ABSTRACT
Objectives:
To assess the clinical and healthcare resource burden among C5 inhibitor (C5i)-treated patients with paroxysmal nocturnal haemoglobinuria (PNH), using patient-reported data.
Methods:
This web-based, cross-sectional survey (01FEB2021–31MAR2021) of adults with PNH treated with eculizumab (France, Germany, UK) or ravulizumab (Germany) included: patient characteristics; treatment patterns/dosage; haematological outcomes (haemoglobin [Hb] levels, transfusions, thrombotic events, breakthrough haemolysis); and medical encounters. Treatment and Hb-level subgroup differences were assessed with statistical significance tests.
Results:
Among 71 patients, 98.6% were C5i-treated for ≥3 months. The majority (with reported Hb levels) had levels ≤12.0 g/dL (85.7%; n = 54/63). The mean Hb level was 10.2 g/dL (standard deviation [SD]: 2.0; median 10.0 g/dL). Treatment with above label-recommended doses was reported by 30.4% (eculizumab) and 5.3% (ravulizumab) of patients. Within the past 12 months among patients treated with C5i for ≥1 year: 24.1% had ≥1 transfusion; 3.2% had ≥1 thrombosis; and 28.6% had ≥1 breakthrough haemolysis. Among all patients, 26.8% and 31.0% reported emergency department/room [ER] and inpatient visits, respectively. Mean annual, per-patient all-cause medical encounters were: 0.5 (ER); 1.9 (inpatient); and overall outpatient visits ranged by setting from 2.0 to 6.4. Most encounters were PNH-related, with means of 0.4 (ER); 1.8 (inpatient); and 1.6–5.4 (outpatient). Primary haematological and medical encounter outcomes were similar between treatment as well as Hb-level subgroups, with almost no statistically significant differences.
Conclusions:
Despite at least 3 months of C5i treatment, high proportions of patients with PNH reported low haemoglobin levels and required transfusions and hospitalizations, which suggests remaining unmet needs.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired haematological disorder associated with serious morbidity and, if untreated, mortality [Citation1,Citation2]. Chiefly characterized by chronic intravascular haemolytic anaemia and thrombosis, classical haemolytic PNH can manifest with a wide range of additional clinical presentations including bone marrow failure resulting in thrombocytopenia, leukopenia and sustained anaemia and sequelae such as renal failure or pulmonary hypertension [Citation1–3]. Typical symptoms include fatigue, shortness of breath, abdominal pain, difficulty swallowing, and erectile dysfunction [Citation1]. Global PNH prevalence is currently unknown, but incidence has been estimated at 1–1.5 cases per million [Citation1]. Estimates of annual incidence and predicted prevalence in the UK are approximately 1 in 770,000 and 1 in 62,500, respectively [Citation4].
PNH is associated with considerable disease burden manifested through adverse humanistic and economic outcomes [Citation1,Citation2,Citation5–7]. An international PNH registry study estimated that fatigue and shortness of breath – both associated with low Hb levels and potentially debilitating – affect approximately 81% and 45% of patients, respectively [Citation1,Citation2]. Patients with PNH and persistently low Hb levels may require medical facility visits for red blood cell (RBC) transfusion [Citation7]. International PNH registry data show that approximately 23% of patients experience PNH-related hospitalizations [Citation5], mainly associated with red blood cell (RBC) transfusions [Citation6]. Breakthrough haemolysis (BTH) is a leading cause requirement for RBC transfusions and increased dosage, which drive overall real-world resource utilization and costs [Citation6,Citation8].
Although spontaneous remission has been observed and allogeneic hematopoietic stem cell transplantation (HSCT) is the only possible cure, true remission is uncommon and observed only after decades of living with the disease [Citation9,Citation10], and HSCT involves high risk and is not recommended for the majority of patients with classical PNH [Citation11]. Thus, the current standard of care for classical haemolytic PNH in the sampled countries is monoclonal antibody C5 inhibitors (C5i, eculizumab and ravulizumab), which target the C5 component of the complement cascade [Citation12]. C5i therapies effectively reduce IVH and thrombotic risk among most treated patients [Citation13–15]. This has changed the PNH treatment landscape considerably through improvement of IVH-associated clinical outcomes, overall survival, and health-related quality of life [Citation7,Citation16–20].
Clinical limitations remain, as some PNH patients experience breakthrough IVH and C3-mediated extravascular haemolysis (EVH) [Citation8,Citation11,Citation12,Citation14,Citation20–25]. PNH is caused by somatic mutations in the phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIGA) gene that can lead to the dominance of PIGA-mutated clones, including mutated RBCs that have a protein deficiency making them susceptible to lysis in the complement cascade [Citation1,Citation26] While designed to mitigate this process, C5 inhibition can lead to EVH, which occurs when C5 inhibition preserves some mutated RBCs that would otherwise be eliminated by IVH; these RBCs become targets for C3-mediated opsonization upstream of C5 in the complement cascade, rendering some C5i-treated patients susceptible to anaemia and subsequent RBC transfusion dependence [Citation21,Citation22,Citation27,Citation28].
