ABSTRACT
Objective
Various hematologic side effects of the Coronavirus Disease 2019 (COVID-19) vaccination has been reported, and most of them are thought to be related to autoimmune pathways. To the best of our knowledge, only few cases of post-COVID-19 vaccination aplastic anemia (AA) have been reported and there is no reported Korean case of COVID-19 vaccine-induced AA yet. We present a case of severe immune-mediated AA that developed after the administration of a messenger ribonucleic acid (mRNA) gene-based spike protein vaccine against COVID-19, which responded well to immunosuppressive therapy, and discuss the probable pathogenesis of AA and the implication of vaccination along with a comparison of previous cases reported.
Methods
A 53-year-old Korean man developed sudden pancytopenia three months after COVID-19 vaccination. To evaluate the cause of pancytopenia, a bone marrow study was performed.
Results
A diagnosis of AA was made through the bone marrow study and he received triple immunosuppressive therapy (IST). After triple IST for five months, his blood cell count was improved and maintained without transfusion and his follow-up bone marrow examination showed improved cellularity.
Conclusion
COVID-19 vaccine might be associated with the development of immune-mediated AA. Prompt hematologic evaluation should be performed when there are symptoms or signs suggestive of cytopenia after COVID-19 vaccination. Although the clinical outcome of post-vaccination AA varies, a good prognosis can be possible for patients with COVID-19 vaccination-induced AA.
Introduction
About a year after the start of the Coronavirus Disease 2019 (COVID-19) vaccination, safety concerns regarding the vaccine have been raised continuously. The hematologic side effects of COVID-19 vaccination include immune thrombocytopenia, autoimmune hemolytic anemia, and the aggravation of pre-existing hematologic diseases such as paroxysmal nocturnal hematuria (PNH) [Citation1–7]. Almost all hematologic manifestations after COVID-19 vaccination in previous studies were thought to be related to autoimmune pathways.
In the majority of sporadic cases, severe and acute aplastic anemia (AA) appear to be immune-mediated [Citation8]. A few studies reported post-vaccination AA [Citation9,Citation10]. To the best of our knowledge, few cases of post-COVID-19 vaccination AA have been reported [Citation1,Citation11–13]. However, a Korean case of COVID-19 vaccine-induced AA has not been reported yet. Here, we present a case of very severe immune-mediated AA that developed after the administration of an mRNA gene-based spike protein vaccine against COVID-19 that responded well to immunosuppressive therapy. In addition, the probable pathogenesis of post-COVID-19 vaccination AA and the implications of vaccination are discussed.
Case description
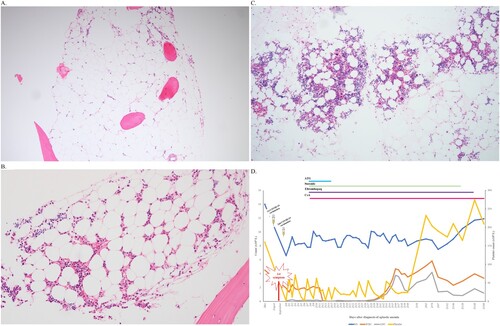
A 53-year-old man was admitted to a tertiary hospital because of bruises on both legs and melena in November 2021. He had no remarkable past or family history. He was not taking any medications. He received the first Moderna COVID-19 vaccine in August 2021 and the second vaccine in September 2021. He first noted the sudden onset of bruises two weeks after the first vaccine dose. He also noticed continuous bleeding after shaving after the second vaccine dose. His most recent complete blood cell counts (CBC) before COVID-19 vaccination (three months before vaccination) were normal (hemoglobin (Hb), 14.0 g/dL (reference range: 13.0–17.5 g/dL); white blood cells (WBC), 4.51 × 109 /L (reference range: 4.0–10.0 × 109 /L); platelets (PLT), 160 × 109 /L (reference range: 150–450 × 109 /L), and he had no symptoms of cytopenia before vaccination. CBC analysis at admission showed the following results: Hb, 6.6 g/dL (reference range: 13.0–18.0 g/dL); WBC, 1.92 × 109 /L (reference range: 4.0–10.0 × 109 /L); absolute neutrophil count (ANC), 0.12 × 109 /L (reference range: 1.4–7.5 × 109 /L); PLT, 2 × 109 /L (reference range: 150–400 × 109 /L); and absolute reticulocytes, 10.6 × 109 /L (reference range: 24–84 × 109 /L), with normal mean corpuscular volume (94.3 fL). All other blood tests (including serum bilirubin, lactate dehydrogenase, vitamins, and iron status) and coagulation tests were within the normal ranges. The peripheral blood smear showed no abnormal immature cells such as blasts or nucleated red blood cells (RBCs). RBC