?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Arsenic trioxide (ATO) might be effective for myelodysplastic syndrome (MDS) by apoptosis induction and demethylation. But ATO has not been widely recommended for small sample and conflicting conclusion of existing trials. This review aimed to systematically evaluate the efficacy of regimens containing ATO for the MDS and explore optimal combination.
Method
Randomized clinical trials (RCTs) about ATO regimens were retrieved from China National Knowledge Infrastructure, Embase and PubMed. With odds ratio (OR) as the effect size, network meta-analysis (NMA) and component network meta-analysis (CNMA) were conducted by R and ‘netmeta’ package, after study selection, quality assessment and data extraction.
Result
Thirty-night RCTs were included with a total of 2125 patients, including 1235 treated by ATO containing regimen. With support therapy alone as reference, no inconsistency and heterogeneity were observed. Although NMA did not demonstrate better efficacy of ATO alone, the result of CNMA indicated that ATO was effective in the improvement of overall remission (ORR) [OR = 2.09(1.61, 2.71)] and complete remission (CR) [OR = 1.66(1.25, 2.21)]. Five ATO-containing regimens reported could effectively improve ORR, some of them benefit in CR or hematological improvement (HI) as well. ATO + Traditional Chinese Medicine (TCM), ATO + Thalidomide (T)+TCM, ATO + Chemotherapy (Chem)+T + TCM were regarded as the optimal combination, which improved both ORR, CR and HI in theory. ATO did not increase the risk of common adverse events compared to supportive therapy [(OR = 0.90(0.67, 1.21)].
Conclusion
ATO may be an effective and well-tolerant option for patients with myelodysplastic syndrome.
1. Introduction
Myelodysplastic syndrome (MDS) is a set of acquired, heterogeneous and clonal disorders, which basic pathology is clonal dysplasia of hematopoietic stem/progenitor cells leading to ineffective hematopoiesis. MDS is characterized by a normal (sometimes increased) count but morphological abnormalities and dysplasia of each hematopoietic cells lineage in the bone marrow, reduction of peripheral blood cells count and a high risk of progression to acute myeloid leukemia.
Current treatment options for MDS are limited, and no extraordinarily effective treatments are available. For relatively low-risk MDS patients, the Chinese or National Comprehensive Cancer Network (NCCN) guideline of diagnosis and treatment recommends blood transfusion, colony stimulating factors, androgens, immunomodulators or immunosuppressor as the primary treatment. For low-risk patients failure to the above treatments, or patient with relatively high-risk disease or higher percentage of blast cells (5–19% in bone marrow), clinical trial, hypomethylating agents (HMAs), low-intensity acute myeloid leukemia (AML)-like chemotherapy regimens such as HAG (homoharringtonine, cytarabine and granulocyte-colony stimulating factor), or even hematopoietic stem cell transplantation is recommended to achieve disease remission.
ATO produced trilineage responses in MDS by several mechanisms of action. These mechanisms included induction of cell cycle arrest, apoptosis and autophagy, generation of reactive oxygen species (ROS), release of cytochrome c and activation of caspases, induction of differentiation and anti-angiogenesis [Citation1]. Nowadays, the induction of apoptosis and demethylation were regarded as two major mechanisms of ATO for MDS treatment. To date, many clinical studies explore the efficacy of ATO for MDS, while most of them were low quality for small study sample. For example, in 2006, a phase II, single-arm, multicentered cohort study conducted on 115 MDS patients showed that, with the use of single-agent ATO, no difference in hematological improvement (HI) rate was observed between the relatively low-risk and relatively high-risk groups (26% vs 17%, P > 0.05) [Citation2]. However, another single-arm multicentered study with a small sample of 51 patients demonstrated a significantly higher HI rate in the relatively low-risk than in the relatively high-risk group (39% vs 9%, P = 0.016) [Citation3]. The small sample sizes of these studies and contradictory conclusion as above were common, which may influence the confidence of clinicians who hesitate the application of ATO. A large cross-sectional survey of 7385 MDS patients in the United States in 2012 showed that only 1.6% of MDS patients were treated with ATO [Citation4]. In 2015, Mayo Clinic counted that less than 4% of 1000 MDS patients were treated with ATO [Citation5]. While in China, there are no relevant statistics. ATO has not been unanimously recommended by national and international guidelines. It is urgent to answer the question that whether ATO is effective and what ATO could benefit. Therefore, in this study, we conducted a systematic review and component network meta-analysis (CNMA) of the published randomized controlled trails (RCTs) of ATO-containing regimens for MDS patients, to provide a new perspective for the application of ATO. This review is the first NMA and CNMA to systematically summarize all recent RCTs results and quantificationally evaluate the precise efficacy of ATO, which offers more information for the utilization of ATO. This protocol was previously registered on PROSPERO (CRD42022298491) by primary authors (Huang Xiaohua and Yang Hongyong).
2. Materials and methods
2.1. Inclusion and exclusion criteria
2.1.1. Inclusion criteria
Literature was included if it met the following four criteria: (1) Study design: Published clinical RCT. (2) Subjects: Patients diagnosed with previously treated or untreated MDS, without limitation on French-American-British (FAB) (except for chronic myelomonocytic leukemia and refractory anemia with excess blasts in transformation) or World Health Organization (WHO) classification. (3) Interventions: The RCT focusing on pairwise comparison of the regimen containing ATO with those not containing was eligible. The regimens could either be ATO alone or in combination with other drugs or blood transfusions, regardless of drug manufacturer and specification. Furthermore, it was also included if the literature was about pairwise comparison of two different ATO combination regimens. (4) Outcome measure: At least one of following primary outcomes should be reported: the quantity of patients achieving complete remission (CR), partial remission (PR), HI. Secondary outcome was adverse events (AEs).
