ABSTRACT
Objectives
Ferroptosis is an iron-dependent, non-apoptotic mode of cell death characterized by excessive accumulation of reactive oxygen species (ROS). It plays an important role in the occurrence, development and treatment of various cancers, but little is known regarding the role of ferroptosis in hematologic malignancies. This review elaborates the regulatory mechanism of ferroptosis and the treatment opportunities for targeting ferroptosis in hematologic malignancies.
Methods:
A systematic literature review through PubMed was conducted to summarize the published evidence on the therapeutic potential of targeting ferroptosis in hematological malignant tumors. Literature sources published in English were searched, using the terms ferroptosis, leukemia, myelodysplastic syndrome, lymphoma and multiple myeloma.
Results:
More and more small molecules have been found to induce ferroptosis in hematologic malignancies through targeted iron metabolism and lipid peroxidation, and some ferroptosis inducers have been proved to have synergistic effect with other chemotherapeutic drugs.
Conclusion:
This paper discusses the significance of ferroptosis in hematologic malignancies and provides a new way for the treatment of hematologic malignancies, and more experimental studies should be conducted in future.
1. Introduction
Ferroptosis, a new regulated cell death (RCD) mode, is first proposed by Dixon et al. [Citation1] in 2012, which varies from cell apoptosis, necrosis, pyroptosis and autophagy in morphology, biochemistry and genetics (). The characteristic morphological changes of ferroptosis are the shrunken mitochondria, condensed internal membrane and reduced or vanished cristae [Citation2]. The molecular mechanism of ferroptosis is mainly dependent on the production and elimination of ROS. Iron metabolism and lipid peroxidation signaling are recognized as central mediators of the process [Citation3]. Emerging evidence shows that ferroptosis is involved in a growing number of oncogenic and anticancer pathways. For example, Dai et al. proved that ferroptosis promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway [Citation4]. However, Badgley et al. proved that ferroptosis play an anti-cancer roles in pancreatic cancer by inducing cysteine depletion [Citation5]. Besides, Bottcher et al. reported that the PGE2 released by ferroptotic cancer cells could disrupt the anti-tumor immunity by targeting NK cells and cDC1s, thus promoting the progression of colon cancer [Citation6]. Most studies have confirmed the pivotal role of ferroptosis in inhibiting or killing diverse cancer cells [Citation7]. Although ferroptosis exhibits a dual effect, pharmacologically triggering ferroptosis is a promising therapeutic therapy. Hematological malignancies are a category of fluid tumors that originate from cells of hematopoietic system or immune system. Systemic iron overload (IO) is common in patients with hematological malignant tumors as a result of ineffective hematopoiesis and repeated blood transfusion. Previous studies have shown that the toxic effect of excessive iron may promote the progression of leukemia [Citation8]. However, recent studies found that triggering ferroptosis based on the high levels intracellular iron has become a promising therapy to preferentially target leukemia cells [Citation9]. Several studies have demonstrated that ferroptosis can inhibit the growth of hematologic malignancies [Citation10–13], making it a promising anticancer strategy. Currently, although iron chelation therapy (ICT) has become an accepted part of the care of some patients with IO based on guideline recommendations, there are no prospective clinical trial data demonstrating a survival benefit in patients with hematological malignant tumors [Citation14]. Different strategies aimed at increasing intracellular iron content may damage the antioxidant capacity of cancer cells, thus entailing ferroptosis. This review summarizes the main molecular regulatory mechanisms of ferroptosis and the latest research progress of targeting ferroptosis in hematological malignant tumors.
Table 1. Features of different kinds of RCD.
