ABSTRACT
Background
The appearance of bite cells associated with methemoglobinemia can be caused by oxidizing drugs such as dapsone in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency or high drug serum levels. Bite cells are often pathognomonic for oxidant injury in patients with G6PD deficiency and suggest active hemolysis.
Case Presentation
We report a case of a woman with no prior history of G6PD deficiency who presented with anemia, methemoglobinemia and bite cells on peripheral blood smear after dapsone therapy for new onset idiopathic urticaria. Laboratory tests for G6PD, blood count and liver function were within normal limits prior to initiation of therapy. During the patient’s hospital course, moderate methemoglobinemia and anemia were identified despite mildly increased serum G6PD level. These pathologies were reversed upon stopping dapsone therapy.
Conclusion
This case highlights the potential for therapeutic levels of dapsone to induce side effects in patients without G6PD deficiency and highlights the importance of routine blood monitoring for anemia and hemolysis during the course of drug therapy.
Introduction
Dapsone is an oral sulfone drug that can cause the appearance of bite cells on peripheral smears in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [Citation1]. Dapsone can be used to treat chronic idiopathic urticaria, a skin condition characterized by the appearance of pruritic, blanchable cutaneous lesions involving the superficial dermis lasting greater than 6 weeks without identifiable triggers [Citation2]. Second-generation H1 antihistamines are typically used as first line therapy for chronic urticaria, and they can be combined with H2 antihistamines, leukotriene modifiers and systemic glucocorticoids for more severe disease [Citation3]. While injectable omalizumab is the preferred therapy for refractory disease, dapsone can be used in the clinical setting of treatment-refractory idiopathic urticaria with neutrophilia, given its ability to disrupt integrin-mediated neutrophil adhesiveness [Citation4–8].
In patients with G6PD deficiency, most commonly A- and Mediterranean variants, dapsone is contraindicated due to its association with methemoglobinemia and hemolytic anemia [Citation9]. It is rare for dapsone to induce severe adverse outcomes in patients without G6PD deficiency when used at therapeutic levels. In the literature, there are reported cases of dapsone-induced methemoglobinemia and hemolytic anemia without known G6PD deficiency in many different contexts including stem cell and renal transplant recipients and immune thrombocytopenia patients [Citation10–13]. We report a similar case of dapsone causing hemolysis and red blood cell morphologic abnormalities in a woman without G6PD deficiency.
Case presentation
A 36-year-old otherwise healthy woman taking no medications presented to her primary care provider with a one-week history of unprovoked facial rash and was treated with a prednisone taper to improve symptoms. After discontinuation of therapy, she developed diffuse, pruritic urticarial rash that started in the extremities and spread to her abdomen (), accompanied by oropharyngeal angioedema and mild shortness of breath. Angioedema raised concern for the potential development of airway obstruction, which prompted pre-emptive treatment with epinephrine. Labs drawn in the emergency department revealed leukocytosis with absolute neutrophilia, potentially secondary to recent prednisone use or systemic autoimmune reaction given the patient’s self-reported history of rheumatoid arthritis. The patient was ultimately diagnosed with acute spontaneous urticaria and was discharged with H1 antihistamines. She was referred to outpatient dermatology clinic, where high dose prednisone was initiated. An extensive autoimmune workup was performed but was negative. After 6 weeks of unresolving symptoms, dapsone and later omalizumab were added to her regimen to allow for continued control of urticaria while prednisone was being tapered. Prior to starting dapsone, she was noted to have an unremarkable complete blood count (CBC) and G6PD levels at normal levels. There was no family history of G6PD deficiency.