Proximal complement inhibitors such as the C3 inhibitor pegcetacoplan (US approval: 14 May 2021; EU approval: Dec 13 2021) have been developed to address this remaining clinical gap, as C3 is the central component involved in complement-mediated EVH and IVH [Citation12,Citation29,Citation30]. Evidence from a pivotal phase 3 trial among adults with PNH and haemoglobin levels <10.5 g/dL despite eculizumab therapy (PEGASUS) showed improved hemoglobin (Hb) levels with pegcetacoplan, as compared with eculizumab [Citation30,Citation31]. However, as pegcetacoplan was first approved in the USA in May 2021 and not yet approved at the time of the study in the EU (approved December 2021), post-licensing surveillance data were unavailable at the time of this study.
The literature on clinical and economic outcomes associated with C5i therapies for PNH have described a high and persistent disease burden in routine practice [Citation2,Citation5,Citation6,Citation8,Citation11,Citation20,Citation28,Citation32]. However, evidence on the impact of suboptimal haemolytic control from the patient perspective remains scarce, especially outside the USA. Thus, we undertook this multinational, cross-sectional study to evaluate the clinical and healthcare resource burden of PNH, as reported by patients treated with eculizumab or ravulizumab in Germany and with eculizumab in France and the UK.
Materials and methods
Study design
We conducted a multinational, web-based, cross-sectional study of patients in France, Germany and the UK from 1 February 2021 through 31 March 2021, recruiting through patient advocacy groups (PAGs; HPN France [France], Stiftung Lichterzellen [Germany], and PNH Support [UK]). Included patients were aged ≥18 years, had a self-reported PNH diagnosis, and were treated with eculizumab or ravulizumab at the time of the study. Patients with multiple myeloma, haemophilia, leukemia, or lymphoma were excluded. To ensure data integrity and survey validity, we incorporated logic programming procedures (e.g. checks on response ranges, consistency, skip patterns, credibility [patterned responses, random answers, short completion time]). As submission required completion of all presented questions, there were no missing data among respondents directed to answer relevant questions (‘I don’t know’ was an option for recall questions).
Based on the sample size calculation, prevalence estimates of this rare condition, and generally high response rates among rare disease populations [Citation32], we sought a minimum of 52 respondents. The sample sizes for the subgroups (ECU, n = 49; RAV, n = 22) were determined to provide at least 80% power to detect an effective size (Cohen’s d = 0.8) at the significance level of α = 0.05, using t-test statistics. This manuscript was prepared in accordance with survey study reporting guidelines (A Consensus-based Checklist for Reporting of Survey Studies [CROSS]) [Citation33].
Study variables
The survey included a screener that determined eligibility based on country of residence, consent regarding adverse event protocol, minimum age, diagnosis, comorbid conditions, and treatment status. Eligible patients progressed to the main survey, which consisted of up to 63 questions covering patient characteristics, treatment patterns, haematological outcomes, and medical encounters. Questions were reviewed by a clinical advisory board consisting of patient advocates from Stiftung Lichterzellen (Germany), Association HPN (France) and PNH Support (UK), as well as practicing specialist clinicians from the United States and Germany and were assessed from the patient perspective as well as national context. Patient characteristics included demographics and clinical characteristics such as weight, mean Hb levels and clinically significant comorbidities. (See for characteristics.) Treatment patterns included time from initiation (categorical), transfusion frequency and dosage. Clinical outcomes included the proportions of patients with Hb levels >12.0 g/dL versus ≤12.0 g/dL, to assess anaemia (at ≤12.0 g/dL, the lower threshold given definitions of ≤12.0 g/dL for women and ≤13.5 g/dL for men) [Citation34]. Hb levels were also assessed above and below the approximate median for international PNH registry patients (<10.5 g/dL and ≥10.5 g/dL) [Citation30,Citation31]. Required transfusions, thrombosis and BTH (subjectively defined as the return of haemolytic activity, such as anaemia, dark urine, a major vascular event, such as a blood clot, or other PNH symptoms) were also assessed (See ). Medical encounters included emergency department/room (ER), inpatient, and outpatient visits (primary care, specialist, telehealth, and other providers). (See Supplementary Table 1 for full survey.) Post-hoc analyses of patients treated for ≥12 months were conducted for Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F] and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [EORTC QLQ-C30] scores, stratified by haematological event status in the past 12 months (patients with ≥1 transfusion or BTH in the past 12 months [Event Group] versus those with neither a transfusion nor a BTH [Non-event Group]).
Figure 1. Patient flow chart. aEthical considerations precluded capture of reasons for voluntary discontinuation and any further data on respondents who quit (see Methods, Ethics Statements and Data Confidentiality).
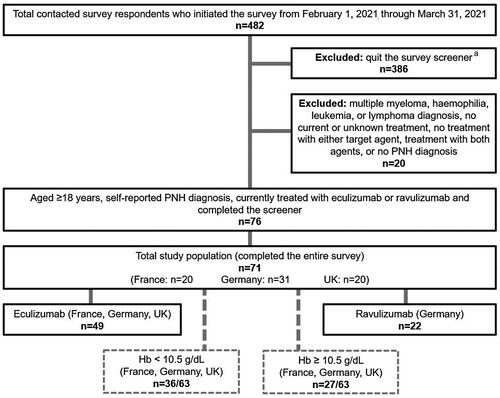
Table 1. Patient characteristics.