2. Alternations of iron metabolism in hematologic malignancies
Iron is an important trace element that are necessary to sustain life, and its level is highly and complex regulated. Epidemiological studies have demonstrated an association between excess iron and increased cancer incidence and risk [Citation15]. Transfusional iron overload always occurs in transfusion dependent hematologic malignancies. It has been reported that acute myeloid leukemia (AML) patients had higher levels of serum ferritin, and hyperferritinemia was significantly associated with a higher cumulative incidence of relapse as well as poorer disease-free and overall survival [Citation16]. Myelodysplastic syndrome is a group of heterogeneous myeloid clonal diseases originating from hematopoietic stem cells. Due to ineffective hematopoiesis and repeated blood transfusions, 50–80% of MDS patients will eventually develop IO [Citation17]. Previous studies have confirmed that MDS patients with higher serum ferritin levels have a poor prognosis[Citation18], and the risk of death increases by 30% for every 500μg/l additional serum ferritin above 1000μg/l [Citation19]. In addition, a multicenter, randomized, double-blind and placebo-controlled trial to assess event-free survival (EFS) and ICT safety in IO patients with low- or intermediate-1-risk MDS confirmed, hazard ratio reduced by 36% (CI, 4% to 58%) and median EFS was prolonged by approximately 1 year with deferasirox versus placebo [Citation20]. Multiple myeloma is a clonal disorder of plasma cells that frequently presents with anemia. One large retrospective analysis of more than one thousand patients found that 73% of myeloma patients were anemic [Citation21]. However, an analysis of 136 consecutive MM patients found that normal iron metabolism and d iron deficiency is more frequent in MM patients than IO [Citation22]. A study found that most of the patients with diffuse large B-cell lymphoma and Hodgkin lymphoma with iron deposits had a higher metabolic tumor volume than did patients without iron deposits [Citation23]. Systemic IO can lead to liver dysfunction, heart failure and atherosclerosis [Citation24], and inhibit the normal hematopoietic function of bone marrow by destroying hematopoietic cells and hematopoietic microenvironment. Iron is a required co-factor for intracellular ribonucleotide reductase that is essential for DNA synthesis. Due to the high rate of proliferation and increased metabolism, cancer cells have an increased need for iron, which promotes the study of iron chelation strategies [Citation25]. So far, there are three iron chelators approved by the European Commission for the treatment of patients with iron overload: Deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX) [Citation26]. Below we summarize the iron chelation strategies against iron overload in hematological malignancies ().
Table 2. Iron chelation strategies in hematological malignancies.
3. Therapeutic strategy: from iron chelation to ferroptosis
3.1 Ferroptosis: role of iron metabolism
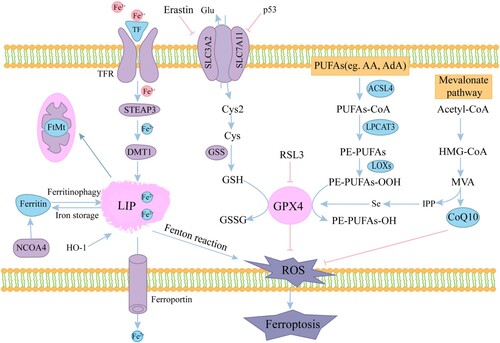
Iron plays an important role in mediating the production of ROS and regulating enzyme activity in lipid peroxidation. Iron metabolism is affected in many aspects, including acquisition, utilization, storage and efflux of iron. Circulating iron(Fe3+), transferrin (TF) and transferrin receptor 1 (TFR1) constitute the TF-Fe3+/TFR1 complex that is subsequently taken into the cell by receptor-mediated endocytosis [Citation38]. In the endosome, Fe3+ is released from the complex, reduced to Fe2+ by six-transmembrane epithelial antigen of the prostate 3 (STEAP3) proteins and transported to the cytoplasmic labile iron pool (LIP) by divalent metal transporter 1 (DMT1) [Citation39, Citation40]. Meanwhile, The TF/TFR1 complex is finally recycled to the cell surface. The iron in LIP is either transported to different parts of the cell for various metabolic needs, or stored in ferritin that includes ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1) [Citation41]. Excessive iron is exported into the circulation via ferroportin 1 (FPN1) which is the sole iron efflux pump yet known and subsequently oxidized by ceruloplasmin (CP) and binded to serum TF [Citation42]. Fe2+ in LIP catalyzes the breakdown of H2O2 to yield hydroxyl radicals through Fenton reaction, which contribute to the ROS pool that could destroy the stability of DNA and the structure of protein and lipid, thus entailing ferroptosis [Citation43]. Multiple regulators of iron metabolism are closely related to ferroptosis. The expression of TFRC is up-regulated in ferroptosis-sensitive cells with RAS mutation, thus enriching the cellular iron pool. The knockdown of TFRC could inhibit erastin-induced ferroptosis [Citation44], supporting the requirement of iron import for ferroptosis. Heme oxygenase 