ABSTRACT
Objective:
We herein describe two cases of de novo lymphoid blastic transformation in patients with no history of chronic-phase chronic myeloid leukemia (CP-CML), both of whom were labeled initially as Philadelphia positive B-Acute Lymphoblastic Leukemia (B-ALL).
Methods:
The first patient was an 18-year-old male who presented with subjective fever, intentional weight loss, generalized fatigue, and headache. Investigations showed leukocytosis (312 × 10^3/ul), thrombocytopenia and anemia. Flowcytometry was consistent with B-ALL, with aberrant expression of CD13 and CD33. He was found to be positive for BCR::ABL by FISH, and karyotype confirmed the presence of the Philadelphia chromosome. He received a pediatric-inspired regimen and achieved remission with negative measurable residual disease (MRD) by flowcytometry, however with persistent cytogenetic abnormality using FISH for BCR::ABL. FISH abnormality was confirmed to be in the myeloid compartment using myeloid segregated FISH, reclassifying the disease to de novo lymphoid blastic phase CML. The second patient was a 52-year-old male who presented with fever and shortness of breath. Bilateral cervical lymphadenopathy and hepatosplenomegaly were identified on examination, and investigations showed leukocytosis (371 × 10^3/ul), anemia, and thrombocytopenia. BCR::ABL rearrangement was identified by FISH, molecular testing, and confirmed with karyotype. He was treated with Mini-CVD and Ponatinib, achieved complete remission with negative MRD by flow cytometry, however molecular studies showed BCR-ABL1 level at 58% IS indicating a persistent cytogenetic abnormality.
Results:
De novo lymphoid blastic-phase CML can therefore be difficult to differentiate from Philadelphia positive B-ALL due to their overlapping clinical and laboratory picture, implying the need to do myeloid compartment evaluation at the time of diagnosis.
Conclusion:
With recent progress in the treatment of Philadelphia positive B-ALL, including the role of transplant with the use of novel agents, a better characterization of this disease entity in retrospective and prospective trials is warranted.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder that progresses through three distinct phases referred to as chronic, accelerated, and blast. Blastic phase CML (BP-CML) is characterized by failure of the blast cells to differentiate, resulting in rapid accumulation of non-functional cells in the marrow and blood, a distinctive hallmark of this phase. Sudden blastic transformation (SBT) has been described in less than 1% of chronic-phase chronic myeloid leukemia (CP-CML) patients that achieve complete cytogenetic remission [Citation1]. Additionally, BP-CML has also been reported during treatment-free remission, without transitioning into the characteristic step-by-step progression known for this hematological malignancy [Citation1,Citation2]. Similarly, de novo blastic-phase CML (BP-CML)-usually lymphoid-has also been described in prior reports with more favorable outcomes [Citation3].
Traditionally there is marked variability in time discerning the diagnosis of chronic-phase disease and the onset of blast crisis, albeit the possibility of a blast crisis still being able to occur before a chronic-phase diagnosis has been made. Prior work by Hovorkova and colleagues showed patients with discordant minimal residual disease (MRD) using Ig/TCR and BCR-ABL1 have a ‘CML-like’ disease where the BCR-ABL1-positive cells are detected in cell subpopulations other than acute lymphoblastic leukemia (ALL) blasts [Citation4]. At cellular level, a minor fraction of BCR–ABL rearranged clones may take over and progress before chronic phase cells have been established, or reestablished in SBT, clonal dominance. Consequently, this can be confused with Philadelphia positive ALL in newly diagnosed patients [Citation5].