Ethics statement and data confidentiality
Email invitations for a 25-minute one-time survey were sent to potential participants by patient organizations; a unique link allowed participants to enter the survey. After providing informed consent, survey participants completed the screener. Survey participation was anonymous and voluntary, with respondents able to easily discontinue and protected anonymity precluding further contact; this prevented capture of reasons for voluntary discontinuation and any further data on these respondents. Patient data were anonymised, stored in a secure server and then transmitted directly to statistical software via a secure file transfer protocol. The survey and procedure were reviewed by the Pearl institutional review board: the US counterpart study was granted an exemption by the US Central Institutional Review Board [Citation27], and the present study was exempt from review board review in the surveyed countries (see Supplementary Table 1 for details). Respondents who qualified and completed the study were compensated for their time and were informed that the study was funded by a (specified) pharmaceutical company.
Statistical analysis
Aggregated data from all countries were used for total study population analysis. According to prespecified analysis in the study protocol, results were stratified by treatment type (eculizumab or ravulizumab);categorized by Hb levels (Hb <10.5 g/dL versus ≥10.5 g/dL); the subgroup cutoff of 10.5 g/dL was selected to generally reflect the median of real-world PNH populations and clinical trial inclusion criteria [Citation28,Citation30,Citation31]. Descriptive statistics were used to analyse the data, with sample sizes, means, medians and standard deviations [SDs] for continuous variables and counts and proportions for categorical variables. Statistical differences between treatment subgroup means in demographic and other dependent variables were assessed using one-way analysis of variance (ANOVA), and differences in proportions were assessed with chi-squared tests. Multiplicity adjustments were not implemented. Sample size power calculations were performed using SAS Studio version 3.81. All statistical analyses were conducted using SPSS version 25 or higher (Armonk, NY: IBM Corp; 2017).
Results
Study population
A total of 482 respondents initiated the initial survey screener, among whom 80.1% (n = 386) quit the survey before completion of the screener and 4.1% (n = 20) were excluded. Among the remaining 76 survey-eligible patients, 93% (n = 71) completed the survey (by treatment: eculizumab 69.0% [n = 49], ravulizumab 31.0% [n = 22]; by country: UK 28.2% [n = 20], France 28.2% [n = 20], Germany 43.6% [n = 31]; by Hb level: 88.7% [n = 63]: <10.5 g/dL 57.1% [n = 36], ≥10.5 g/dL 42.9% [n = 27]) ().
Patient characteristics
The total study population were mostly women (66.2%; n = 47), and the mean (SD) age was 43.0 years (±13.1). The mean weight (SD) of the total study population was 73.4 kg (±14.9) and was statistically significantly lower for eculizumab versus ravulizumab (69.7 kg [±13.4] versus 78.2 kg [±15.6], respectively; p = 0.045). There were no other statistically significant differences between the treatment subgroups. The mean age at PNH diagnosis was 29.8 years (±11.6). Among patients with known Hb levels (n = 63), 98.4% (all but one) had their last check ≤6 months before the survey, and 84.1% had their most recent check ≤1 month before the survey. The mean Hb level was 10.2 g/dL (median: 10.0; ±2.0); aplastic anaemia was the most common related comorbidity (36.6%; n = 26) ().
Hemoglobin level subgroup analyses
Among the <10.5 g/dL subgroup, there were significantly larger proportions of women (83.3% [n = 30] versus 55.6% [n = 15]; p = 0.016) as compared with the ≥10.5 g/dL subgroup. All other characteristics generally aligned with trends in the total study population, without additional statistically significant differences (at valid sample sizes) ().
Treatment patterns and outcomes
Patterns
Almost all patients (98.6%; n = 70) reported treatment durations of ≥3 months, and 88.7% (n = 63) reported durations of ≥12 months. The mean treatment intervals among eculizumab- and ravulizumab-treated patients were 15.8 (median: 14.0; ±8.4) and 55.4 (median: 56.0; ±2.1) days between doses, respectively. The majority of eculizumab-treated patients (83.7%) received treatment at intervals of 14 days per label indications, with the remainder treated in longer intervals, and majorities of eculizumab- and ravulizumab-treated patients (81.3% and 81.0%, respectively) reported no change in interval or dosage in the past 12 months (excluding loading doses; since initiation for patients treated for ≤12 months). Results for latest-recorded Hb level subgroups followed similar trends (Supplementary Table 2).
Across prescribing weight categories (40–59 kg, 60–99 kg, ≥100 kg), one (5.3% [n = 1/19]) of the responding patients treated with ravulizumab was prescribed an above-label dose (at last dose). Despite fixed label-recommended dosage (900 mg) among eculizumab-treated patients, 30.4% (n = 14/46) of those who responded reported an above-label (last) dose (1200–1600 mg) ().
Table 2. Dosage.