1 (HMOX1/HO-1) catabolizes heme into three products: carbon monoxide (CO), biliverdin, and free iron. Erastin induces HO-1 expression in HT-1080 fibrosarcoma cells and overexpressed HO-1 accelerates Erastin-triggered ferroptotic cell death, whereas Zinc protoporphyrin IX (ZnPP), a HO-1 inhibitor, prevented Erastin-triggered ferroptosis [Citation45]. In addition, the overexpression of mitochondrial ferritin (FTMT) in neuronal cells could inhibit erastin-induced ferroptosis [Citation46]. Nuclear Receptor Coactivator 4 (NCOA4) is a selective cargo receptor that mediates the autophagic degradation of ferritin (‘ferritinophagy’) to maintains intracellular iron homeostasis. Meanwhile, NCOA4 levels are altered by iron status [Citation47]. Mancias et al. further revealed that the iron-dependent regulation of NCOA4 levels is a result of iron-dependent recognition of NCOA4 by HERC2 E3 ubiquitin ligase [Citation48]. The knockdown of NCOA4 blocks ferritin degradation and suppresses erastin-induced ferroptosis, whereas the overexpression of NCOA4 promotes ferroptosis [Citation49]. Cellular iron homeostasis is tightly regulated at the posttranscriptional level by iron regulatory proteins (IRPs). Iron responsive element binding protein 2 (IREB2/ IRP2) has been identified as a necessary gene for erastin-induced ferroptosis in HT-1080 and CALU-1 cells, Silencing IREB2 confers protection against erastin-induced ferroptosis [Citation1]. Its expression products maintain the stability of intracellular LIP by regulating iron metabolism genes, such as TFRC and FTH1. Heat shock proteins (HSPs), a class of functionally related stress proteins, have the ability to regulate iron metabolism. For example, heat shock protein family B small member 1 (HSPB1) can inhibit TFR1-mediated iron uptake. In addition, the up-regulation of CISD1(a kind of mitochondrial iron export protein) suppresses erastin-induced ferroptosis in human hepatocellular carcinoma cells by limiting mitochondrial iron uptake [Citation46, Citation50].
3.2 Ferroptosis: role of lipid peroxidation
Lipid peroxidation is a key initiator of the ferroptotic cascade. Free polyunsaturated fatty acids (PUFAs) must first be esterified as membrane phospholipids before they can be oxidized to lipid peroxides by ROS to become ferroptotic signals [Citation51]. PUFAs metabolism involves two important enzymes, acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3). ACSL4 catalyzes PUFAs and CoA to synthesize polyunsaturated fatty acid coenzyme A (PUFA-CoA), which is then further catalyzed by LPCAT3 into phosphatidylethanolamines(PE)-PUFAs [Citation52]. Therefore, knockdown ACSL4 or LPCAT3 may suppress ferroptosis by depleting the substrates for lipid peroxidation. Doll et al. [Citation53] also confirmed that downregulation of ACSL4 expression or inhibition of ACSL4 activity with thiazolidinediones can block the production and diffusion of lipid hydroperoxides(PUFAs-OOH), thus effectively inhibiting ferroptosis. Lipoxygenase (LOXs), an iron-containing enzyme, can catalyze PE-PUFAs to PE-PUFAs-OOH, resulting in the occurrence of ferroptosis. LOXs activity can be inhibited by tocopherol and tocopherol and Knockdown LOX can suppress erastin-induced ferroptosis [Citation51, Citation54, Citation55]. System Xc-, a glutamate-cystine reverse transport system(composed of two subunits:SLC3A2 and SLC7A11), is mainly responsible for the transport of intracellular glutamate (Glu) and extracellular cystine (Cys2) in a 1:1 manner, then the latter is rapidly converted to cysteine (Cys) for the synthesis of Glutathione (GSH) [Citation56]. Ferroptosis-inducing agents (FINs) can inhibit the intake of cystine and reduce the synthesis of GSH by down-regulating SLC3A2 [Citation55]. In addition, Tumor suppressor protein p53 can also directly inhibit SLC7A11 transcription [Citation57]. GSH, as an intracellular antioxidant buffer, maintains the redox balance in vivo with the assistance of glutathione peroxidase 4 (GPX4). GSH depletion leads to the conversion of PE-PUFAs to PE-PUFA-OOH catalyzed by LOXs, which drives ferroptosis [Citation58]. GPX4, a necessary antioxidant enzyme, can convert two molecules of GSH to oxidized glutathione (GSSG) and reduce PE-PUFA-OOH to PE-OH [Citation59], thus inhibiting ferroptosis. Ras-selective lethal small molecule 3 (RSL3) can directly silence GPX4 to inhibit its activity and overexpression of GPX4 could inhibit RSL3-induced ferroptosis [Citation10]. In addition, the deletion of GPX4 in mice can lead to embryonic death [Citation60]. GPX4, as a proteins containing selenocysteine (Sec), is also controlled by the mevalonate (MVA) pathway which can be inhibited by Statins [Citation61]. Coenzyme Q10 (CoQ10), an endogenous antioxidant produced through MVA pathway, can also prevent ferroptosis by blocking the lipid peroxidation process [Citation62].