2.1.2. Exclusion criteria
We excluded study if it met one of the following criteria: (1) Cohort study, non-randomized concurrent controlled trial, case report, review, comment, experimental study only. (2) The subjects were diagnosed with myelodysplastic/myeloproliferative neoplasms (MDS/MPN). (3) It was unavailable to acquire both primary and secondary outcome result of each group.
2.2. Literature retrieval
Two researchers (Huang Xiaohua and Yang Hongyong) designed the retrieve protocol. The search terms were as follows: ‘myelodysplastic syndrome’, ‘myelodysplastic’, ‘myelodysplasia’, ‘MDS’, ‘RAEB’, ‘refractory anemia’, ‘arsenic trioxide’, ‘ATO’, ‘As2O3’. Another two researchers (Liu Ruixuan and Liu Yuan) conducted a systematic retrieve in China National Knowledge Infrastructure (CNKI), WangFang database (WangFang), China Science and Technology Journal Database (VIP), Chinese BioMedical Literature Database (CBM), Embase, PubMed, Springerlink, Wiley online library, Cochrane library, Web of Science from inception to March 9, 2023. With PubMed as the example, the search query was (‘2000/01/01’ [Date – Publication] : ‘2023/03/09’ [Date – Publication]) AND (clinical trial [Publication Type]) AND (((‘Myelodysplastic Syndromes’ [Mesh] OR ((((myelodysplastic[Title] OR myelodysplasia[Title]) OR MDS[Title]) OR RAEB[Title]) OR refractory anemia[Title])) AND (‘Arsenic Trioxide’ [Mesh] OR ((ATO[Title/Abstract] OR arsenic trioxide[Title/Abstract]) OR As2O3[Title/Abstract])))). If there were two or more literature being published in different date but reporting a same trail, the latest versions were accepted. For duplicate literature, the one with complete data was enrolled. We managed the literature by software EndNote 20.0.
2.3. Literature selection and data extraction
Cross-checking was performed independently by two researchers (Liu Ruixuan and Liu Yuan) after screening the literature in strict accordance with inclusion and exclusion criteria, with disagreements resolved by discussion or adjudication by a third researcher (Yang Hongyong). A table previously designed (Huang Xiaohua) by software EpiData Manager 4.4.2.0 was utilized for data extraction. Datum was extracted by two researchers (Liu Ruixuan and Liu Yuan) independently with assistance of software EpiData Entry Client 4.4.2.0, and datum cross-check was also performed before quality assessment and statistical analysis. The extracted information included: (1) the first author and the publication year, (2) the sample size, demographic characteristics of treatment and control group, (3) the regimen of each group and the efficacy stratification, (4) the quantity of patients with CR, PR, HI and AEs in each group.
2.4. Quality assessment and statistical method
The quality of the eligible literature was assessed using the risk of bias assessment tool version 2 recommended by the Cochrane Handbook for Systematic Review of Interventions [Citation6]. The odds ratio (OR) and its 95% confidence interval (95%CI) were used as the effect size to calculate relative efficacy of each treatment for CR, overall remission (ORR = PR + CR), HI and AEs. We performed both standard NMA and CNMA following the frequentist approaches [Citation7–9]. Multi-arm trials were included in the network meta-analysis. The correlation of treatment effects on different comparisons was accounted for by re-weighting all comparisons of each multi-arm studies.
First, stratified by different outcomes, network graphs were plotted to clarify the relationship between interventions and determine reference group.
Second, in NMA, value and
were reported to quantify the inconsistency and heterogeneity, subsequently with test of them and reporting the Q, P-value. If
<50%, insignificant heterogeneity would accept, and common effect model NMA would be conducted. Otherwise, the random effect model NMA would be utilized. Then we synthesized evidence by integrating direct and indirect estimation for each comparison into a single summary effect [OR(95%CI)]. The NMA league tables of each primary outcome were shown, and relative rank would be estimated by surface under the cumulative ranking curve (SUCRA).
Third, in CNMA, the additive model without any interactive item was the initial CNMA model, and the difference between CNMA and NMA would be estimated by Cochran Q test. If the initial model was significantly different from the NMA model, the addition of interactive item (ATO interacting with one or more other drugs) to the initial CNMA model would be considered, until the insignificant heterogeneity observed. Then overall effect of each component was estimated, subsequently with the calculation of arbitrary complex intervention's effect, which was ATO in combination with one or more other drugs, in order to explore the optimal ATO containing regimens in theory. The CNMA league tables of complex intervention were shown.
Finally, the risk of AEs was estimated by CNMA. Subgroup analysis based on remission criteria and sensitivity test of primary outcome by estimating the effect after excluding the high-risk trails were also performed.
All the above NMA and CNMA result would be visually demonstrated by forest plots. Contour-enhanced funnel plot was drawn and publication bias would be estimated by examining the asymmetry using the Harbord method.
All the above statistical analyses were performed using the R (version 4.2.1) and ‘netmeta’ package (version 2.5-0), and P < 0.05 was considered a statistically significant difference.