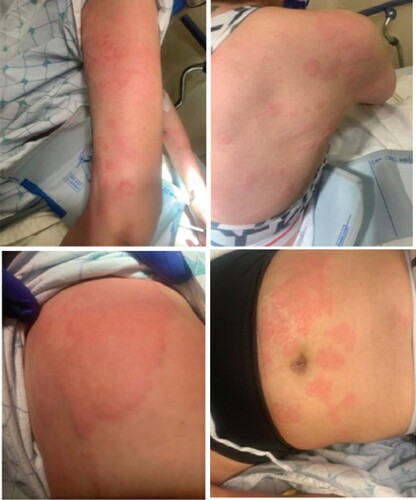
Figure 1. Urticaria was present on the patient on presentation, characterized by diffuse, erythematous, and blanchable wheals involving the superficial dermis. (Top left, clockwise) Urticarial wheals were present on the patient’s arms, back, abdomen, and shoulders.
Five months after onset of urticaria, the patient presented to the emergency department for intermittent epistaxis, which resolved spontaneously. Her prothrombin time, partial thromboplastin time and platelet counts were within normal limits. During evaluation, her oxygen saturation was noted to be in the upper 80% range on room air. She required two liters of oxygen to maintain adequate saturation. Venous blood gas revealed decreased oxygen content and increased methemoglobin at 7% (, Top). Dapsone was thought to be the offending agent and was discontinued.
Figure 2. (Top) Serial patient venous blood gas measurements of oxygen content and methemoglobin levels were made upon hospital admission and at the two-week outpatient visit. Patient data show rapidly decreasing methemoglobin levels associated with increasing oxygen content in the days following termination of dapsone therapy. Values above the red dotted line refer to normal oxygen content. Values below the blue dotted line refer to normal methemoglobin levels. (Bottom) Patient data showing serial hemoglobin measurements during the course of dapsone therapy as indicated by values in the shaded area. Initiation of dapsone therapy is indicated by an arrow and vertical dotted line. Discontinuation of dapsone occurred during admission and is indicated by an arrow and vertical dotted line on day 0. Serial hemoglobin measurements following discontinuation of dapsone therapy are indicated by values to the right of the vertical dotted line. Values above the horizontal black dotted line refer to normal hemoglobin levels.
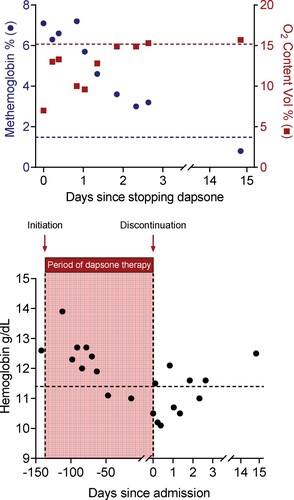
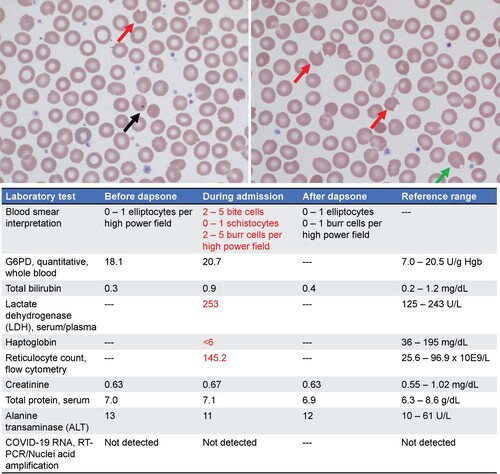
The patient was admitted to the hospital for further observation. She was discovered to have normocytic anemia with normal iron studies, folate and B12 levels. Hemoglobin was trending down prior to admission, which could not be fully explained by intermittent epistaxis (, Bottom Left). This was accompanied by an elevated reticulocyte count, raising suspicion for hemolysis, which was further supported by elevated LDH, low haptoglobin and total bilirubin levels above her baseline (, Bottom). Peripheral blood smear revealed moderate bite cells and Howell-Jolly bodies, which were not observed prior to initiation of dapsone (, Top). The patient was not noted to have splenomegaly, and G6PD levels were mildly elevated (, bottom). Interestingly, two weeks following discharge her hemoglobin was normal (, Bottom Right) and her blood smear no longer showed bite cells, schistocytes, or Howell-Jolly bodies (, Blood Smear Interpretation). After discontinuation of dapsone therapy, methemoglobin levels trended down, and oxygen content trended up (, Top). The resolving clinical picture suggests that dapsone caused the patient’s anemia, methemoglobinemia, and abnormal peripheral smear features. The patient was discharged uneventfully for outpatient follow up, where her urticaria continued to be well controlled on omalizumab.
