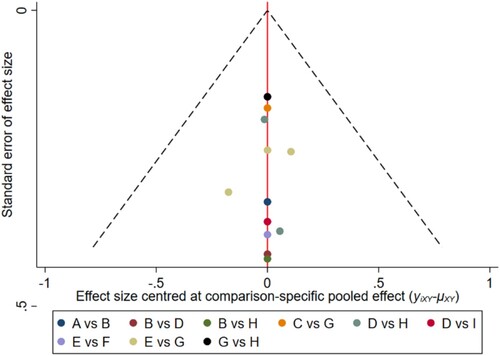
Figure 11. The network funnel plots of pairwise comparisons of regimens on 48 months OS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Len, F = LenPred, G = Placebo, H = Thal, I = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of bortezomib-thalidomide comparing thalidomide. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
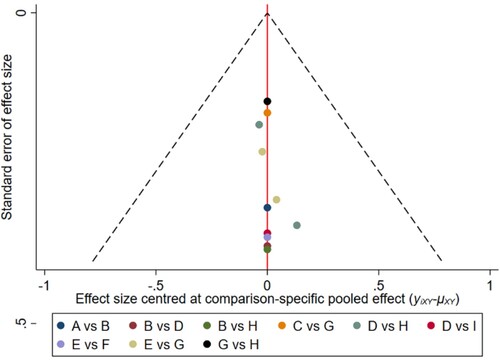
Figure 12. The network funnel plots of pairwise comparisons of regimens on 6 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomibLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
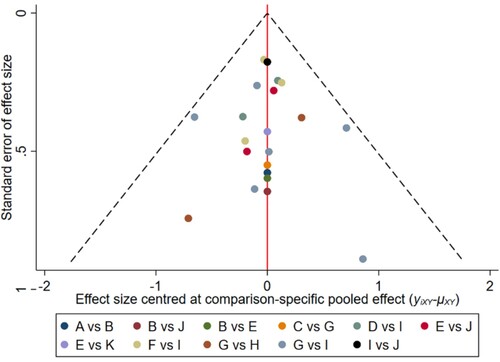
Figure 13. The network funnel plots of pairwise comparisons of regimens on 12 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomiLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
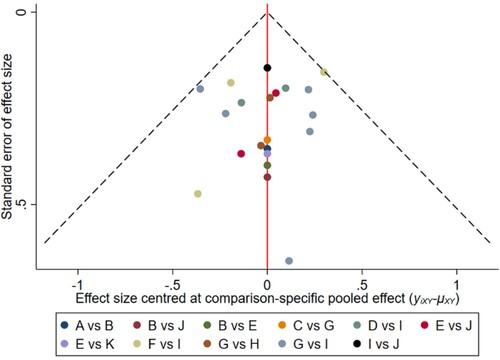
Figure 14. The network funnel plots of pairwise comparisons of regimens on 18 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomiLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
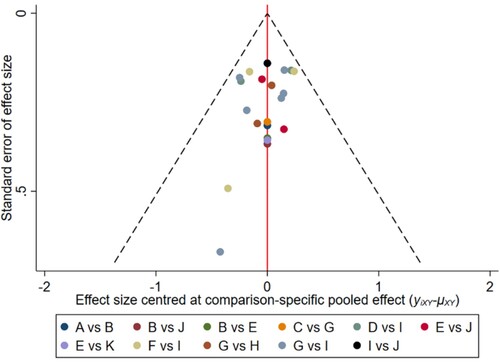
Figure 15. The network funnel plots of pairwise comparisons of regimens on 24 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomiLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of lenalidomide comparing placebo. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
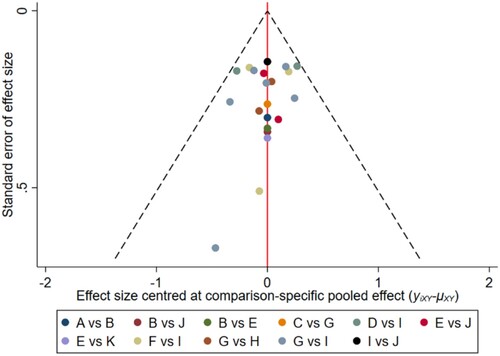
Figure 16. The network funnel plots of pairwise comparisons of regimens on 30 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomiLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of ixazomib comparing placebo. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
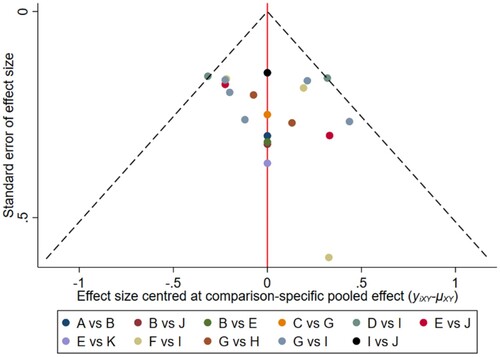
Figure 17. The network funnel plots of pairwise comparisons of regimens on 36 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = CarfilzomiLen, D = Daratumumab, E = IFN, F = Ixazomib, G = Len, H = LenPred, I = Placebo, J = Thal, K = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of thalidomide-interferon comparing interferon. CarfilzomibLen: Carfilzomib-lenalidomide; BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
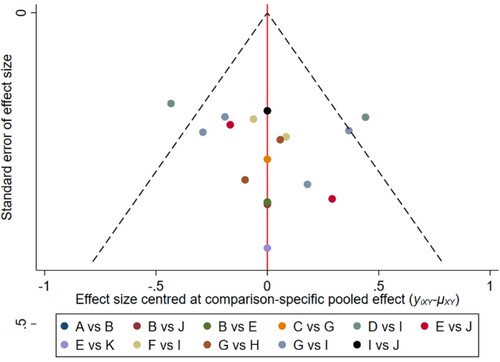
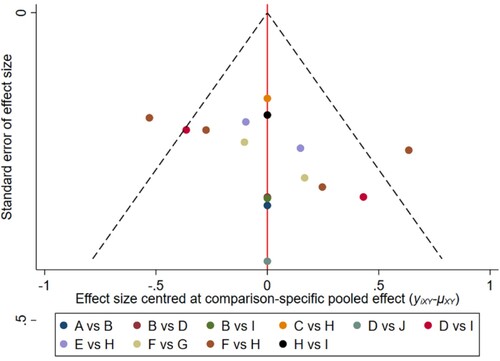
Figure 18. The network funnel plots of pairwise comparisons of regimens on 42 months PFS rate. Abbreviation: A = BortPred, B = BortThal, C = Daratumumab, D = IFN, E = Ixazomib, F = Len, G = LenPred, H = Placebo, I = Thal, J = ThalIFN. The network funnel plots shows that there exist small sample effects in the studies of thalidomide-interferon comparing interferon. BortThal: bortezomib-thalidomide; IFN: interferon; LenPred: lenalidomide- prednisone; Thal: thalidomide; ThalIFN: thalidomide- interferon.
4. Discussion
To the best of our knowledge, this is the first network meta-analysis to compare relative efficacy of all current available maintenance therapies for MM.