3. Result
3.1. Literature retrieval result
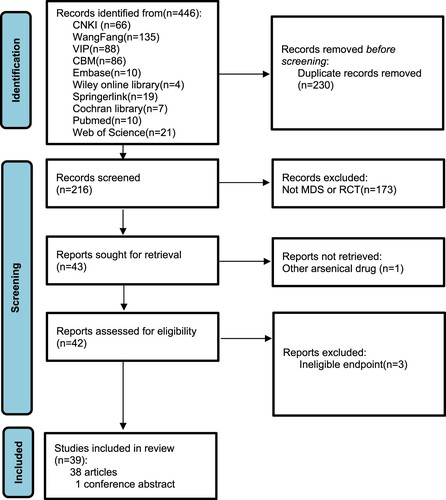
A total of 446 literature were identified from the initial retrieval. After removing 230 duplicate records, 216 potentially eligible literature were screened. Full text of 173 records were not further retrieved after reading the title and abstract for their ineligible diagnosis or study design. One literature was excluded because the arsenical agent mentioned in the abstract actually was not ATO, and another three literature were also excluded for incomplete message of outcome. Finally, 39 randomized controlled trials were included (38 articles and 1 conference abstract). The process of literature screening is shown in .
3.2. Basic characteristic of eligible trials
Most of the including RCTs were public in Chinese, except an abstract [Citation10] and an article [Citation11], with a total of 2125 participants, including 1235 patients treated by ATO containing regimen. ATO and another 5 ATO combination regimens were reported: ATO + Chem (Chemotherapy), ATO + HMA, ATO + T (Thalidomide), ATO + TCM (Traditional Chinese Medicine) and ATO + T + TCM. More details are shown in and .
Table 1. Patient characteristics and trail quality assessment.
Table 2. Treatment and efficacy.
3.3. Quality assessment
There were 14 RCTs [Citation12–14,Citation18–21,Citation24,Citation31,Citation34,Citation43,Citation46–48] described the method of randomization, but did not describe the allocation concealment method in detail. One of the RCTs explicitly stated that it was an open-label trial [Citation23], and one was single masking trail [Citation10]. The risk of bias assessment indicated that most included RCTs’ quality was not satisfied. Details were presented in .
3.4. Result of network meta-analysis
3.4.1. Network graph
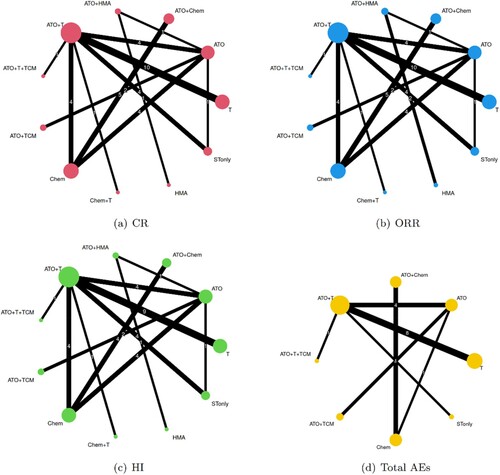
Six ATO containing regimens as mentioned above, and five another treatment constructed the intervention network. Categorized by different outcomes, the networks of primary and secondary endpoint were as in , and network of total AEs was sparser than networks of primary outcome, which meant the considerable missing data. However, supportive therapy only (STonly) was common in all outcomes, and therefore, STonly was set as the reference group for both NMA and CNMA.
3.4.2. Test of inconsistency and heterogeneity
All ,
and Q values were small enough to support the assumption of consistency, and no significant heterogeneity was observed. Therefore, the common effect model was considered.
3.4.3. The efficacy and relative rank of the treatment
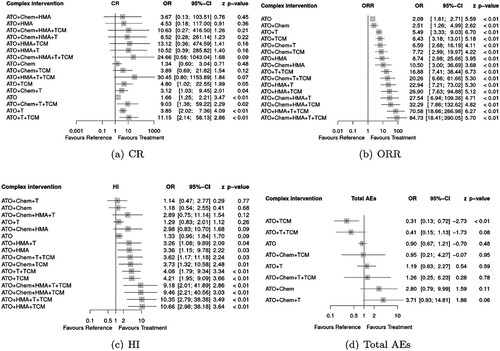
The result of NMA suggests that compared with STonly, ATO alone was not associated with significant improvement of CR [OR = 0.69(0.20, 2.34)], ORR [OR = 1.89(0.74, 4.85)] and HI [OR = 1.20(0.60, 2.42)] rates. Nevertheless, except for ATO + Chem, another four ATO combination regimens were effective and beneficial in ORR [ATO + HMA: OR = 7.92(1.94, 32.31), ATO + T: OR = 5.32(2.47, 11.50), ATO + T + TCM: OR = 9.76(2.24, 42.56), ATO + TCM: OR = 7.07(2.10, 23.83)], and ATO + TCM also associated with improving HI [OR = 4.87(1.63, 14.52)] (). Compared with MDS patients treated by regimens without ATO, the addition of ATO meant advanced efficacy in some circumstance [for ORR, ATO + Chem vs Chem: OR = 2.04 (1.30, 3.20), ATO + HMA vs HMA: OR = 3.08 (1.10, 8.66), ATO + T vs T: OR = 2.05 (1.44, 2.91), for CR, ATO + T vs T: OR = 1.90 (1.28, 2.83)] (see Supplemental tables S1–S3). Among all treatments reported in the including RCTs, it was predicted that ATO + T, ATO + T + TCM, ATO + TCM were the optimal combination for enhancement of CR, ORR, HI respectively, according to the result of SUCRA (). We surprisingly found the poor efficacy of ATO + Chem and Chem, leading to the concerning and reconsideration of chemotherapy (some low intensive AML regimens) application.