Figure 3. (Top) Patient’s peripheral blood smear at hospital admission. Red arrows indicate bite cells. Black arrow indicates a Howell-jolly body. Green arrow indicates a blister cell. (Bottom) Pertinent patient laboratory values before initiating, during treatment and after termination of dapsone therapy. Reference ranges are included to indicate normal values. Red text highlights abnormal lab values.
Discussion
The ‘bite cell’ is an aberrant feature of mature red blood cells seen on peripheral smear characterized by removal of one or more portions of the biconcave disc rim of red blood cells by splenic macrophages at sites where hemoglobin has been abnormally deposited [Citation1]. Occasionally, ‘blister cells’, which have a large vacuole along the periphery, may also form alongside ‘bite cells.’ Oxidative injury of red blood cells can result in hemoglobin sulfhydryl group oxidation, which can precipitate the formation of these abnormal red blood cell features. They are most commonly associated with hemolysis and methemoglobinemia in patients with G6PD deficiency exposed to triggers such as medications including dapsone [Citation14,Citation15]. Dapsone is a sulfone antibiotic used as first line therapy for leprosy and second line therapy for Pneumocystis pneumonia [Citation16]. It can also be used as a second line therapy for chronic spontaneous urticaria [Citation2,Citation17]. Dapsone is converted to the metabolite hydroxylamine, which can accumulate in red blood cells and generate free radicals that deplete glutathione stores, leading to oxidative damage of the red blood cell membrane [Citation18]. G6PD is a key enzyme that participates in restoring glutathione. In the rare case of dapsone overdose, hydroxylamine can accumulate to excess levels to overwhelm the protective effect of glutathione and cause oxidative damage to red blood cells even in the presence of normal G6PD levels [Citation11].
This patient had normal G6PD levels and was started on a therapeutic level of dapsone. The patient and had no prior history of red blood cell morphologic abnormalities. Her other home medications would not have predisposed her to hemolysis and her general blood chemistry values were within normal limits (Supplemental Table 1). Yet, after starting dapsone, her hemoglobin trended down (, Bottom Left). A small retrospective case series of 100 leprosy patients taking 100 mg of dapsone per day showed a hemoglobin drop of 3 g/dl in 16% of participants, a finding was that was associated with older age [Citation19]. This patient’s peripheral blood smear showed bite cells and blister cells (, Top). Interestingly, her smear also showed Howell-jolly bodies, a finding most commonly seen in patients without a spleen [Citation20]; the patient was not asplenic and she showed no other evidence for splenic insufficiency. We speculate that in a clinical setting in which numerous Heinz bodies of precipitated hemoglobin are being cleared, the removal of red blood cell nuclear fragments may be transiently impaired (i.e. the capacity for efficient splenic clearance of nuclear fragments is exceeded).