The results of our analysis indicated that daratumumab showed significant efficacy on OS at 36 months compared with placebo, nevertheless lenalidomide occurs more rapidly than daratumumab. No OS benefit was suggested with the rest of regimens. Potential reasons were that lenalidomide is second-generation IMiDs which mainly act on myeloma cells and bone marrow hematopoietic microenvironment, thus playing a dual role of killing tumor cells and immune regulation. However, daratumumab exerts its anti-tumor effect through a variety of mechanisms, such as complement-dependent cytotoxicity, antibody-dependent phagocytosis and induction of apoptosis [Citation25]. OS benefited from daratumumab which continuously suppresses the myeloma cells over a long period of time is needed to have a positive effect on OS. Post-transplant maintenance with lenalidomide is commonly utilized as it is the only US Food and Drug Administration-approved drug for this indication. Our results supported the FDA decision. Meta-analysis conducted by Balitsky et al. [Citation26]. demonstrated that maintenance therapy increased PFS in transplant ineligible MM patients, without any improvement in OS. It is not surprising that our analysis was in maxing heterogeneous populations, such as patients who transplant candidates, not transplant candidates and elder patients with general population. Meanwhile, OS is known to be affected by a number of factors including induction and consolidation therapy, co-morbidities and functional status of patients.
As for PFS, lenalidomide-prednisone showed significant efficacy on PFS at 12 months and sustained until 42 months, On the contrary, lenalidomide-carfilzomib showed significant efficacy on PFS as early as at 6 months. the results of pair-wise comparisons indicated that lenalidomide-carfilzomib were associated with greater improvements in PFS compared to lenalidomide, daratumumab and ixazomib. carfilzomib combination regimens were very effective for relapsed or refractory MM (RRMM) [Citation27]. The FORTE study showed a higher rate of conversion to MRD negativity in the KR arm [Citation15]. The results of this current meta-analysis showed that the introduction of carfilzomib not only increased the remission rate of patients, but also helped patients to achieve deeper remission, and then improve the survival prognosis. There is currently strong recommendation of carfilzomib-based regimens in maintenance of MM.
Prior to our study, the most up to date NMAs showed that lenalidomide maintenance appears to be the most effective first line maintenance treatments for NDMM [Citation28]. Our analysis was slightly different from previous studies. we included recent new regimens, updated results and different sample sizes. Hence, our analysis was more convincing.