Figure 3. Forest plot of treatment efficacy: ATO: Arsenic trioxide, Chem: Chemotherapy, HMA: Hypomethylating agent, T: Thalidomide, TCM: Traditional Chinese Medicine, STonly: Support therapy only. CR: complete remission, PR: partial remission, HI: hematological improvement, AEs: adverse events. NMA: network meta-analysis, CNMA: component network meta-analysis.

Table 3. The relative rank of different treatment (SUCRA)
3.5. Result of component network meta-analysis
3.5.1. Test of inconsistency and heterogeneity
The assessment of CNMA inconsistency should further consider the difference between NMA and CNMA models. Regardless of any outcomes, the Cochrane Q values of the standard NMA model and additive CNMA model were close (P > 0.05), suggesting insignificant difference of them, which support the hypothesis of consistency in CNMA as well. Therefore, the interactive CNMA model was not necessarily considered. No significant heterogeneity was observed for of all outcomes both less than 50%, and the common effects model was applied.
3.5.2. Effect of components
Based on the assumption of CNMA, the effect of a treatment consisting of several components is the additive sum of treatment’s component effect. Each component’s effect can be estimated even though without certain trail about one component. This is CNMA unique advantage. In this review, the effect of ATO, HMA, Chemotherapy, thalidomide and TCM was offered respectively (). It suggested that ATO significantly improved ORR rate [OR = 2.09 (1.61, 2.71)], CR rate [OR = 1.66 (1.25, 2.21)] compared with supportive therapy. Compared with other component, HMA, thalidomide and TCM demonstrated the best effect in ORR [OR = 4.18 (1.47, 11.89)], CR [OR = 2.32 (1.23, 4.37)] and HI [OR = 3.17 (1.58, 6.37)] respectively. All components were not relative to increasing risk of total AEs, while AEs risk of HMA failed to estimate because of missing data.
Table 4. The model characteristic of different endpoint and the effect of component
3.5.3. The efficacy of all ATO regimens
CNMA demonstrated a different result from NMA. Compared with STonly, not only ATO alone but also 5 ATO-containing regimens could effectively improve ORR. More patients who treated with ATO + T [OR = 3.85 (2.02, 7.36)], ATO + TCM [OR = 4.80 (1.02, 22.55)], ATO + T + TCM [OR = 11.15(2.14, 58.13)] achieved CR, and HI was more common in ATO + HMA [OR = 3.36(1.15, 9.78)], ATO + T + TCM [OR = 4.08(1.79, 9.34)], ATO + TCM [OR = 4.21(1.95, 9.09)] group than STonly. The narrower confidence interval was observed in CNMA, which represented a preciser estimation so that the conclusion of CNMA was different from NMA (). Based on the assumption of CNMA, the effect of arbitrary combination of components could be estimated. Referred to , all ATO containing regimens were effective. Although the total effect is the sum of components’ effect, the more components did not represent the better efficacy. For example, a 5 drug combination: ATO + Chem + HMA + T + TCM had a higher rate of CR, but it was not significantly different from ST only [OR = 24.66 (0.58, 1043.04)], while the ATO + T + Chem + TCM could effectively increase the CR rate [OR = 9.03 (1.38, 59.22)]. Therefore, we categorized all possible ATO containing regimens into four groups:
Improve ORR, CR and HI, which regarded as the optimal combination:, ATO + TCM, ATO + T + TCM, ATO + Chem + T + TCM
Improve ORR and CR: ATO, ATO + T, ATO + Chem + T
Improve ORR and HI: all regimens containing ATO and TCM except groups 1–2 and ATO + HMA, ATO + HMA + T
Only improve ORR: the rest combination except for groups 1–3.
Figure 4. Forest plot for CNMA: Supportive therapy was treated as reference. ATO: Arsenic trioxide, Chem: Chemotherapy, HMA: Hypomethylating agent, T: Thalidomide, TCM: Traditional Chinese Medicine. CR: complete remission, PR: partial remission, HI: hematological improvement, AEs: adverse events.
Pairwise comparison referred to supplemental material (see supplemental tables S4–S6).
3.6. Common adverse events
The incidence of adverse effects reported in the included research was shown in supplemental figure S1. In general, single-agent ATO did not increase total adverse events compared to supportive therapy [(OR = 0.90(0.67, 1.21)], and ATO + TCM significantly reduced the risk of total AEs [OR = 0.31(0.13, 0.72)]. Besides, marked reduction of infection was observed in ATO + HMA [OR = 0.14(0.04, 0.51)] group, which could also be regarded as one of the clinical benefits of ATO plus HMA for patients with MDS. We tried to estimate and predict the total AEs risk of all possible combination regimens with ATO by CNMA as well (). Because of missing data, only eight complex interventions’ effect size could be calculated. It was suggested that all possible combination regimens containing ATO did not relative to higher risk of total AEs.