Case 1
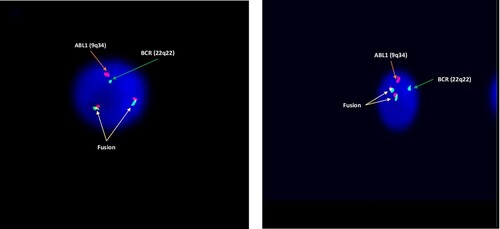
A 17-year-old previously healthy male presented to the Emergency Department complaining of a subjective fever for 5 days, which was associated with generalized fatigue and headache. The patient reported intentional weight loss as well as night sweats for two months before the presentation. On initial evaluation, there was no hepatosplenomegaly or lymphadenopathy. Complete blood count (CBC) showed marked leukocytosis with total white blood cell count (WBC) 312 × 10^3/ul, a hemoglobin level of 5.6 gm/dl, and a platelet count of 26 × 10^3/mm3. Peripheral blood smear displayed 90% blast cells. Renal function tests, liver function tests, and serum electrolytes were all unremarkable. Bone marrow aspirate and biopsy demonstrated hypercellular marrow (cellularity of 100%), heavily infiltrated by immature mononuclear cells replacing the normal trilineage hematopoiesis. Flow cytometry showed a population of around 85% blasts, expressing CD45dim, CD19, CD34, CD58 CD38, and TdT. There was also a partial expression of CD10, CD22, and CD79A, along with aberrant expression of CD13 and CD33. CD20 as well as all other myeloid and T-lineage antigens tested were negative. FISH for BCR::ABL rearrangement was positive in 100% of the scored nuclei. Conventional cytogenetics confirmed the presence of Philadelphia chromosome (46,XY,t(9;22)(q34;q11.2)[15]). Polymerase chain reaction (PCR) testing for BCR-ABL1 was unable to detect BCR-ABL1 fusion b2/a2 and b3/a2 (p210) or e1/a2 (p190) transcripts, even upon repetition, suggesting atypical BCR-ABL1 fusion transcript. Next Generation Sequencing reveled RUNX1 mutation. The case was initially labeled as Philadelphia positive B-ALL. After initial cytoreduction, the patient was induced with the pediatric-inspired protocol (inclusive of Cyclophosphamide, Cytarabine, Daunorubicin, Vincristine, Methotrexate, and Pegaspargase) with Dasatinib, which was later switched to Ponatinib. This switch was primarily driven by a concern of persistent peripheral blood blasts on day 14. Ponatinib started at a dose of 45 mg per day, which was then adjusted to 30 mg as the patient achieved complete remission. FISH analysis at the time of remission showed 12% positivity, however, MRD was negative by flow cytometry. This prompted a myeloid segregated FISH analysis confirming the rearrangement to be on the myeloid compartment []. Disease was reclassified as de novo lymphoid BP-CML. The patient was planned and worked up for allogenic stem cell transplant (SCT). Unfortunately, the patient had no match sibling or unrelated donor, and after discussion with the patient, he elected to delay Haplo-SCT. He was started on blinatumomab along with ponatinib, and currently on cycle 5 of blinatumomab/ponatinib without evidence of disease (7 months from the initial diagnosis).