Outcomes
Haematological outcomes for patients treated with C5i for ≥12 months
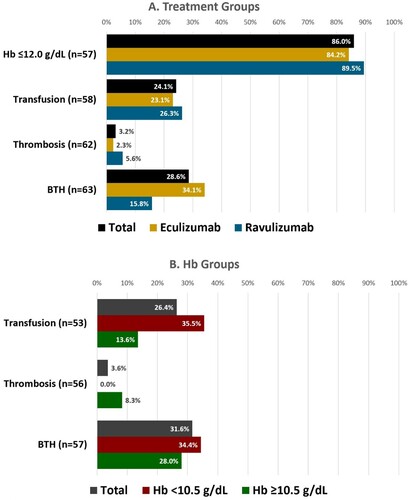
Among 63 patients who recalled HB levels in the total study population, 85.7% had most recent levels of ≤12.0 g/dL at the time of the survey (n = 54) (data not shown). Among 57 patients who recalled Hb levels and were treated for ≥12 months, 86.0% (n = 49) had most recent levels of ≤12.0 g/dL at the time of the survey (eculizumab [n = 32]: 84.2%; ravulizumab [n = 17]: 89.5%). Fifty-eight (n = 58) patients recalled their transfusion history, among whom 24.1% (n = 14) had ≥1 transfusion within the previous 12 months (23.1% [n = 9] and 26.3% [n = 5] for eculizumab and ravulizumab, respectively). Sixty-two (n = 62) patients recalled their thrombosis history; 3.2% (n = 2) had ≥1 thrombotic event in the previous 12 months (2.3% [n = 1] and 5.6% [n = 1] for eculizumab and ravulizumab, respectively). Sixty-three (n = 63) patients recalled their breakthrough haemolysis (BTH) history; 28.6% (n = 18) had ≥1 breakthrough haemolytic event within the previous 12 months (34.1% [n = 15] and 15.8% [n = 3] for eculizumab and ravulizumab, respectively). There were no statistically significant differences between subgroups ().
Figure 2. Haematological outcomes. BTH, breakthrough haemolysis; Hb, (serum) hemoglobin.
Notes: Data reflect events in the past 12 months, among query respondents treated for ≥12 months. Breakthrough haemolysis was defined in the survey as: ‘ … the return of haemolytic disease activity e.g. return of (my) symptoms/ anaemia / major vascular event e.g. a blood clot.’
Hemoglobin level subgroup analyses
The Hb level subgroups results followed directional trends favoring the Hb ≥ 10.5 g/dL group (numerically lower proportions) for transfusions and BTH, although the Hb ≤10.5 g/dL group reported more thrombosis; there were no statistically significant differences ().
Post-hoc haematological event status subgroup analyses
Post-hoc analyses of the ≥12 month-treated population with requisite data (n = 59) found the Event Group (n = 24) had significantly lower mean FACIT-F (52-point scale; lower = worse) (30.8 [±13.5] versus 38.2 [±14.2]; p = 0.049) and significantly lower mean EORTC QLQ-C30 scores (100-point scale; lower = worse) (60.4 [±21.5] versus 72.1 [±20.0]; p = 0.036), as compared with the Non-event Group (n = 35) (Supplementary Table 3).
Medical encounters
All-cause medical encounters
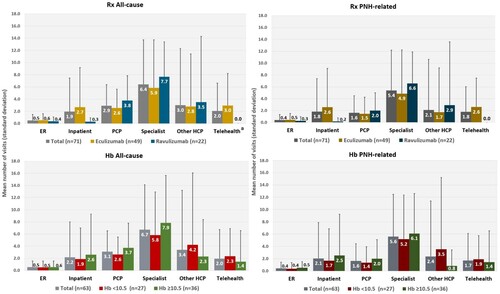
Among the total study population (n = 71) within the previous 12 months, the mean numbers of all-cause encounters were: ER visits: 0.5 (±1.0); inpatient visits (hospitalizations): 1.9 (±5.5); and overall outpatient visits ranging by setting from 2.0 (±4.6) to 6.4 (±7.3). The eculizumab group had significantly more telehealth visits compared with the ravulizumab group (3.0 [±5.3] versus 0.0 [±0.0]; p = 0.011), but there were no other statistically significant differences ().
PNH-related medical encounters
Among the total study population (n = 71) within the previous 12 months, the mean numbers of PNH-related encounters were: ER visits: 0.4 (±1.0); inpatient visits:1.8 (±5.5); and overall outpatient visits ranging by setting from 1.6 (±2.9) to 5.4 (±6.8). There were no significant differences between treatment subgroups. () A total of 12 patients (16.9%) reported ≥1 ER visit, with a mean of 2.3 (±1.0) visits; and 15 patients (21.1%) reported ≥1 inpatient visit, with a mean of 8.7 (±9.5) visits. Overall, the proportions of patients with ≥1 outpatient visit ranged by setting from 11 (15.5%; other providers) to 56 (78.9%; specialists), with mean visits ranging from 3.38 ([±3.36]; primary care providers [PCP]) to 13.6 ([±18.4]; other providers) (Supplementary Table 4).
Among the patients with PNH-related ER visits (n = 12), the most common reason was breakthrough haemolysis (66.7%), followed by chest pain (16.7%). The most common reason for inpatient visits was breakthrough haemolysis (40.0%), followed by fatigue (20.0%). There were no significant differences between subgroups at valid sample sizes (Supplementary Table 4).
Hemoglobin level subgroup analyses
Directional trends were generally consistent with total study population results. There were no significant differences between subgroups (, Supplementary Table 4).
Discussion
This is the first cross-sectional, multinational study of European patient-reported clinical and economic outcomes among individuals living with PNH. Our results show that despite all but one patient being treated for at least three months, most (85.7%) were anaemic (at Hb levels at or below 12.0 g/dL). Moreover, among patients who were treated for at least one year (88.7%), more than a quarter experienced BTH, and nearly a quarter required transfusions. Medical resource use was also considerable, and BTH was by far the most common reason for ER visits and hospitalizations. As overall trends were similar across subgroups, these data generally align with and expand upon previous studies suggesting persistent unmet need associated with C5i therapy [Citation2,Citation5,Citation6,Citation11,Citation20,Citation28,Citation32].