The feasibility of long-term lenalidomide therapy is limited due to tolerability and increasing rates of discontinuation due to hematologic toxicity, notably grade 3/4 neutropenia as well as risk of SPMs [Citation29]. In fact, some patients choose not to take oral lenalidomide for maintenance because of its toxicity and prefer a non-oral medication.
Daratumumab is the first-in-class human monoclonal antibody that targets CD38. After January, 2022, since daratumumab was approved as national essential drugs, economic burden was reduced. daratumumab 16 mg/kg intravenously every 4 weeks or every 8 weeks (a reduced frequency compared with standard daratumumab long-term dosing according to patient's tolerability) as maintenance therapy for patients were accepted [Citation17,Citation23]. The main toxicity of daratumumab consists of infusion-related reactions (IRRs); in addition, the CD38 expression of the airway muscle cells partially explains the main symptoms that involve the respiratory tract, with throat irritation, cough [Citation30]. However, treatment discontinuation due to IRRs is uncommon. In clinical practice, to prevent severe respiratory adverse events, standard premedication of daratumumab with steroids, antihistamines, and antipyretics administration is recommended. Switching from an intravenously administered to a subcutaneous therapy to minimize the treatment burden may make prolonged daratumumab therapy more feasible in the community settings [Citation31]. Furthermore, daratumumab does not need dose adjustment in patients with renal impairment. Therefore, daratumumab may be an alternative option for certain patients.
The second-generation PI, carfilzomib is an irreversible proteasome inhibitor. It selectively blocks the chymotrypsin-like activity of the 20S proteasome [Citation32]. This causes cell cycle arrest, apoptosis, and cell death, showing superiority over bortezomib with negligible neurological toxicity. But, a greater efficacy from lenalidomide-carfilzomib was associated with an increased side effect profile of cardiac toxicity as well [Citation15,Citation32]. Parenteral administration which brings a lot of inconvenience to MM patients and economic burden may limit the feasibility of maintenance therapy with this regimen. Monitoring patients for clinical signs or symptoms of cardiac failure or cardiac ischemia is necessitated. Evaluate promptly if cardiac toxicity is suspected. Adjust total fluid intake as clinically appropriate in patients with baseline cardiac failure or who are at risk for cardiac failure. Meanwhile, modify dosing based on toxicity. Taking these into account: MM patients who were eligible for transplantation with capability of finance or commercial Health Insurance may be the candidate for this regimen.
Given that these therapy regimens are long-term treatments. Efficacy, safety and tolerability concerns should be considered when selecting a long-term treatment approach regimen. We hope that all regimens can be listed in National Essential Drugs Content in China to reduce the economic burden of patient. MM may become a chronic disease with personalized maintenance regimens by reducing toxicity and de-escalating therapy.
4.1. Limitation
First, we devised a very comprehensive search strategy, but unfortunately, we could have missed unpublished literature resulting in publication bias limitations.
Second, our NMA was limited to the PFS and OS outcomes, and we did not include other outcomes that might be of interest, such as adverse events, quality of life, cost-effectiveness. The impact of induction and consolidation therapy, other potential confounding factors, including different patient baseline characteristics, for example, cytogenetic risk, or renal function on OS and PFS was also not considered in this analysis. The final outcome may be influenced by induction and consolidation rather than the maintenance therapy itself.
Third, there was a limited number of studies, particularly for recent new regimens. Some of the included studies had very small sample sizes, which added to overall uncertainty of the NMA results.
5. Conclusion
In conclusion, post-ASCT maintenance therapy with lenalidomide continued is the current standard of care based on the available clinical trial data, the most likely next step will be to incorporate carfilzomib into the maintenance setting. Physicians also need to be vigilant about the risk of adverse event and cost-economic in MM patients treated with lenalidomide-carfilzomib. More head-to-head clinical trials are needed to confirm our findings.
Acknowledgements
We thank the authors of the included studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gerecke C, Fuhrmann S, Strifler S, et al. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113(27-28):470–476. doi:10.3238/arztebl.2016.0470.
- Shank BR, Brown VT, Schwartz RN. Multiple myeloma maintenance therapy: a review of the pharmacologic treatment. J Oncol Pharm Pract. 2015;21(1):36–51. doi:10.1177/1078155213514468.
- Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Ann Oncol. 2000;11(11):1427–1436. doi:10.1023/a:1026548226770.
- Kagoya Y, Nannya Y, Kurokawa M. Thalidomide maintenance therapy for patients with multiple myeloma: meta-analysis. Leuk Res. 2012;36(8):1016–1021. doi:10.1016/j.leukres.2012.04.001.
- Alonso R, Cedena MT, Wong S, et al. Prolonged lenalidomide maintenance therapy improves the depth of response in multiple myeloma. Blood Adv. 2020;4(10):2163–2171. doi:10.1182/bloodadvances.2020001508.
- Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791. doi:10.1056/NEJMoa1114138.