We considered several possibilities as to why the patient experienced dapsone-induced hemolysis and developed morphologic abnormalities of red blood cells. We considered undiagnosed G6PD deficiency as well as functional insufficiencies in kidney, liver, or spleen. The patient had no family history, supporting blood tests, or prior diagnoses suggesting these issues. G6PD deficiency is uncommon in women as the gene is X-linked and usually requires two mutations to result in severe disease [Citation21]. It is be possible that the patient has a heterozygous G6PD mutation that affects protein function, and such carriers can exhibit hemolysis when exposed to oxidizing agents including dapsone [Citation22,Citation23]. Such carriers can have significant subset of red cells that are G6PD deficient, and flow cytometry is able to distinguish G6PD deficient and G6PD replete red blood cells and thereby determine risk of drug-induced hemolysis in these individuals [Citation24]. To date, we have not performed genetic testing or flow analysis to definitively assess this possibility but given the absence of a family history of G6PD deficiency such heterozygosity is not likely. Another consideration was the possibility of a pre-existing hemolytic anemia that may have elevated the patient’s G6PD levels; however, the patient’s baseline hemoglobin was within normal limits. It could also be possible that the patient has a rare, undiagnosed hemoglobinopathy and indeed, dapsone use in the setting of hemoglobinopathy Hasharon has been reported to cause methemoglobinemia and hemolysis in one case report [Citation25].
Finally, we considered the possibility that chronic dapsone use may have been the sole cause of methemoglobinemia and hemolysis, which can rarely occur in patients on high doses of dapsone or with functional mutations in drug elimination pathways such as cytochrome b5 reductase [Citation26]. One case report highlighted the ability of dapsone to cause methemoglobinemia in up to 15% of patients and hemolysis in up to 20% of patients on a daily dose of 100 mg [Citation27,Citation28]. However, these patients also had other significant comorbidities such as leprosy, which may have influenced the interpretation of these results. Given the lack of findings suggesting G6PD deficiency, we believe that this case is a rare presentation of dapsone-induced methemoglobinemia and hemolysis.
Conclusions
This case highlights that even at low doses, dapsone may induce methemoglobinemia and hemolysis accompanied by abnormal peripheral blood smear findings. Heightened clinical awareness for downward-trending hemoglobin levels as an indicator of toxicity during dapsone treatment may help in the early diagnosis of drug-induced methemoglobinemia and hemolysis. Peripheral blood smear may further aid in clinical diagnosis in addition to current guidelines [Citation29].
Author contributions
YH and SCK wrote the manuscript. YH, MG, MA, ADL, HJP, JSG, SCK collected and interpreted data and did literature research. YH, MG, MA, ADL, LEB, HJP, JSG, SCK revised and approved the manuscript.
Supplemental Material
Download MS Word (19.3 KB)Acknowledgements
We thank the patient for kindly giving us permission to present her case in this report and for being helpful in answering questions. We thank UCSF laboratory medicine technologists for helping to procure patient specimens. In particular, we thank Ray Chan for answering questions regarding the processing of specimens. GraphPad Prism 8 was used for making figures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are presented within this manuscript.
Additional information
Funding
References
- Yoo D, Lessin LS. Drug-associated “bite cell” hemolytic anemia. Am J Med 1992;92:243–248. doi:10.1016/0002-9343(92)90071-I
- Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Front Immunol. 2019;10:627. doi:10.3389/fimmu.2019.00627
- Powell RJ, Du Toit GL, Siddique N, et al. BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin Exp Allergy. 2007;37:631–650. doi:10.1111/j.1365-2222.2007.02678.x