Patient characteristics were generally consistent with baseline findings for the International PNH Registry population (in 2013 and 2017), as well as populations from retrospective observational studies and a counterpart survey study conducted in the USA [Citation2,Citation5,Citation6,Citation32,Citation35]. Although we observed a proportion of women somewhat higher than the range reported in the literature (66.2% versus 53.0%–55.7%), this may reflect the membership of the patient organizations that disseminated the survey, which tend to have more women [Citation36,Citation37].
The notable proportion of patients prescribed above-label doses (>900 mg) of eculizumab (30.4%) is generally consistent with a range of approximately 21%–46% reported in previous real-world studies [Citation28,Citation32,Citation35], and may be related to the prevalence of BTH reported among the 12-month treated eculizumab group (34.1%). As clinicians report increasing dosage in response to BTH [Citation8], these dosage findings suggest unmet need. The single (5.3%) ravulizumab-treated patient reporting above-label dosage is in general range of clinician expectations for dose adjustment (10%) [Citation8]. In contrast, the US counterpart study observed above-label dosing among 33.3% and 18.2% of 40–60 kg and 61–100 kg subgroup patients, respectively; this discrepancy may be attributable to systemic differences between national systems (ie, closer label adherence in Europe), or population differences among commercial insurance enrolees [Citation32,Citation38].
Whilst to our knowledge our comparative real-world haematological outcomes are novel, the general trends and overall unmet need we observed are largely consistent with previous findings. Our total study population and eculizumab subgroup median Hb level results align with international PNH registry data and a UK real-world study (10.0 g/dL versus 9.8 and 10.9 g/dL, respectively) [Citation2,Citation28]. Likewise, our ravulizumab subgroup transfusion results align with a 52-week open label trial extension (26.4% versus 26.3%) [Citation39].
However, our eculizumab subgroup transfusion results are somewhat lower than the range for generally comparable periods in US claims and UK electronic health data analyses (23.1% versus 36.4%–36.2%) [Citation6,Citation28], possibly due to study design differences. Our observed treatment group trends for BTH are directionally consistent with head-to-head trial data, wherein ravulizumab-treated patients experienced slightly less BTH overall [Citation23]. Our eculizumab subgroup BTH results were slightly above the range reported in the literature for long-term treatment (34.1% versus 5%–27%), although this may be attributable to differing definitions and study populations [Citation40]. Likewise, our ravulizumab subgroup BTH results are somewhat above the range reported in a one-year trial extension (15.8% versus ∼4.0%–7.3%), possibly due to stricter inclusion criteria and an LDH-based definition in that study [Citation39]. Nonetheless, it is important to note that 43.7% (n = 31) of the study population reported history of comorbid bone marrow disorders (BMDs; aplastic anaemia, myelodysplastic syndrome, or other bone marrow disorders), and misattribution of Hb reductions with pathogeneses other than BTH may have led to over-estimation. This overall variation in results, endpoint definitions and populations speaks to the heterogeneous presentation of the disease, the scarcity of data and the need for continued research, including investigations of the specific impact of associated bone marrow disorders.
To our knowledge, our post-hoc analysis of FACIT-F and EORTC QLQ-C30 scores stratified by haematological event status is novel, but the findings generally accord with previous real-world studies showing associations between PNH and considerably reduced health-related quality of life (HRQoL) as compared with the general population, including previously published related analyses by this author group [Citation2,Citation32,Citation41,Citation42]. While the significantly lower (worse) scores observed among the Event Group were expected, the Non-event group scores are notable in relation to the general population. Minimum clinically important differences (MCIDs) in FACIT-F mean scores range from 3 to 5 points; [Citation43,Citation44] while the differences we observed between event groups were both statistically significant and clinically important, even the Non-event Group scores were beyond the upper limit of the MCID range, as compared with the general population (38.2 versus 43.5) [Citation43]. Although Non-event Group mean EORTC global health status scores were closer to the general population (72.1 versus 75.0) [Citation45], and there are currently no MCIDs for the EORTC QLQ-C30 validated for PNH, overall these findings suggest that PNH has a considerable negative impact on HRQoL regardless of the occurrence of BTH or transfusion requirements. This observation of widespread burden accords with previously published findings by this author group that mean FACIT-F and EORTC QLQ-C30 scores do not differ significantly between HB level subgroups (<10.5 g/dL versus ≥10.5 g/dL) [Citation42].