The mechanism and regulators of ferroptosis are shown in .
Figure 1. Mechanisms of ferroptosis. The circulated iron (Fe3+) combined with transferrin (TF) enters into cells mediated by transferrin receptor (TFR). Fe3+ is reduced to Fe2+ by iron oxide reductase STEAP3 in the endosome and transported to the cytoplasmic LIP by DMT1. FTH heavy chain is transported to autophagosome after binding with NCOA4, and the degraded iron is released into the cytoplasm. Excessive iron is carried in the extracellular space through FPN. Free iron can also form ROS via the Fenton reaction. System Xc- (consisting of SLC7A11 and SLC3A2) exports glutamine and imports cystine into the cell. Cystine is then transformed into cysteine for GSH synthesis. ACSL4 catalyzes PUFAs and CoA to synthesize PUFA-CoA, which is then further catalyzed by LPCAT3 into PE-PUFAs. Then LOXs oxidizes PE-PUFAs to PE-PUFAs-OOH. Redox enzymes GPX4 use GSH to reduces the endogenous neutralization of PUFAs-OOH to PUFAs-OH, ultimately reducing ROS accumulation.
4. Targeting ferroptosis in hematological malignancies
Many studies have shown that ferroptosis is involved in the development and treatment of cancer and plays an important role in anti-tumor activity. LeJiang et al. [Citation57] proved that p533KR mutants, which are defective in p53-dependent cell cycle arrest, apoptosis and senescence, regulate cysteine metabolism and lead to ferroptosis in tumor cells by inhibiting the expression of SLC7A11. In addition, ferroptosis can be induced in a variety of tumors, including hepatocellular carcinoma, renal cancer, melanoma, breast cancer, pancreatic cancer, neuroblastoma, ovarian cancer, gastric cancer, lung cancer, colorectal cancer and hematological malignancy. Different cancer cells have different sensitivity to ferroptosis, among which DLBCL (hematological malignancies) and renal carcinoma, are more susceptible to erastin-induced ferroptosis [Citation7]. Below we summarize the role of ferroptosis in hematological malignancies, including leukemia, MDS, DLBCL, and MM ().
Table 3. FINs in hematologic malignancies.
4.1 Leukemia
A study found that the expression of TFR1 in leukemic cells is higher compared to their normal counterparts, and poorly differentiated primitive AML blasts tend to express higher levels of TFR1 than partially differentiated AML blasts [Citation74]. It can be inferred that TFR1 may be involved in the clonal development of AML. However, it is still controversial whether TFR1 is a poor prognostic marker of AML. Studies have shown that the survival curves of cells between high and low expression levels of TFR1 are very similar [Citation75]. Similarly, TFR2 is also overexpressed in AML and its expression levels were significantly correlated with serum iron. Moreover, elevated mRNA levels of TFR2-α are associated with a better prognosis in AML patients [Citation76, Citation77]. In addition, Lipocalin2 (LCN2), also known as neutrophil gelatinase-associated Lipocalin, is a protein involved in iron uptake [Citation78]. The levels of LCN2 is up-regulated in AML patients with complete remission (CR) but down-regulated in patients with refractory AML, indicating that the elevated expression of LCN2 contributes to a favorable prognosis of AML [Citation79]. Bertoli et al. [Citation80] confirmed that serum ferritin is an independent prognostic factor for AML, and the overexpression of FTH in AML patients is related to chemotherapy resistance. In addition, the expression of FPN1 is down-regulated in most AML cell lines and primary AML samples [Citation81]. Furthermore, it has been found that low levels of FPN1 are associated with favorable prognosis in AML [Citation82]. These findings suggest that intracellular iron accumulation may contribute to the death of leukemic cells. Moreover, quite many achievements have been made in targeted iron metabolism therapy for leukemia, includes iron chelators and FINs. Ferroptosis has attracted considerable interest as a potential and extensive anticancer strategy. Recent studies have shown that FINs can induce leukemic cells ferroptosis. Erastin-induced ferroptosis in AML cell lines is related to high mobility group protein 1 (HMGB1) cytoplasmic translocation, RAS-JNK/p38 pathway and subsequent TFR1 up-regulation [Citation63]. Furthermore, autophagy could regulate iron homeostasis by affecting ferritin expression and LIP level and alter sensitivity of ALL cells to erastin [Citation64]. Autophagy also regulates VDAC3 ubiquitination by FBXW7 to promote erastin-induced ferroptosis in acute lymphoblastic leukemia [Citation65]. Erastin can also enhanced the anticancer activity of cytarabine or doxorubicin in leukemia cells, while ferrostatin-1 weakened this effect [Citation83–85]. Similarly, RSL3 inactivates GPX4 by depletion of GSH, sensitizing leukemic cells to Smac mimic BV6-induced cell death [Citation66]. In addition, dihydroartemisinin (DHA) can increase iron in LIP by swallowing ferritin, thereby promoting the accumulation of ROS and leading to ferroptosis in leukemic cells [Citation68]. It is worth mentioning that sorafenib, an ferroptosis inducer, has been clinically approved for the treatment of AML with FLT3-ITD mutation [Citation67, Citation86]. Kunxia Cao et al. [Citation71] found that FTO inhibitor-loaded GSH-bioimprinted nanocomposites (GNPIPP12MA) target the FTO/m6 a pathway synergized GSH depletion for enhancing anti-leukemogenesis, and induce ferroptosis by disrupting intracellular redox status. Studies confirmed that magnetic iron oxide nanoparticles Fe3O4 MNPs has obvious cytotoxic effect on Jurkat leukemic cells and cytarabine coated on Fe3O4@SiO2 nanoparticles can enhance the antileukemic effect of cytarabine [Citation70]. Therefore, the induction of targeted ferroptosis in leukemic cells is a promising new therapeutic method.
4.2 Myelodysplastic syndrome (MDS)
According to current guidelines, ICT has become part of the care of patients with low-risk MDS, but is not recommended for patients with high-risk MDS. Moreover, multiple retrospective and cohort studies also showed that overall survival (OS) of patients with chelation is significantly higher than that of patients with non-chelation. However, no published prospective randomized controlled trials have shown that ICT benefits survival in patients with MDS so far [Citation34]. As for the mode of ferroptosis induced by excessive iron, it has been verified in a variety of tumors, and a recent study also confirmed that ferroptosis has worked in MDS cells. LV et al. [Citation11] demonstrated that dexitabine can induce ferroptosis in MDS cells by reducing the level of GSH and GPX4 activity, and erastin can enhance the cytotoxic effect of dexitabine on MDS cells, while DFO can reverse this effect. This finding may provide new ideas for the treatment of MDS patients with IO, but the specific mechanism of ferroptosis in MDS cells remains to be further studied. Whether ICT or iron supplementation therapy, there is an urgent problem to be solved, that is, how to accurately target tumor cells to reduce the adverse effects on the whole body.
4.3 Diffuse large B-cell lymphoma (DLBCL)
Diffuse large B cell lymphoma is a type of cancer cell that is highly sensitive to ferroptosis regulated by GPX4. Erastin and RSL3 induce ferroptosis in DLBCL cell lines by inducing lipid ROS production, and Vitamin E (a lipophilic antioxidant) can inhibit this process [Citation10]. Erastin also exerted an antitumor effect by inhibiting system Xc- induce ferroptosis in a DLBCL xenograft model [Citation72]. In addition, YukoKinowaki et al. [Citation87] demonstrated that the increased ferroptotic sensitivity of DLBCL cells may be due to its weakness in the sulfur transfer pathway. Furthermore, the overexpression of GPX4 can reduce ROS-induced cell death in cultured LCL-K cells, which is related to the poor prognosis of DLBCL patients and is an independent prognostic factor. GPX4 is one of the important regulatory factors of ferroptosis, so we consider that the poor prognosis of patients with overexpression of GPX4 may be related to the inhibition of ferroptosis. A study confirmed that artesunate could induce ferroptosis in DLBCL cells by impairing STAT3 signaling [Citation88]. In addition, Anja Schmitt et al. [Citation89] confirmed that dimethyl fumarate induces ferroptosis by the peroxidation of phospholipids in DLBCL and has synergistic effect with ferroptosis suppressor protein 1(FSP1) inhibitor. These findings may provide fresh perspective for the treatment of DLBCL.