morphology was not remarkable. Serologic tests for hepatitis viruses and human immunodeficiency virus and autoimmune tests were all negative except for cytomegalovirus (CMV) immunoglobulin G (IgG). The bone marrow (BM) examination showed markedly hypocellular marrow (less than 5% cellularity) without any morphologic abnormalities or increases in the number of blasts (A). The cytogenetic results were 45,X,-Y[Citation4]/46,XY,t(11;14)(p10;p10)[Citation3]/46,XY[Citation13]. Flow cytometric analysis showed no definite PNH clones (0.02% RBC, 0.14% granulocytes, and 0.60% monocytes; all were within the reference ranges of the local laboratory). The diagnosis of very severe aplastic anemia was made. The patient received triple immunosuppressive therapy (IST: anti-thymocyte globulin (rabbit) 260 mg/day for 5 days, cyclosporin A 700 mg/day for 15 days, 800 mg for the next 10 days, 1200 mg for the next 2 days, 1400 mg for the next 2 days, 700 mg for the next 10 days, 500 mg for the next 10 days, 300 mg for the next 150 days, and eltrombopag 75 mg/day for 180 days), and his blood cyclosporin level was continuously maintained within the therapeutic range (150–300 ng/mL). During triple IST, he experienced Escherichia coli sepsis and CMV colitis. However, he recovered after appropriate antibiotic and antiviral therapy. At the last follow-up (April 2022), he was still maintained on triple IST. He showed improved blood counts (Hb 10.3 g/dL, ANC 1.01 × 109 /L, and PLT 219 × 109 /L) without transfusion. His follow-up BM examination showed improved cellularity (from 5% to 30%, average: 15%) (B). The patient never experienced COVID-19 during his hospital course.
Figure 1. (A) Bone marrow biopsy at the time of aplastic anemia diagnosis. (B) Bone marrow biopsy and (C) clot section at follow-up. (D) Changing blood count patterns in our patient. ** ANC, absolute neutrophil count; ATG, anti-thymocyte globulin; CsA, cyclosporin A; HB, hemoglobin; PLT, platelet; WBC, white blood cells.
Discussion
Until now, several hematologic adverse effects from COVID-19 vaccinations have been reported, with variable severity and outcomes [Citation1–7,Citation9,Citation10]. The precise cause of hematologic adverse effects, mostly cytopenia, is currently unclear. However, most studies have suggested an underlying immunologic mechanism [Citation2–4,Citation6,Citation14,Citation15]. The triggering of autoimmune responses to host proteins has been considered a mechanism in the development of COVID-19 vaccine-induced thrombotic thrombocytopenia [Citation6]. A similar mechanism of AA after COVID-19 vaccination was proposed in previous reports [Citation1,Citation11]. The current literature revealed a few hypotheses regarding the relationship between spike protein received in mRNA vaccines and adverse effects [Citation16]. There are possible interactions between spike protein and several intra-/extracellular signaling pathways, which result in various post-vaccination adverse effects [Citation16]. According to proteomic analysis, severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) spike protein could bind to hemoglobin and its metabolites, and lead to further adverse medical diseases [Citation16,Citation17]. A previous study reported a possible association between AA relapse and COVID-19 vaccination [Citation12]. In that study, enhanced CD8+ T-cell-dependent activation and immune-mediated response to an mRNA-based COVID-19 vaccine were suggested as mechanisms for the increased AA relapse risk.
Secondary AA can be caused by infections, drugs, or various diseases. The pathophysiology and treatment of AA are somewhat different depending upon the cause [Citation8]. For example, cytotoxic drugs can damage marrow chemically and physically. Treatment for such damage includes supportive care, hematopoietic growth factors, and hematopoietic stem cell transplantation (HSCT) [Citation8]. Idiopathic and various immune-associated diseases can destroy marrow immunologically. Such destruction can be treated by immunosuppressive therapy and HSCT [Citation8]. Our patient responded well to conventional immunosuppressive therapy for AA and maintained tolerable blood counts without transfusions several months after diagnosis, further supporting the autoimmune mechanism of AA stimulated by the COVID-19 vaccine [Citation8]. This is the strongest evidence for an immune mechanism of AA after COVID-19 vaccination.