- Seth S, Khan DA. The comparative safety of multiple alternative agents in refractory chronic urticaria patients. J Allergy Clin Immunol Pract. 2017;5:165–170.e2.e2. doi:10.1016/j.jaip.2016.08.010
- Ozdemir M, Engin B, Toy H, et al. Treatment of plaque-type localized scleroderma with retinoic acid and ultraviolet A plus the photosensitizer psoralen: a case series. J Eur Acad Dermatol Venereol. 2008;22:519–521. doi:10.1111/j.1468-3083.2007.02390.x
- Morgan M, Cooke A, Rogers L, et al. Double-blind placebo-controlled trial of dapsone in antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2:601–606. doi:10.1016/j.jaip.2014.06.004
- Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res 2014;306:103–124. doi:10.1007/s00403-013-1409-7
- Booth SA, Moody CE, Dahl MV, et al. Dapsone suppresses integrin-mediated neutrophil adherence function. J Invest Dermatol. 1992;98:135–140. doi:10.1111/1523-1747.ep12555654
- Pamba A, Richardson ND, Carter N, et al. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120:4123–4133. doi:10.1182/blood-2012-03-416032
- Morris A, Bain BJ, Atta M, et al. A puzzling case of methemoglobinemia. Am J Hematol 2017;92:1103–1104. doi:10.1002/ajh.24747
- Olteanu H, Harrington AM, George B, et al. High prevalence of Dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Bone Marrow Transplant 2012;47:399–403. doi:10.1038/bmt.2011.83
- Mitsides N, Green D, Middleton R, et al. Dapsone-induced methemoglobinemia in renal transplant recipients: more prevalent than previously thought. Transpl Infect Dis. 2014;16:37–43. doi:10.1111/tid.12161
- Colella MP, Orsi FA, Alves ECF, et al. A retrospective analysis of 122 immune thrombocytopenia patients treated with dapsone: efficacy, safety and factors associated with treatment response. J Thromb Haemostasis. 2021;19:2275–2286. doi:10.1111/jth.15396
- Belfield KD, Tichy EM. Review and drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am J Health-Syst Pharm. 2018;75:97–104. doi:10.2146/ajhp160961
- Opsahl M, Chen W. Blister and bite cells in G6PD deficiency. Int J Lab Hematol. 2022;44:55–56. doi:10.1111/ijlh.13689
- Paniker U, Levine N. Dapsone and sulfapyridine. Dermatol Clin. 2001;19:79–86, viii. doi:10.1016/S0733-8635(05)70231-x.
- Liang SE, Hoffmann R, Peterson E, et al. Use of dapsone in the treatment of chronic idiopathic and autoimmune urticaria. JAMA Dermatol. 2019;155:90–95. doi:10.1001/jamadermatol.2018.3715
- Grossman SJ, Jollow DJ. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther. 1988;244:118–125.
- Byrd SR, Gelber RH. Effect of dapsone on haemoglobin concentration in patients with leprosy. Lepr Rev. 1991;62:171–178. doi:10.5935/0305-7518.19910020
- Scafidi JM, Gupta V. Histology, Howell Jolly bodies. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Beutler E, Yeh M, Fairbanks VF. The Normal human female as a mosaic of X-chromosome activity: studies using the gene for G-6-PD-deficiency as a marker. Proc Natl Acad Sci USA. 1962;48:9–16. doi:10.1073/pnas.48.1.9
- Chu CS, Bancone G, Nosten F, et al. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J 2018;17:101. doi:10.1186/s12936-018-2248-y
- Todd P, Samaratunga IR, Pembroke A. Screening for glucose-6-phosphate dehydrogenase deficiency prior to dapsone therapy. Clin Exp Dermatol 1994;19:217–218. doi:10.1111/j.1365-2230.1994.tb01168.x
- Bancone G, Kalnoky M, Chu CS, et al. The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci Rep. 2017;7:9822. doi:10.1038/s41598-017-10045-2
- Ganer A, Knobel B, Fryd CH, et al. Dapsone-induced methemoglobinemia and hemolysis in the presence of familial hemoglobinopathy Hasharon and familial methemoglobin reductase deficiency. Isr J Med Sci. 1981;17:703–704.
- Keerty D, Eaton K, Haynes E. Dapsone-induced hypoxia. Cureus. 2020;12. doi:10.7759/cureus.9334
- Puavilai S, Chutha S, Polnikorn N, et al. Incidence of anemia in leprosy patients treated with dapsone. J Med Assoc Thai. 1984;67:404–407.
- Singh S, Sethi N, Pandith S, et al. Dapsone-induced methemoglobinemia: “Saturation gap”-the key to diagnosis. J Anaesthesiol Clin Pharmacol. 2014;30:86–88. doi:10.4103/0970-9185.125710
- Iolascon A, Bianchi P, Andolfo I, et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol 2021;96:1666–1678. doi:10.1002/ajh.26340