3.7. Subgroup analysis, publication bias and sensitive analysis
Considering about similar efficacy of chemotherapy to STonly and absence of STonly in trials using remission criteria ①, the inactive reference switched to chemotherapy in subgroup analysis. It indicated that patients benefited from ATO in ORR in all remission criteria subgroup, but CR rate was not significantly improved according to criteria ③, which conflicted to previous analysis (see Supplemental Table S7). While 10 high risk studies were excluded, the main result and conclusion did not change. ATO still was better than ST only in the enhancement of ORR [OR = 2.18 (1.63, 2.92)] and CR [OR = 1.88 (1.34, 2.64)] rates. Other combination effect reported in inclusion RCTs did not significantly change. That meant a robust conclusion that ATO and its combination with other drug were effective therapy for patients with MDS (see Supplemental Figure S2). Publication bias did not exist because the funnel plot asymmetry wasn’t observed (t = 0.19, P = 0.852) (see Supplemental Figure S3).
4. Discussion
4.1. Key findings and implication
According to the result of CNMA, ATO was provided an effective drug for MDS, and some combination regimens containing ATO present a more encourage remission rate than those not containing. ATO also did not increase the risk of common AEs. Therefore, it is believed that ATO can improve efficacy and is a well-tolerant option for patients with MDS.
Another notable finding was poor efficacy of chemotherapy. The chemotherapy mentioned in our study was low intensity regimens, such as HAG, CAG or low-dose subcutaneous cytarabine. Those regimens are still mentioned in some MDS guidelines, but it has been regarded as unfavorable option in most countries for its limit efficacy. A phase III trial in 2007 noted that the remission rate of low-dose cytarabine group was only 18% [Citation53]. The meta-analysis indicated that the CR rate of HAG and CAG for patients with MDS was 45.0% and 45.7% respectively, which was both significantly inferior to AML [Citation54,Citation55]. It was perhaps because the long-time hematopoiesis suppression result from low intensity chemotherapy counteracts the benefit, due to MDS patients characterized refractory pancytopenia and myelodysplasia, although low intensity chemotherapy can reduce bone marrow blast somehow.
We noted that three versions of remission criteria were involved. Criteria ① was a widely-used consensus in China. In 2001, criteria ② was proposed by international work group, which was the first generally accepted standard in worldwide, and revised version (criteria ③) was published in 2006. The blast threshold in both bone marrow and peripheral blood of CR and PR of three criteria was same, and all criteria required peripheral blood recovery to normal level. However, there was a difference in remission duration. Patients who acquired CR and PR responded at least 6 months, 2 months and 4 weeks respectively in criteria ①②③. Threshold of HI of erythroid response (HI-E) was 30, 20 and 15 g/L hemoglobin increase for at least 8 weeks respectively in criteria ①②③. The definition of HI of neutrophil (HI-N) and HI of platelet (HI-P) was similar. In general, early version criteria was stricter than later one, so patients who achieved remission defined by early criteria were accordance with later criteria as well, and compared with former criteria, more patients might be labelled ‘effective’ referred to later, undemanding standard. That might be reluctant actually because of poor response and prognosis of MDS. Too strict or too undemanding criteria might result in weakening discrimination ability, which might impede the new agent research for criteria. Fortunately, ATO presented robust efficacy in ORR in our review, regardless of remission criteria, so the efficacy of ATO might be trustful.
4.2. Potential pharmacological mechanisms
It’s believed that cells’ apoptosis induction and methylation regulation play important roles in MDS treated by ATO. For inducing apoptosis, an experimental research demonstrated that ATO inhibited the proliferation and induced apoptosis of high risk MDS cell line SKM-1 and MUTZ-1, mainly by inducing ROS production in tumor cell, which leads to induction of DNA, protein damages, and by decreasing B-cell leukemia/lymphoma-2 (BCL-2) expressions [Citation56,Citation57]. The mechanism mentioned above will be strengthened when ATO in combination with decitabine, therefore, some researchers developed a bone-targeting nanoparticle to co-deliver decitabine and ATO in order to enhance the efficacy and circulation time [Citation58]. An ex-vivo study of 12 MDS patients indicated that ATO could up-regulate some pro-apoptotic genes, such as Harakiri (HRK), BCL2 Antagonist/Killer 1 (BAK1), Caspase-5, BCL2 Associated Agonist Of Cell Death (BAD), TNF Receptor Superfamily Member 1A (TNFRSF1A), BCL2 like 14 (BCL2L14) and down-regulate caspase recruitment domain 18 (CARD18). For details, 69% of pro-apoptotic genes down-regulation was observed in patients with low risk disease, while 91% of pro-apoptotic genes up-regulation was seen in patients with high risk disease [Citation59]. For methylation regulation, one study found that ATO resulted in positive effects by decreasing methylation of the tumor suppressor Id4 in MDS patients [Citation60], but more experimental researches are required to confirm the detailed mechanism of ATO in demethylation. On the other hand, aberrant hypomethylation also was regarded as an important role in MDS. An experimental research showed that almost 50.88% genes were aberrantly hypomethylated in MDS patients compared with healthy individuals. Therefore, the improvement of hypomethylation was a potential target of MDS. Encourage efficacy of ATO in combination with TCM was also observed in our study. ATO + TCM with or without another drug was effective and had a relatively superior rank to other regimens (SUCRA). Some study indicated that TCM with arsenical drug could improve aberrant hypomethylation by increasing DNA methyltransferase 1 (DNMT1) expression [Citation61]. Therefore, ATO is more likely a methylation regulator, which provides an innovative pathway of MDS treatment. The above studies provide molecular biological evidence for the treatment of MDS with ATO.