In our patient, bruising started two weeks after the first dose of vaccine, and bleeding started just after the second dose of vaccine. According to the literature, spike protein can circulate in the blood from one hour after mRNA vaccination, and the serologic response arises about three weeks after vaccination [Citation16]. Measuring the blood spike protein antigen titer could have helped to elucidate the mechanism of AA in our patient, although false-negative results can be seen since the spike protein is a modified antigen and all testing involves the initial antigen [Citation18]. However, the test was not performed. Based on the current literature, the possible cause of AA in our patient may have been an interaction with spike protein, a serologic response, or both when considering the time that the hematologic symptoms occurred. In this case, we should also consider the possibility that this was a spike protein-derived temporal condition mimicking AA. However, since our patient responded well to immunosuppressive treatment for AA, we thought that the possibility of true and definite AA caused by the above-mentioned possible mechanisms was high.
It was also necessary to determine whether or not AA was already present. Before vaccination, our patient had no history of infection, drug intake, or other possible causes of AA. According to the literature, the first symptoms related to cytopenia or the first CBC abnormalities were reported as early as the day following vaccination, and as late as three months after [Citation1,Citation11–13]. AA was usually diagnosed 1–2 weeks after receiving other forms of vaccines [Citation9,Citation19]. Our patient’s blood test results were normal three months before vaccination, but he experienced the sudden onset of bruising two weeks after the first vaccination and was diagnosed with AA two months after the second dose of the COVID-19 vaccine. Our patient’s timeline is consistent with that of other patients reported in the literature [Citation1,Citation11–13]. Therefore, this case was most likely COVID-19 vaccine-induced AA rather than the expression of pre-existing disease.
In our patient, the chromosomal abnormality t(11;14)(p10;p10) was found. Generally, the t(11;14)(q13;q32) abnormality can be found in plasma cell myeloma and other hematologic malignancies, but there was no evidence of other hematologic malignancies, including myeloma, in the patient’s bone marrow study. The t(11;14)(p10;p10) abnormality has not been reported to be associated with any specific hematologic malignancies. No specific genes have been identified in this location but clonal mosaicism of the chromosome has been found in many normal tissues. Some benign clonal populations are common in AA [Citation8]. Therefore, the significance of this chromosomal abnormality was inconclusive. Close bone marrow study follow-ups and chromosomal analysis should be done to evaluate the clinical significance of a chromosomal abnormality. Next-generation sequencing (NGS) can detect mutations associated with myeloid malignancies in nearly 20% of the patients with AA [Citation20]. Unfortunately, we could not perform an NGS study. However, a genetic abnormality is not an essential requirement for the diagnosis of AA. Therefore, we could diagnose AA in our patient.
The clinical and laboratory characteristics of the cases reported so far, as well as those in our case, are summarized in [Citation1,Citation11–13]. All reported patients were males who had received an mRNA gene-based spike protein vaccine against COVID-19. The onset time varied from one day to 2–3 months after vaccination. However, our patient experienced the sudden onset of bruising two weeks after the first vaccination. Therefore, the onset time was thought to be within one month after vaccination. The severity of AA after COVID-19 vaccination was severe without definite PNH clones. However, the clinical course of each case was different. The three previously reported patients showed insufficient responses to IST, and one patient expired. Actually, the only curative treatment for AA is HSCT. It is always the preferred treatment, especially in a young patient with immune aplastic anemia [Citation8]. However, IST is the standard treatment modality for older patients who do not undergo HSCT. About 20–30% of the patients who responded to IST showed a complete response [Citation8]. Contrary to the two previously reported patients, our patient responded well to IST. He maintained an improved blood count without transfusion. His bone marrow cellularity also improved. It is currently unclear which factors contributed to these prognostic differences. Different vaccine manufacturers and/or chromosomal abnormalities might have contributed to the differences in the clinical courses. Therefore, the possibility of a good prognosis in patients with immune-mediated AA after COVID-19 vaccination could be considered, although there was insufficient information to develop a comprehensive theory.