Our medical encounter results show PNH-related encounters were the main driver of overall healthcare resource utilization among the total study population, ranging from 77% (ER) to 94% (hospitalizations) of mean all-cause visits; BTH was by far the most common reported reason for ER and inpatient visits, which suggests the likelihood of required RBC transfusions being a major cost factor. Moreover, mean utilization was considerably higher among patients with at least one encounter in each setting. Comparable real-world data is scarce, but our results show higher utilization than a US claims study of patients with PNH prescribed eculizumab (mean follow-up: 1.6 years), which also found PNH-related inpatient and outpatient visits to be considerable drivers of overall utilization, but at lesser magnitude (33% of hospitalizations and 27% of outpatient visits), with PNH-related ER visits comprising only a negligible proportion of all-cause ER visits [Citation6]. Similarly, our eculizumab subgroup results for mean all-cause ER and combined outpatient visits (PCP, specialist, other providers) are somewhat higher (ER: 0.6 ± 1.1 versus 0.3 ± 0.8; [combined] outpatient: 14.2 versus 10.3), and our results for hospitalizations are considerably higher (2.7 ± 6.5 versus 0.6 ± 1.0) [Citation6]. Results for PNH-related encounters followed similar directional trends of greater magnitude (ER: 0.5 ± 1.1 versus 0.0 ± 0.3; hospitalizations: 2.6 ± 6.5 versus 0.2 ± 0.5; [combined] outpatient: 10.7 versus 2.8). In the context of the Covid pandemic, which likely dissuaded in-person service utilization among the study population, these discrepancies may reflect differences in national healthcare systems: ie, patients in universal payer systems may be less likely to avoid hospitalization [Citation46]. Moreover, our use of patient advocacy groups for survey dissemination may have selected for patients with greater clinical burden and on treatment for longer than a commercially-insured US dataset of a treatment-naïve population. Regardless, the considerable resource use observed in both studies speaks to unmet need and calls for continued investigation.
The prevailing directional trends we observed favoring ravulizumab versus eculizumab are largely consistent with head-to-head trial data and likely reflect ravulizumab’s longer half-life [Citation47,Citation48]. However, the general lack of statistical differences between treatment subgroups suggests unmet needs germane to C5 inhibitor treatment at large. Similarly, the general similarity between Hb subgroups suggests considerable disease burden across a wide range of PNH haemolysis severity, and further, that classic symptoms like fatigue may occur across a wide range of Hb levels. This suggests clinicians should carefully consider patient-reported need in treatment decision-making. In this context, our results add to growing evidence of persistent clinical and economic burden of disease specifically associated with C5 inhibitor treatment, which calls for continued research and more effective treatment.
While our self-reported survey design precluded quantification, clinical trial data suggest EVH may have been a major factor in outcomes [Citation30,Citation31]. Future research is needed to quantify IVH versus EVH burden in routine practice. Likewise, as optimization of pharmacological treatment options is currently the most practical way forward [Citation9–11], there is a need for investigation of complement inhibition with upstream targets such as C3, factor B, and factor D; our results may inform future real-world studies on new agents such as pegcetacoplan as data become available.
Limitations
Our results should be interpreted in the context of certain general limitations of survey studies. For example, self-reporting is subject to response biases and recall errors [Citation49,Citation50]. The wording of some questions may have been open to divergent interpretation, and diagnoses, lab measures, and healthcare procedures and encounters could not be confirmed. The convenience sampling design may also have introduced bias: as willing respondents who received the study invitation from patient organizations likely have more burdensome cases compelling both group and survey participation, results are not necessarily generalizable to the overall patient population.
Interpretation should also consider study-specific limitations. For instance, PNH rarity limited sample sizes, and a large proportion of patients who responded to the survey invitation did not complete the screener for reasons that were not capturable, which may limit generalizability. Study scope considerations precluded stratification of results by the presence of bone marrow disorders, and reports of BTH-related events could not be clinically confirmed; moreover, concomitant medications for bone marrow disorders were not recorded. Results should be interpreted accordingly. Real-world sampling of patients prescribed ravulizumab was only possible in Germany, and results were not adjusted for differing patient characteristics. In addition, results should be interpreted in the context of the Covid-19 pandemic: although several survey questions were designed to differentiate PNH-related outcomes from Covid-related outcomes, at the time of the study data on possible pathogenesis connections between the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and PNH were not yet available [Citation51–55], and the study did not exclude or stratify patients with known Covid infections. Likewise, accessibility and safety issues surrounding COVID 19 may have reduced overall medical visits or extended treatment intervals, and the reasons for extended intervals were not captured; thus, results may underestimate disease burden under normal circumstances. In addition, other concomitant infections that may have affected haematological outcomes were not assessed. Finally, outcomes were not stratified by time on treatment; future evaluations of treatment outcomes over multiple timepoints would be scientifically instructive.
Conclusions
The results of this survey show that despite treatment of at least three months with C5 inhibitor therapy (eculizumab or ravulizumab), 87.5% remained anaemic (at or below haemoglobin levels of 12.0 g/dL); among patients treated at least 12 months, 24.2% required transfusions and 28.6% experienced BTH. (BTH). We observed a comparatively high level of medical encounters overall, with 26.8% and 31.0% of patients reporting emergency and inpatient visits, respectively, predominantly because of BTH. These results suggest persistent clinical and healthcare utilization burden among patients with PNH treated with C5 inhibitors, which underscores the need for alternative treatments to improve haemolytic control and reduce health care resource burden.
Competing interests
J.P. has received honoraria and consulting fees from Alexion, Amgen, Apellis Pharmaceuticals, Biologix, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Gilead, Grünenthal, F. Hoffmann-La Roche Ltd, MSD, Novartis, Pfizer, Sobi, and SwixxBiopharma; F.SF. has received honoraria and consulting fees from Alexion, Novartis and Sobi, as well as research funding (in affiliation with APHP Saint Louis Hospital) from Alexion and Novartis; P.B. is a part-time employee of Stiftung Lichterzellen, which is a paid consultant to Apellis Pharmaceuticals and Sobi; M.P. is the Chair of PNH Support (in a voluntary capacity), which has received a grant and consulting fees from Apellis Pharmaceuticals and consulting fees from Sobi, and M.P. has participated on an advisory board for Sobi as a volunteer; J.M. was a full-time employee of Kantar Health, a paid consultant to Apellis Pharmaceuticals, at the time of the study and currently has no conflicts to declare; H.C. is a full-time employee of Cerner Enviza (previously Kantar Health, a paid consultant to Apellis Pharmaceuticals); K.W., Z.H., J.N. and E.P. are full-time employees of Sobi, and E.P. and K.W. are shareholders; and R.D. and J.F. are full-time employees of Apellis Pharmaceuticals.