- Dimopoulos MA, Špička I, Quach H, et al. Ixazomib as postinduction maintenance for patients With newly diagnosed multiple myeloma not undergoing autologous stem cell transplantation: The phase III TOURMALINE-MM4 trial. J Clin Oncol. 2020;38(34):4030–4041. doi:10.1200/jco.20.02060.
- Zweegman S, Stege CAM, Haukas E, et al. Ixazomib-Thalidomide-low dose dexamethasone induction followed by maintenance therapy with ixazomib or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation; results from the randomized phase II HOVON-126/NMSG 21.13 trial. Haematologica. 2020;105(12):2879–2882. doi:10.3324/haematol.2019.240374.
- Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC myeloma IX trial: survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030–6038. doi:10.1158/1078-0432.Ccr-12-3211.
- Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide before and after autologous stem cell transplantation for transplant-eligible patients of all ages in the randomized, phase III, Myeloma XI trial. Haematologica. 2021;106(7):1957–1967. doi:10.3324/haematol.2020.247130.
- Rosiñol L, Oriol A, Teruel AI, et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: a PETHEMA/GEM trial. Leukemia. 2017;31(9):1922–1927. doi:10.1038/leu.2017.35.
- Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (London, England). 2019;393(10168):253–264. doi:10.1016/s0140-6736(18)33003-4.
- Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617–1629. doi:10.1016/s1470-2045(15)00389-7.
- Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017;4(9):e431–e442. doi:10.1016/S2352-3026(17)30140-0.
- Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22(12):1705–1720. doi:10.1016/s1470-2045(21)00535-0.
- Bringhen S, D’Agostino M, Paris L, et al. Lenalidomide-based induction and maintenance in elderly newly diagnosed multiple myeloma patients: updated results of the EMN01 randomized trial. Haematologica. 2020;105(7):1937–1947. doi:10.3324/haematol.2019.226407.
- Moreau P, Hulin C, Perrot A, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378–1390. doi:10.1016/s1470-2045(21)00428-9.
- van de Donk NW, van der Holt B, Minnema MC, et al. Thalidomide before and after autologous stem cell transplantation in recently diagnosed multiple myeloma (HOVON-50): long-term results from the phase 3, randomised controlled trial. Lancet Haematol. 2018;5(10):e479–e492. doi:10.1016/s2352-3026(18)30149-2.
- Ludwig H, Adam Z, Tóthová E, et al. Thalidomide maintenance treatment increases progression-free but not overall survival in elderly patients with myeloma. Haematologica. 2010;95(9):1548–1554. doi:10.3324/haematol.2009.020586.
- Mateos MV, Oriol A, Martínez-López J, et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood. 2012;120(13):2581–2588. doi:10.1182/blood-2012-05-427815.
- Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. doi:10.1056/NEJMoa1402888.
- Brioli A, Manz K, Pfirrmann M, et al. Frailty impairs the feasibility of induction therapy but not of maintenance therapy in elderly myeloma patients: final results of the German Maintenance Study (GERMAIN). J Cancer Res Clin Oncol. 2020;146(3):749–759. doi:10.1007/s00432-019-03101-z.
- Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet (London England). 2020;395(10218):132–141. doi:10.1016/s0140-6736(19)32956-3.
- Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–1769. doi:10.1056/NEJMoa1112704.
- Nooka AK, Kaufman JL, Hofmeister CC, et al. Daratumumab in multiple myeloma. Cancer. 2019;125(14):2364–2382. doi:10.1002/cncr.32065.
- Balitsky AK, Karkar A, McCurdy A, et al. Maintenance therapy in transplant ineligible adults with newly-diagnosed multiple myeloma: A systematic review and meta-analysis. Eur J Haematol. 2020;105(5):626–634. doi:10.1111/ejh.13496.
- Chen R, Chen B, Zhang X, et al. Efficacy of carfilzomib in the treatment of relapsed and (or) refractory multiple myeloma: a meta-analysis of data from clinical trials. Discov Med. 2016;22(121):189–199.
- Gay F, Jackson G, Rosiñol L, et al. Maintenance treatment and survival in patients With myeloma: A systematic review and network meta-analysis. JAMA Oncol. 2018;4(10):1389–1397. doi:10.1001/jamaoncol.2018.2961.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J Clin Oncol. 2017;35(29):3279–3289. doi:10.1200/jco.2017.72.6679.
- van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29. doi:10.1182/blood-2017-06-740944.
- Paul B, Hamadeh I, Atrash S, et al. Daratumumab subcutaneous formulation for the treatment of multiple myeloma. Expert Opin Biol Ther. 2020;20(11):1253–1259. doi:10.1080/14712598.2020.1806231.
- Engelhardt M, Szymaniak-Vits M, Ajayi S, et al. Carfilzomib. Recent Results Cancer Res. 2018;212:265–283. doi:10.1007/978-3-319-91439-8_13.