Table 1. Clinical and laboratory features of patients with COVID-19-vaccine-induced aplastic anemia.
In conclusion, the COVID-19 vaccine might be associated with the development of immune-mediated cytopenia and/or AA. Prompt hematologic evaluation should be performed when there are symptoms or signs suggestive of cytopenia after COVID-19 vaccination. Although the clinical outcomes of patients with post-vaccination AA varied from insufficient recovery despite appropriate medical treatment and HSCT to hematologic improvement with only medical treatment, patients with COVID-19 vaccination-induced AA may have good prognoses. The exact factors contributing to the differences in clinical outcomes are currently unknown. Further studies with large and prospective cohorts are needed to determine the relationship between COVID-19 vaccination and AA.
Conflicts of interest
None declared.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Soonchunhyang University (No. 2022-03-015).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cecchi N, Giannotta JA, Barcellini W, et al. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. 2021.
- Fattizzo B, Giannotta JA, Cecchi N, et al. SARS-CoV-2 vaccination in patients with autoimmune cytopenias: The experience of a reference center. Am J Hematol. 2021;96(11):E413–E6.
- Kim G, Choi EJ, Park HS, et al. A case report of immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. J Korean Med Sci. 2021;36(43):e306), doi:10.3346/jkms.2021.36.e306.
- Osmanodja B, Schreiber A, Schrezenmeier E, et al. First diagnosis of thrombotic thrombocytopenic purpura after SARS-CoV-2 vaccine - case report. BMC Nephrol. 2021;22(1):411), doi:10.1186/s12882-021-02616-3.
- Gerber GF, Yuan X, Yu J, et al. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood. 2021;137(26):3670–3. doi:10.1182/blood.2021011548.
- Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. 2022;44(1):e10–e2. doi:10.1111/ijlh.13535.
- Portuguese AJ, Sunga C, Kruse-Jarres R, et al. Autoimmune- and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Adv. 2021;5(13):2794–8. doi:10.1182/bloodadvances.2021004957.
- Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–56. doi:10.1056/NEJMra1413485.
- Donnini I, Scappini B, Guidi S, et al. Acquired severe aplastic anemia after H1N1 influenza virus vaccination successfully treated with allogeneic bone marrow transplantation. Ann Hematol. 2012;91(3):475–6. doi:10.1007/s00277-011-1278-0.
- Angelini P, Kavadas F, Sharma N, et al. Aplastic anemia following varicella vaccine. Pediatr Infect Dis J. 2009;28(8):746–8. doi:10.1097/INF.0b013e31819b6c1f.
- Tabata S, Hosoi H, Murata S, et al. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J Autoimmun. 2022;126:102782, doi:10.1016/j.jaut.2021.102782.
- Röth A, Bertram S, Schroeder T, et al. Acquired aplastic anemia following SARS-CoV-2 vaccination. Eur J Haematol. 2022;109(2):186–94. doi:10.1111/ejh.13788.
- Sridhara S, Nair R, Stanek M. Severe aplastic anemia after receiving SARS-CoV-2 moderna mRNA vaccination. J Hematol. 2022;11(1):34–9. doi:10.14740/jh954.
- Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–7. doi:10.1002/ajh.26132.
- Shah SRA, Dolkar S, Mathew J, et al. COVID-19 vaccination associated severe immune thrombocytopenia. Exp Hematol Oncol. 2021;10(1):42), doi:10.1186/s40164-021-00235-0.
- Mouliou DS, Dardiotis E. Current evidence in SARS-CoV-2 mRNA vaccines and post-vaccination adverse reports: knowns and unknowns. Diagnostics (Basel). 2022;12(7.
- Lechuga GC, Souza-Silva F, Sacramento CQ, et al. SARS-CoV-2 proteins bind to hemoglobin and Its metabolites. Int J Mol Sci. 2021;22(16.
- Mouliou DS, Gourgoulianis KI. False-positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives. Expert Rev Respir Med. 2021;15(8):993–1002. doi:10.1080/17476348.2021.1917389.
- Hendry CL, Sivakumaran M, Marsh JC, et al. Relapse of severe aplastic anaemia after influenza immunization. Br J Haematol. 2002;119(1):283–4. doi:10.1046/j.1365-2141.2002.379111.x.
- Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124(17):2698–704. doi:10.1182/blood-2014-05-574889.