Our study proves the efficacy of ATO for MDS, however, ATO is administrated for injection only that is inconvenient for application somehow. The active component of ATO is and some oral arsenic drug has been widely utilized, such as Realgar (As2S2). The compound of Realgar in combination with indigo has been approved for treating APL in China. It’s believed that Realgar acts as a controlled-release formulation of the same reactive centers that are generated by ATO. A recent meta-analysis suggested that oral Realgar compound was as effective as ATO injection for APL. For the lower bioavailability of Realgar, its nanoparticles with higher bioavailability in theory is under investigation [Citation62].
4.3. Advancement and limitations of study
Our study indicated that although insignificant heterogeneity was observed between CNMA and NMA in general, the effectiveness of ATO proved by CNMA conflicted to ineffective estimation from NMA. The contradiction above was associated with the difference of effect size calculation method of CNMA and NMA. When calculating the ATO effect size, standard NMA acquired the summary estimation through synthesis of direct and indirect pairwise comparison of ATO, while CNMA would further utilize the statistical information provided by all comparison containing ATO. Therefore, a preciser estimation and more shrinking confidence interval was offered by CNMA, and the conclusion CNMA was believed more reliable [Citation7].
Thalidomide is a kind of immunomodulator, which was widely used for MDS before new generation immunomodulator: lenalidomide. Although our study had proven effectiveness of thalidomide and its combination with ATO, it was believed that thalidomide is more inferior to lenalidomide, which has been approved for MDS with 5q- at present. It was pitiful that no RCT of ATO and lenalidomide direct comparison was recorded, so ATO and lenalidomide combinative effect could not be estimated in our study. We assumed that the synergistic effectiveness of ATO and immunomodulator might exist according to our CNMA result, hence ATO in combination with lenalidomide may be a hopeful regimen in future.
The quality of most RCTs included in our review was concerned, for unclear description of the protocol. Therefore, more high-quality trails were still required. Previous studies have shown a positive correlation between blood concentration of arsenic and efficacy. We attempted to investigate optimal as well as safe dosage, but it was limited because the drug doses used in most included studies was 10 mg. Similarly, we did not conduct the risk categories subgroup analysis because of lack of data.
5. Conclusion
ATO may be effective and with low toxicity for MDS, and some combination of ATO with other drugs remarkably improves the effectiveness in theory. Hence, ATO may deserve more consideration from hematological clinicians. However, the benefit of ATO still need more high-quality study to confirm and clarify.
Author contributions
Xiaohua Huang and Hongyong Yang designed the study. Yuan Liu and Ruixuan Liu retrieved the database, select the literature and input the data. Xiaohua Huang and Xiaoqiu Zou performed the statistical analysis and wrote the manuscript. Hongyong Yang checked the result. All authors approved the final version of manuscript.
Supplemental Material
Download MS Word (4.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bokorová R, Fuchs O, Myslivcova D, et al. Arsenic trioxide upregulates cereblon mRNA expression and potentiates sensitivity of SKM-1 and MDS-L cells to lenalidomide. Blood. 2019;134(Supplement_1):5397. doi:10.1182/blood-2019-126443
- Vey N, Bosly A, Guerci A, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006 Jun;24(16):2465–2471. doi:10.1200/JCO.2005.03.9503
- Schiller GJ, Slack J, Hainsworth JD, et al. Phase II multicenter study of arsenic trioxide in patients with myelodysplastic syndromes. J Clin Oncol. 2006 Jun;24(16):2456–2464. doi:10.1200/JCO.2005.03.7903
- Zandberg DP, Huang TY, Ke X, et al. Treatment and outcomes for chronic myelomonocytic leukemia compared to myelodysplastic syndromes in older adults. Haematologica . 2013 Apr;98(4):584–590. doi:10.3324/haematol.2012.062547
- Gangat N, Patnaik MM, Begna K, et al. Primary myelodysplastic syndromes: The Mayo Clinic experience With 1000 patients. Mayo Clin Proc. 2015 Dec;90(12):1623–1638. doi:10.1016/j.mayocp.2015.08.022
- Sterne JAC, Savovic J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019 Aug;366:l4898. doi:10.1136/bmj.l4898
- Rucker G, Petropoulou M, Schwarzer G. Network meta-analysis of multicomponent interventions. Biom J. 2020 May;62(3):808–821. doi:10.1002/bimj.201800167
- Shim SR, Kim SJ, Lee J, et al. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. doi:10.4178/epih.e2019013
- Rucker G, Schmitz S, Schwarzer G. Component network meta-analysis compared to a matching method in a disconnected network: A case study. Biom J. 2021;63(2):447–461. doi:10.1002/bimj.201900339
- Kropf PL, Shameem R, Pancari PA, et al. Improved survival for MDS/CMML patients treated with the combination of decitabine (DAC) and arsenic trioxide (ATO) in a phase II adaptive three arm randomization study: DAC alone Vs. DAC +/-Carboplatin or ATO. Blood [Conference Abstract]. 2016;128(22):3170. doi:10.1182/blood.V128.22.3170.3170
- Wei W, Zhou F, Zhang Y, et al. A combination of thalidomide and arsenic trioxide is effective and well tolerated in patients with myelodysplastic syndromes. Leukemia Res. 2012;36(6):715–719. doi:10.1016/j.leukres.2011.12.023
- Bu YW. The clinical research of arsenic trioxide combined with thalidomide in the treatment of patients with intermediate-1 MDS. J Jining Med Univ. 2016;39(04):245–247.