Case 2
A 52-year-old previously healthy male presented to the hospital complaining of fever and shortness of breath (SOB). An annual exam conducted 18 months before presentation along with laboratory investigations proved unremarkable. Initial evaluation revealed bilateral cervical lymphadenopathy and hepatosplenomegaly. CBC showed marked leukocytosis with WBC 371 × 10^3/ul, a hemoglobin level of 7.8 gm/dl, and a platelet count of 20 × 10^3/mm3. Peripheral blood smear displayed hyperleukocytosis with a marked leukoerythroblastic blood picture and around 34% circulating blasts. Bone marrow examination demonstrated hypercellular marrow (cellularity reaching 100%) with total replacement of the normal trilineage hematopoiesis by sheets of immature mononuclear cells. Flow cytometry showed a population of cells with dim to negative CD45 expression and low SSC, accounting for approximately 38% of all cells. The cells were positive for CD19, CD10, CD34 (partial), TdT, CD38 and CD79a. CD20 as well as other myeloid and T-lineage antigens were negative. FISH showed BCR::ABL rearrangement in 96% of the scored nuclei. Conventional cytogenetics confirmed the presence of Philadelphia chromosome (46,XY,t(9;22)(q34;q11.2)[10]). Quantitative PCR for BCR-ABL1 revealed BCR-ABL1 p210 detected at a level of 456% IS. The patient’s presentation with SOB and oxygen requirement indicated a potential leukocytosis, for which he was initially cytoreduced with Hydroxyurea, Cytarabine (2 gm IV flat dose), and Dexamethasone. He developed tumor lysis syndrome (TLS) which was rescued with Rasburicase. As the patient’s condition felt tenuous, following discussion with the patient, he was induced with Cyclophosphamide, Vincristine, and Dexamethasone (mini hyper-CVD) with Ponatinib. Following induction with the regimen, bone marrow examination revealed a cellular marrow (of 40%) with trilineage hematopoiesis displaying 1% blasts by morphology and MRD negativity by flow cytometry. However, molecular studies showed BCR-ABL1 level at 58% IS indicating a persistent cytogenetic abnormality, and suggesting lymphoid blastic phase CML. The patient subsequently continued protocol and achieved remission with MRD negativity using Ponatinib with a sequential combination of blinatumomab and mini-hyper-CVD regimen [].
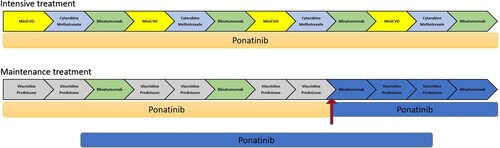
Figure 2. Treatment schema for the second case, in which mini hyper-CVD/cytarabine-methotrexate alternate with blinatumomab during induction and consolidation. The patient is currently proceeding through maintenance (red arrow) with planned cycles highlighted in blue. The patient received 16 intrathecal chemotherapies during his treatment course. We are planning to continue ponatinib (15mg) for a minimum of 5 years. mini-CVD: cyclophosphamide vincristine and dexamethasone.
Discussion
De novo lymphoid BP-CML can be difficult to differentiate from Philadelphia positive B-ALL. Hints to the diagnosis of de novo lymphoid BP-CML are inclusive of the presence of splenomegaly, basophilia or myeloid left-shift maturation, as well as the P210 BCR-ABL transcript.
As a result of recent advancements in the treatment landscape of Philadelphia-positive B-ALL with additional novel therapeutics like blinatumomab, inotuzumab ozogamicin, chimeric antigen receptor T cell, as well as the use of third-generation tyrosine kinase inhibitor Ponatinib, these patients have significantly improved outcomes [Citation6,Citation7]. Whether the same can be applied to de novo lymphoid BP-CML is yet to be explored. The role of stem cell transplant in Philadelphia-positive B-ALL achieving complete molecular tesponse (CMR) at 3 months is questionable at this time, but in the case of de novo lymphoid blastic phase CML the data is limited [Citation8–11]. The rate of achieving 3 months CMR is likely to be lower in de novo lymphoid BP-CML when compared to Philadelphia-positive B-ALL, given the likelihood of a more resistant disease, and disease reservoir in the myeloid compartment. Whether these patients need transplants, the role of CMR, and the optimal time needed to achieve CMR, are questions yet to be answered for this disease cohort.
A recently reported D-ALBA study of a chemotherapy-free frontline induction and consolidation treatment in Ph-positive ALL with dasatinib and blinatumomab was associated with high molecular response and survival with few grades ≥3 adverse events [Citation12]. In another trial of blinatumomab with ponatinib, 24 newly diagnosed patients were treated with an overall response rate (CR/CRi) of 100% and CMR of 91%, with an estimated 2-year event-free survival (EFS) and overall survival (OS) of 95% respectively. In contrast to the D-ALBA trial where almost 40% of patients received SCT, none of the newly diagnosed patients went for transplant in this trial reported by Short et al. [Citation11]. In this study, 3 patients with lymphoid BP-CML were treated as frontline.