Supplemental Material
Download MS Word (87 KB)Supplemental Material
Download MS Word (87 KB)Acknowledgements
Medical writing support was provided by Michael Kane for Apothecom, a paid consultant to Swedish Orphan Biovitrum AB, and was funded by the latter.
Data availability
Anonymised patient source data are not available; all relevant analysed data are included in the manuscript and supplementary materials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Anonymised patient source data are not available; all relevant analysed data are included in the manuscript and supplementary materials.
Additional information
Funding
References
- Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017 Nov;3:17028. doi:10.1038/nrdp.2017.28.
- Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the international PNH registry. Ann Hematol. 2020 Jul;99(7):1505–1514. doi:10.1007/s00277-020-04052-z.
- Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015 Sep;95(3):190–198. doi:10.1111/ejh.12543.
- Orphanet: Paroxysmal nocturnal hemoglobinuria [Internet]. Orphanet Journal of Rare Diseases. [cited 2021 Mar 24]. Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=GB&Expert=447.
- Schrezenmeier H, Muus P, Socié G, et al. Baseline characteristics and disease burden in patients in the international paroxysmal nocturnal hemoglobinuria registry. Haematologica. 2014 May;99(5):922–929. doi:10.3324/haematol.2013.093161.
- Cheng WY, Sarda SP, Mody-Patel N, et al. Real-world healthcare resource utilization (HRU) and costs of patients with paroxysmal nocturnal hemoglobinuria (PNH) receiving eculizumab in a US population. Adv Ther. 2021 Aug;38(8):4461–4479. doi:10.1007/s12325-021-01825-4.
- Bektas M, Copley-Merriman C, Khan S, et al. Paroxysmal nocturnal hemoglobinuria: patient journey and burden of disease. J Manag Care Spec Pharm. 2020 Dec;26(12-b Suppl):S8–S14. doi:10.18553/jmcp.2020.26.12-b.s8.
- Tomazos I, Sierra JR, Johnston KM, et al. Cost burden of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria receiving ravulizumab versus eculizumab. Hematology. 2020 Dec;25(1):327–334. doi:10.1080/16078454.2020.1807226.
- Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995 Nov;333(19):1253–1258. doi:10.1056/nejm199511093331904.
- Korkama ES, Armstrong AE, Jarva H, et al. Spontaneous remission in paroxysmal nocturnal hemoglobinuria—return to health or transition into malignancy? Front Immunol. 2018;9:1749. doi:10.3389/fimmu.2018.01749.
- Bektas M, Copley-Merriman C, Khan S, et al. Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs. J Manag Care Spec Pharm. 2020 Dec;26(12-b Suppl):S14–S20. doi:10.18553/jmcp.2020.26.12-b.s14.
- Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. doi:10.3389/fimmu.2019.01157.
- Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004 Feb;350(6):552–559. doi:10.1056/NEJMoa031688.
- Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011 Jun;117(25):6786–6792. doi:10.1182/blood-2011-02-333997.
- Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013 Jun;121(25):4985–4996. doi:10.1182/blood-2012-09-311381.
- Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014 Oct;124(18):2804–2811. doi:10.1182/blood-2014-02-522128.
- Brodsky RA. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2010 Jun;95(6):855–856. doi:10.3324/haematol.2010.023176.
- Alexion. Solirus (eculizumab) [package insert]. U.S. Food and Drug Administration website. [cited 2021 Aug]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125166s422lbl.pdf.
- Alexion. Soliris (eculizumab) [package leaflet]. European Medicines Agency website. [cited date]. Available from: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu309653.
- Griffin M, Kelly R, Pike A. A review of the treatment landscape in paroxysmal nocturnal haemoglobinuria: where are we now and where are we going? Therapeutic Advances in Rare Disease. 2020;1:2633004020959349. doi:10.1177/2633004020959349.
- Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010 Apr;95(4):567–573. doi:10.3324/haematol.2009.007229.
- Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv Exp Med Biol. 2013;735:155–172. doi:10.1007/978-1-4614-4118-2_10.
- Brodsky RA, Peffault de Latour R, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2021 Jan;106(1):230–237. doi:10.3324/haematol.2019.236877.
- Ravulizumab [package insert]. Boston, MA: Alexion Pharmaceuticals, Inc.; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761108s000lbl.pdf.
- Ravulizumab [package leaflet]. Boston, MA: Alexion Pharmaceuticals, Inc.; 2019. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ultomiris.
- Risitano AM, Peffault de Latour R. How we(‘ll) treat paroxysmal nocturnal haemoglobinuria: diving into the future. Br J Haematol. 2022 Jan;196(2):288–303. doi:10.1111/bjh.17753.
- Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009 Apr;113(17):4094–4100. doi:10.1182/blood-2008-11-189944.
- McKinley CE, Richards S, Munir T, et al. Extravascular hemolysis due to C3-loading in patients with PNH treated with eculizumab: defining the clinical syndrome. Blood. 2017;130:3471–3471.