- Zeng YJ, Liang ZY, Peng GR, et al. Turmeric enhanced the efficacy of arsenic trioxide combining with thalidomide for patients with myelodysplastic syndrome. Jiangxi J Trad Chinese Med. 2021;52(11):31–33.
- Zeng ZY. Efficacy and safety of Arsenious Acid and Thalidomide in the treatment of myelodysplastic syndrome. Contemp Med. 2018;24(11):76–78.
- Chen QW, Zhang L, Lu JH, et al. The clinical observation of arsenic trioxide combined with Jianpibushenjiedu Fomula for elderly patients with MDS-RAEB. Liaon J Trad Chinese Med. 2011;38(9):1841–1843.
- Dang HB. The analysis of thalidomide combinded with arsenic trioxide for 38 patients with myelodysplastic syndrome. Chongqing Med. 2014;43(14):1791–1793.
- Deng LL. Efficacy analysis of arsenic trioxide combining with thalidomide for patients with intermediate risk-1 myelodysplastic syndrome. Diet Health. 2018;5(39):78.
- Guo SL. Applicating arsenic trioxide for the treatment of myelodysplastic syndrome-refractory anemia with excess blasts. China Pract Med. 2012;7(24):195–196.
- Guo TJ, Gong JM, Wu BJ, et al. Clinical observation on arsenic trioxide combined with cytarabine in the treatment of 38 patients with myelodysplastic syndrome. China Med Herald. 2012;9(9):80–1,4.
- Huang PC, Si YH, Mo SY. Effect of arsenic trioxide combined with thalidomide in the treatment of myelodysplastic syndrome. Guide China Med. 2020;18(14):77–78.
- Li HY. Comparative analysis of arsenic trioxide and HAG for the treatment of myelodysplastic syndrome. J North Pharm. 2017;14(02):174–175.
- Li HM, Liu LN, Mei ZY, et al. Analysis of effect of ultra-low dose of decitabine and arsenic acid in treatment of myelodysplastic syndrome. J Med Forum. 2018;39(1):1–26.
- Li SM. Clinical trial of arsenic trioxide in the treatment of patients with middle and high risk myelodysplastic syndrome. Chin J Clin Pharmacol. 2017;33(23):2364–2367.
- Li YQ, Liang ZW, Luo GZ. Compound Zaofan pill combined with low dose cytarabine, arsenic trioxide treatment syndrome randomized controlled trial of abnormal comprehensive geriatric bone marrow hyperplasia. J Pract Tradition Chin Intern. 2013;27(06):102–104.
- Liang YN LISL. Efficacy analysis of arsenic trioxide plus thalidomide for myelodysplastic syndrome. J Med Theor Pract. 2013;26(17):2312–2313.
- Liang YN, Qin YL. Analysis of the clinical effect of arsenious acid combine with thalidomide for treating myelodysplastic syndrome. J Yanan Univers(Med Sci). 2015;13(2):32–34.
- Liu H. The clinical value of As2O3+ Ara-c in treating the patients with MDS. J Hunan Norm Univer(Med Sci). 2016;13(6):20–23.
- Liu WX, He T, Wang T, et al. The clinical efficacy observation of patients with myelodysplastic syndrome treated by arsenic trioxide combining with thalidomide. China Health Care Nutrition. 2013;10:589–590.
- Luo ZY. Combination treatment of arsenic trioxide and thalidomide for patients with myelodysplastic syndrome: efficacy and adverse events. Contempor Med. 2017;23(03):33–34.
- Su JM, Zhang C, Rong TJ, et al. Combination treatment of arsenic trioxide and thalidomide for patients with myelodysplastic syndrome. Shanxi Med J. 2017;46(05):559–562.
- Sun F, Gao MJ, Yong YL. The 30 instances clinical observation of “Bushen Jiedu Recipe” and arsenious acid in treating myelodysplastic syndrome. Liaon J Tradition Chin Med. 2014;41(8):1656–1658.
- Tian Y. Clinical study on myelodysplastic syndrome. China Clin Practl Med. 2010;04(5):32–33.
- Wang FY. Combination of thalidomide, calcitriol and andarsenic trioxide in the treatment of myelodysplastic syndrome. Hainan Med J. 2009;20(11):42–44.
- Wang GJ, Tian Y. Effect of arsenious acid combined with thalidomide on patients with myelodysplastic syndromes. J Med Forum. 2018;39(7):55–57.
- Wang GY, Ma HH, Zhao JS. Clinical value study about the combining application of arsenic trioxide and thalidomide for 85 cases with myelodysplastic syndrome. Cardiovas Diseas J Integrat Trad Chin Wester Med(Electron). 2019;7(26):187–188.
- Wang JX, Zhang QR. The regulative effect of arsenic trioxide plus thalidomide for myelodysplastic syndrome. Modern J Integrat Tradition Chin Western Med . 2013;22(24):2679–2681.
- Wu CN, Song GC, Zhang J. Efficacy of arsenic trioxide in the treatment of elderly patients with high risk of bone marrow hyperplasia syndrome. Sichuan Med J. 2017;38(01):66–68.