In our report, the first case had no presenting basophilia or splenomegaly, while the second had both, which should have been alarming for BP-CML. Given several prior reports, we believe myeloid segregated FISH should be done at the time of diagnosis to identify those patients better. In this report, the first patient was planned for transplant; however, due to the lack of matched donor, SCT was delayed after discussing the option of Haplo-SCT with the patient. The second patient also elected not to go for a transplant and continued to be in MRD negative remission, although with a short follow-up (20 months).
In conclusion, de novo lymphoid blastic phase CML is an entity that needs to be better studied and differentiated from Ph-positive ALL. Retrospective and sub-analysis of prospective trials for this specific cohort are warranted.
Author contributions
MH compiled and summarized the data. MH and MA analyzed the data and wrote the article. ST and AA reviewed and analyzed morphology, flowcytometry and cytogenetic data. MA, MM, IT, AA, HA, SZ, IT and NA treated patients. All authors contributed, reviewed, and edited the manuscript.
Informed consent
Obtained.
Data availability
The authors declare that data supporting the findings of this study are available within the article.
Disclosure statement
MA: Honoraria: Johnson & Johnson, Pfizer, Astellas, Novartis, Amgen, AstraZeneca, AbbVie, Advisory board: Johnson & Johnson, Biologix. Research support: Abbvie, AstraZeneca. Other authors declare no conflict of interest with this manuscript.
References
- Jabbour E, Kantarjian H, O'Brien S, et al. Sudden blastic transformation in patients with chronic myeloid leukemia treated with imatinib mesylate. Blood. Jan 2006;107(2):480–482. doi:10.1182/blood-2005-05-1816.
- Alfayez M, Richard-Carpentier G, Jabbour E, et al. Sudden blastic transformation in treatment-free remission chronic myeloid leukaemia. Br J Haematol. 2019;187(4):543–545.
- Ohanian M, Kantarjian HM, Quintas-Cardama A, et al. The clinical impact of time to response in De Novo accelerated phase chronic myeloid leukemia (CML-AP). Blood. 2012;120(21):72–72. doi:10.1182/blood.V120.21.72.72.
- Hovorkova L, Zaliova M, Venn NC, et al. Monitoring of childhood ALL using. Blood. 2017;129(20):2771–2781. doi:10.1182/blood-2016-11-749978.
- Sloma I, Jiang X, Eaves AC, et al. Insights into the stem cells of chronic myeloid leukemia. Leukemia. Nov 2010;24(11):1823–1833. doi:10.1038/leu.2010.159.
- Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016;122(23):3650–3656. doi:10.1002/cncr.30231.
- Jabbour E, Sasaki K, Short NJ, et al. Long-term follow-up of salvage therapy using a combination of inotuzumab ozogamicin and mini-hyper-CVD with or without blinatumomab in relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Cancer. 2021;127(12):2025–2038. doi:10.1002/cncr.33469.
- Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507. doi:10.1182/blood-2016-03-707562.
- Short NJ, Kantarjian H, Jabbour E. Optimizing the treatment of acute lymphoblastic leukemia in younger and older adults: new drugs and evolving paradigms. Leukemia. 11 2021;35(11):3044–3058. doi:10.1038/s41375-021-01277-3.
- Sasaki K, Kantarjian HM, Short NJ, et al. Prognostic factors for progression in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia in complete molecular response within 3 months of therapy with tyrosine kinase inhibitors. Cancer. 2021;127(15):2648–2656. doi:10.1002/cncr.33529.
- Short NJ, Kantarjian H, Konopleva M, et al. Updated results of a phase II study of ponatinib and blinatumomab for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2021;138(Supplement 1):2298–2298. doi:10.1182/blood-2021-153795.
- Foà R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613–1623. doi:10.1056/NEJMoa2016272.