4.4 Multiple myeloma (MM)
Rajkumar et al. [Citation12] demonstrated the effectiveness of iron toxicity on the growth of cancer cells in the MM mouse model. In addition, studies have confirmed that the MM cell lines are sensitive to iron toxicity, and the combination of bortezomib and iron can more effectively inhibit the survival of MM cells and the progression of disease than those used alone [Citation90, Citation91]. Iron significantly increased the production of malondialdehyde (MDA), a by-product of lipid peroxidation in MM cell lines (MM.1S, U266, H929 and OPM-2) [Citation92], which is a recognized index of ferroptosis. In addition, Zhong et al. [Citation73] found that GPX4 and SLC7A11, as key regulators of ferroptosis, are highly expressed in primary MM cells, and a new immunosuppressant Fingolimod (FTY720) can down-regulate their expression at mRNA and protein levels. FTY720 induces ferroptosis and autophagy through PP2A/AMPK pathway, while deferrioxamine mesylate (DFOM) and ferristatin-1 (Fer-1) can reverse FTY720-induced ferroptosis in MM cells. Apart from that, artemether has also been shown to be effective against MM [Citation93]. In summary, we speculate that ferroptosis may be involved in the development and treatment response of MM.
5. Conclusion
Ferroptosis, a form of iron-dependent non-apoptotic cell death, is an ideal target for biochemical research. This paper discusses the significance of ferroptosis in hematologic malignancies and provides a new way for the treatment of hematologic malignancies. Iron metabolism plays a critical role in hematologic malignancies, and more studies are needed to evaluate the possibility of targeted iron therapy from iron chelation to ferroptosis. In addition, non-targeted iron supplementation may increase the tumorigenicity of tumors and cause harmful systemic effects, so how to target iron supplementation to tumor cells to induce ferroptosis is a complex problem to be solved. More and more small molecules have been found to induce ferroptosis directly or indirectly through targeted iron metabolism and lipid peroxidation, and some ferroptosis inducers have been proved to effectively inhibit the growth of tumor cells in combination with other chemotherapeutic drugs. Among them, iron-based nanomaterials have accurate targetability, which can reduce the adverse effects of ferroptosis on normal tissues and cells. Although the research on ferroptosis mediated tumor therapy and drug design is still in its infancy, the research in this area is receiving more and more attention, and the induction of ferroptosis is expected to become a promising anti-cancer strategy.
Ethics approval and consent to participate
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072.
- Li J, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2).
- Lu B, et al. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 2017;8:992.
- Dai E, et al. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. 2020;11(1):6339.
- Badgley MA, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–89.
- Bottcher JP, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037.
- Mou Y, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34.
- Grignano E, et al. From iron chelation to overload as a therapeutic strategy to induce ferroptosis in leukemic cells. Front Oncol. 2020;10:586530.
- Kajarabille N, Latunde-Dada GO. Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J Mol Sci. 2019;20(19).
- Yang WS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317–331.
- Lv Q, et al. Abnormal ferroptosis in myelodysplastic syndrome. Front Oncol. 2020;10:1656.
- Rajkumar SV, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology. 2014;15(12):E538–E548.
- Battipaglia G, et al. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3 mutated acute myeloid leukemia: an update. Clin Lymphoma Myeloma Leukemia. 2019;19(8):506–508.
- Franke GN, et al. Iron overload and its impact on outcome of patients with hematological diseases. Mol Aspects Med. 2020;75:100868.
- Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk – a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23(1):12–31.
- Lebon D, et al. Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk Res. 2015;39(8):818–821.
- Shenoy N, et al. Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood. 2014;124(6):873–881.
- Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011;90(1):1–10.
- Malcovati L, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–7603.
- Angelucci E, et al. Iron chelation in transfusion-dependent patients with low- to intermediate-1-risk myelodysplastic syndromes: a randomized trial. Ann Intern Med. 2020;172(8):513–522.
- Kyle RA, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33.