- Yang HP, Zhao XQ, Hu M. Arsenic trioxide associated with large-dose ascorbic acid, thalidomide in treatment of 20 patients with myelodysplastic syndrome. J Hen Univ Sci Techn (Med Sci). 2011;29(04):280–282.
- Yu SM. An explorative research of patients with myelodysplastic syndrome treated by thalidomide, arsenic trioxide and Rocaltrol. Guide China Med. 2017;15(08):68–69.
- Zhang J, Xu JG. Comparison of between arsenic trioxide and HAG regimen in the treatment of myelodysplastic syndrome. Sichuan Med J. 2012;33(10):1793–1795.
- Zhang QR, Chen LS, Wang JX, et al. Effects of arsenic trioxide plus thalidomide on immune function in patients with myelodysplastic syndrome. Clinic Med China. 2013;29(12):1243–1246.
- Zhang QR, Chen LS, Zhang GH, et al. The efficacy observation of the regimen majoritve with arsenic trioxide for elderly high risk patients with myelodysplastic syndrome. Chin J Clinic (Electron Edit). 2011;5(16):4895–4896.
- Zhao XQ, Wu YL, Yang HP. Clinical analysis of thalidomide, arsenic trioxide and calcitriol treating myelodysplastic syndrome]. Chinese J. Trauma Disabil Med. 2016;24(18):17–18.
- Zhao XQ, Yang HP. Exploration and analysis of the therapeutic value of arsenic trioxide combined with thalidomide for patients with myelodysplastic syndrome. J ShanDong First Med Univers ShanDong Academ Med Sci. 2017;38(04):414–415.
- Zhong JW, Luo C, Zhao CF, et al. Effect observation of thalidomide combined with arsenious acid in the treatment of myelodysplastic syndrome. Chinese J Pharmacoepidemiol. 2017;26(4):234–236.
- Zhu CX. Investigate the effect of arsenic trioxide combined with thalidomide in the treatment of myelodysplastic syndrome. China Foreig Med Treatm. 2020;39(13):99–101.
- Zuo AL. A combination of arsenic trioxide and CAG for patients with myelodysplastic syndrome. Tianjin Med J. 2010;38(11):1007–1008.
- Zhang YZ, Xie SY, Sun Y. Effects of decitabine combined with arsenic trioxide regimen on immune function in patients with myelodysplastic syndrome undergoing chemotherapy. Contemp Med. 2022;28(13):148–150.
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. doi:10.6004/jnccn.2017.0007
- Zhang ZN. Diagnosis and efficacy criteria of hematological disease (version 2). Yang CL, editor. Beijing: China Science Publishing and Media Ltd; 1998.
- Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000 Dec;96(12):3671–3674.
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood 2006 Jul 108(2):419–425. doi:10.1182/blood-2005-10-4149
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007 Mar;109(6):1114–1124. doi:10.1002/cncr.22496
- Wei G, Ni W, Chiao JW, et al. A meta-analysis of CAG (cytarabine, aclarubicin, G-CSF) regimen for the treatment of 1029 patients with acute myeloid leukemia and myelodysplastic syndrome. J Hematol Oncol. 2011;14(4):46. doi:10.1186/1756-8722-4-46
- Xie M, Jiang Q, Li L, et al. HAG (Homoharringtonine, Cytarabine, G-CSF) regimen for the treatment of acute Myeloid Leukemia and Myelodysplastic syndrome: A meta-analysis with 2,314 participants. PLoS One. 2016;11(10):e0164238.
- Hua HY, Gao HQ, Sun AN, et al. Arsenic trioxide and triptolide synergistically induce apoptosis in the SKM–1 human myelodysplastic syndrome cell line. Mol Med Rep. 2016;14(5):4180–4186. doi:10.3892/mmr.2016.5779
- Huang L, Liu Z, Jiang H, et al. Decitabine shows synergistic effects with arsenic trioxide against myelodysplastic syndrome cells via endoplasmic reticulum stress-related apoptosis. J Investig Med. 2019 Oct;67(7):1067–1075. doi:10.1136/jim-2018-000953
- Wu X, Hu Z, Nizzero S, et al. Bone-targeting nanoparticle to co-deliver decitabine and arsenic trioxide for effective therapy of myelodysplastic syndrome with low systemic toxicity. J Control Release. 2017 Dec;268:92–101. doi:10.1016/j.jconrel.2017.10.012
- Galimberti S, Guerrini F, Salvi F, et al. Arsenic trioxide and ascorbic acid interfere with the BCL2 family genes in patients with myelodysplastic syndromes: an ex-vivo study. J Hematol Oncol. 2012 Sep;5:53. doi:10.1186/1756-8722-5-53
- Shao X, Lu R, Guan X, et al. [Effects of arsenic trioxide on Id4 methylation status in bone marrow mononuclear cells and its clinical efficacy for myelodysplastic syndrome]. Zhonghua Xue Ye Xue Za Zhi. 2014 Mar;35(3):247–250.
- Zhou QB, Zhu QZ, Wang HZ, et al. Traditional Chinese medicine containing arsenic treated MDS patients effectively through Regulating Aberrant Hypomethylation. Evid Base Complem Altern Med. 2020;2020:7469809.
- Hollow SE, Johnstone TC. Realgar and arsenene nanomaterials as arsenic-based anticancer agents. Curr Opin Chem Biol. 2023 Feb;72:102229. doi:10.1016/j.cbpa.2022.102229