- Pegcetacoplan [package insert]. Waltham, MA: Apellis Pharmaceuticals, Inc.; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215014s000lbl.pdf.
- Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021 Mar;384(11):1028–1037. doi:10.1056/NEJMoa2029073.
- Röth A HB, Griffin M, et al. Effect of pegcetacoplan on quality of life in patients with paroxysmal nocturnal hemoglobinuria from the Pegasus phase 3 trial comparing pegcetacoplan to eculizumab. Blood. 2020;136(Suppl. 1):10–12.
- Dingli D, Matos JE, Lehrhaupt K, et al. The burden of illness in patients with paroxysmal nocturnal hemoglobinuria receiving treatment with the C5-inhibitors eculizumab or ravulizumab: results from a US patient survey. Ann Hematol. 2022 Feb;101(2):251–263. doi:10.1007/s00277-021-04715-5.
- Sharma A, Minh Duc NT, Luu Lam Thang T, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021 Oct;36(10):3179–3187. doi:10.1007/s11606-021-06737-1.
- Badireddy M, Baradhi KM. Chronic anemia. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534803/.
- Cheng WY, Sarda SP, Mody-Patel N, et al. Real-world eculizumab dosing patterns among patients with paroxysmal nocturnal hemoglobinuria in a US population. Clinicoecon Outcomes Res. 2022;14:357–369. doi:10.2147/CEOR.S346816.
- Kofahl C, Dierks M, Schulz-Nieswandt F. SelbstHILfe in Deutschland. Available from: https://www.nakos.de/data/Andere/2018/UKE-Broschuere-SHILD-Studie.pdf.
- PNH Support, UK. Data on file.
- Placeholder: chance data brief comparative regulatory frameworks.
- Schrezenmeier H, Kulasekararaj A, Mitchell L, et al. One-year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naïve to complement inhibitor therapy: open-label extension of a randomized study. Ther Adv Hematol. 2020;11:2040620720966137. doi:10.1177/2040620720966137.
- O’Connell TBM, Johnson S, Tu L, et al. Cost-utility analysis of ravulizumab compared with eculizumab in adult patients with paroxysmal nocturnal hemoglobinuria. Pharmacoeconomics. 2020;38(9):981–994.
- Escalante CP, Chisolm S, Song J, et al. Fatigue, symptom burden, and health-related quality of life in patients with myelodysplastic syndrome, aplastic anemia, and paroxysmal nocturnal hemoglobinuria. Cancer Med. 2019 Feb;8(2):543–553. doi:10.1002/cam4.1953.
- Panse J, de Fontbrune F S, Burmester P, et al. The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors in France, Germany and the United Kingdom: patient-reported insights on symptoms and quality of life. Eur J Haematol. 2022 Jun. doi:10.1111/ejh.13816.
- Montan I, Löwe B, Cella D, et al. General population norms for the functional assessment of chronic illness therapy (FACIT)-Fatigue scale. Value Health. 2018 Nov;21(11):1313–1321. doi:10.1016/j.jval.2018.03.013.
- Cella D, Johansson P, Ueda Y, et al. Clinically important difference for the FACIT-Fatigue scale in paroxysmal nocturnal hemoglobinuria: a derivation from international PNH registry patient data. Blood. 2021;138(Supp. 1):1952.
- Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014 Jul;53(7):958–965. doi:10.3109/0284186x.2013.879998.
- National Research C, Institute of M. The national academies collection: reports funded by national institutes of health. In: Woolf SH, Aron L, editors. US health in international perspective: shorter lives, poorer health. National Academies Press (US); 2013. PMID: 24006554.
- Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019 Feb;133(6):540–549. doi:10.1182/blood-2018-09-876805.
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019 Feb;133(6):530–539. doi:10.1182/blood-2018-09-876136.
- Walentynowicz M, Schneider S, Stone AA. The effects of time frames on self-report. PLoS One. 2018;13(8):e0201655. doi:10.1371/journal.pone.0201655.
- Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi:10.2147/jmdh.S104807.
- Gerber GF, Yuan X, Yu J, et al. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood. 2021 Jul;137(26):3670–3673. doi:10.1182/blood.2021011548.
- Hines A, Hakim N, Barrientos J. COVID-19 infection presenting as paroxysmal nocturnal hemoglobinuria. Clin Case Rep. 2021 Aug;9(8):e04636. doi:10.1002/ccr3.4636.
- Pike A, Muus P, Munir T, et al. COVID-19 infection in patients on anti-complement therapy: the Leeds national paroxysmal nocturnal haemoglobinuria service experience. Br J Haematol. 2020 Oct;191(1):e1–e4. doi:10.1111/bjh.17097.
- Pravdic Z, Mitrovic M, Bogdanovic A, et al. COVID-19 presented with deep vein thrombosis in a patient with paroxysmal nocturnal haemoglobinuria. Hamostaseologie. 2021 Oct;41(5):397–399. doi:10.1055/a-1554-6432.
- Schüller H, Klein F, Lübbert M, et al. Hemolytic crisis in a patient treated with eculizumab for paroxysmal nocturnal hemoglobinuria possibly triggered by SARS-CoV-2 (COVID-19): a case report. Ann Hematol. 2021 Mar;100(3):841–842. doi:10.1007/s00277-020-04318-6.