- Konig C, et al. Prevalence of iron overload vs iron deficiency in multiple myeloma: resembling or different from MDS– and stem cell transplant (SCT)–patients? Clin Lymphoma Myeloma Leuk. 2013;13(6):671–680.
- Cottereau AS, et al. Whole-body diffusion-weighted MR imaging of iron deposits in hodgkin, follicular, and diffuse large B-cell lymphoma. Radiology. 2018;286(2):560–567.
- Greenberg PL, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(1):60–87.
- Kovacevic Z, et al. The medicinal chemistry of novel iron chelators for the treatment of cancer. Curr Top Med Chem. 2011;11(5):483–499.
- Weber S, et al. The clinical significance of iron overload and iron metabolism in myelodysplastic syndrome and acute myeloid leukemia. Front Immunol. 2020;11:627662.
- Furukawa T, et al. Iron deprivation decreases ribonucleotide reductase activity and DNA synthesis. Life Sci. 1992;50(26):2059–2065.
- Yang Y, et al. Effects of deferoxamine on leukemia in vitro and its related mechanism. Med Sci Monit. 2018;24:6735–6741.
- Leardi A, et al. Desferioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. Br J Haematol. 1998;102(3):746–752.
- Yalcintepe L, Halis E. Modulation of iron metabolism by iron chelation regulates intracellular calcium and increases sensitivity to doxorubicin. Bosn J Basic Med Sci. 2016;16(1):14–20.
- Shapira S, et al. Deferasirox selectively induces cell death in the clinically relevant population of leukemic CD34(+)CD38(-) cells through iron chelation, induction of ROS, and inhibition of HIF1alpha expression. Exp Hematol. 2019;70:55–69.
- Ohyashiki JH, et al. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009;100(5):970–977.
- Li N, et al. Synergistic inhibitory effects of deferasirox in combination with decitabine on leukemia cell lines SKM-1, THP-1, and K-562. Oncotarget. 2017;8(22):36517–36530.
- Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: that is the question! Blood Rev. 2018;32(5):368–377.
- Devin J, et al. Targeting cellular iron homeostasis with ironomycin in diffuse large B-cell lymphoma. Cancer Res. 2022;82(6):998–1012.
- Kamihara Y, et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/beta-catenin signaling in human multiple myeloma. Oncotarget. 2016;7(39):64330–64341.
- Pullarkat V, et al. Iron chelators induce autophagic cell death in multiple myeloma cells. Leuk Res. 2014;38(8):988–996.
- Wang Y, et al. Iron metabolism in cancer. Int J Mol Sci. 2018;20(1).
- Colins A, et al. Mathematical modeling of intestinal iron absorption using genetic programming. PLoS One. 2017;12(1).
- Zhou LF, et al. Alterations in cellular iron metabolism provide more therapeutic opportunities for cancer. Int J Mol Sci. 2018;19(5).
- Brown RAM, et al. Altered iron metabolism and impact in cancer biology, metastasis, and immunology. Front Oncol. 2020;10:476.
- Torti SV, et al. Iron and cancer. Annu Rev Nutr. 2018;38:97–125.
- Lachaier E, et al. Ferroptosis, a new form of cell death relevant to the medical treatment of cancer. M S-Med Sci. 2014;30(8-9):779–783.
- Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245.
- Kwon MY, et al. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6(27):24393–24403.
- Wang YQ, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Am J Hematol. 2017;92(8):E459–E459.
- Mancias JD, et al. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105+.
- Mancias JD, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. eLife. 2015;4.
- Hou W, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428.
- Yuan H, et al. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844.
- Kagan VE, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90.
- Dixon SJ, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10(7):1604–1609.
- Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98.
- Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482(3):419–425.
- Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285.
- Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–176.
- Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62.
- Yang WS, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966–E4975.
- Lv HH, et al. Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid Med Cell Longevity. 2019;2019.
- Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11-12):2195–2209.
- Angeli JPF, Conrad M. Selenium and GPX4, a vital symbiosis. Free Radical Biol Med. 2018;127:153–159.
- Mullen PJ, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16(11):718–731.
- Ye FH, et al. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRAS(Q)(61L) cells. Am J Cancer Res. 2019;9(4):730 + .
- Zhu T, Fan Y. Autophagy regulates the sensitivity of acute lymphoblastic leukemia cells to ferroptosis activator by influencing iron homeostasis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(5):1380–1386.
- Zhu T, et al. Autophagy regulates VDAC3 ubiquitination by FBXW7 to promote erastin-induced ferroptosis in acute lymphoblastic leukemia. Front Cell Dev Biol. 2021;9:740884.
- Schoeneberger H, et al. Impairment of antioxidant defense via glutathione depletion sensitizes acute lymphoblastic leukemia cells for smac mimetic-induced cell death. Oncogene. 2015;34(31):4032–4043.
- Metzelder SK, et al. Long-term survival of sorafenib-treated FLT3-ITD-positive acute myeloid leukaemia patients relapsing after allogeneic stem cell transplantation. Eur J Cancer. 2017;86:233–239.
- Du J, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radical Biol Med. 2019;131:356–369.
- Fox JM, et al. Artemisinin-derived dimer ART-838 potently inhibited human acute leukemias, persisted in vivo, and synergized with antileukemic drugs. Oncotarget. 2016;7(6):7268–7279.
- Namvar F, et al. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomed. 2014;9:2479–2488.
- Cao K, et al. Glutathione-bioimprinted nanoparticles targeting of N6-methyladenosine FTO demethylase as a strategy against leukemic stem cells. Small. 2022;18(13):e2106558.
- Zhang Y, et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26(5):623–633.
- Zhong Y, et al. FTY720 induces ferroptosis and autophagy via PP2A/AMPK pathway in multiple myeloma cells. Life Sci. 2020;260:118077.
- Liu Q, et al. Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia. Leuk Lymphoma. 2014;55(4):892–898.
- Wu B, et al. Clinical value of high expression level of CD71 in acute myeloid leukemia. Neoplasma. 2016;63(5):809–815.
- Kawabata H, et al. Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells. Blood. 2001;98(9):2714–2719.
- Nakamaki T, et al. Elevated levels of transferrin receptor 2 mRNA, not transferrin receptor 1 mRNA, are associated with increased survival in acute myeloid leukaemia. Br J Haematol. 2004;125(1):42–49.
- Bauvois B, Susin SA. Revisiting neutrophil gelatinase-associated lipocalin (NGAL) in cancer: saint or sinner? Cancers (Basel). 2018;10(9).
- Yang WC, et al. Higher lipocalin 2 expression may represent an independent favorable prognostic factor in cytogenetically normal acute myeloid leukemia. Leuk Lymphoma. 2013;54(8):1614–1625.
- Bertoli S, et al. Ferritin heavy/light chain (FTH1/FTL) expression, serum ferritin levels, and their functional as well as prognostic roles in acute myeloid leukemia. Eur J Haematol. 2019;102(2):131–142.
- Trujillo-Alonso V, et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol. 2019;14(6):616–622.
- Gasparetto M, et al. Low ferroportin expression in AML is correlated with good risk cytogenetics, improved outcomes and increased sensitivity to chemotherapy. Leuk Res. 2019;80:1–10.
- Dachert J, et al. RSL3 and Erastin differentially regulate redox signaling to promote Smac mimetic-induced cell death. Oncotarget. 2016;7(39):63779–63792.
- Probst L, et al. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol. 2017;140:41–52.
- Yu Y, et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol. 2015;2(4):e1054549.
- Battipaglia G, et al. Efficacy and feasibility of sorafenib As a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3 mutated acute myeloid leukemia. Blood. 2016;128(22).
- Kinowaki Y, et al. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab Invest. 2018;98(5):609–619.
- Chen Y, et al. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell Signal. 2021;88:110167.
- Schmitt A, et al. Dimethyl fumarate induces ferroptosis and impairs NF-kappaB/STAT3 signaling in DLBCL. Blood. 2021;138(10):871–884.
- Campanella A, et al. Iron increases the susceptibility of multiple myeloma cells to bortezomib. Haematologica. 2013;98(6):971–979.
- Bordini J, et al. Induction of iron excess restricts malignant plasma cells expansion and potentiates bortezomib effect in models of multiple myeloma. Leukemia. 2017;31(4):967–970.
- Bordini J, et al. Iron causes lipid oxidation and inhibits proteasome function in multiple myeloma cells: a proof of concept for novel combination therapies. Cancers (Basel). 2020;12(4).
- Holien, T., et al., Lymphoma and myeloma cells are highly sensitive to growth arrest and apoptosis induced by artesunate. Eur J Haematol. 2013. 91(4): p. 339-346.
