ABSTRACT
Acute aerobic exercise has a facilitative effect on task components with varied inhibitory control demands. However, the effects on specific neural processes evoked by an inhibitory control task remain underexplored. In this study, we examined the effects of acute aerobic exercise on midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation, two EEG frequency markers that index distinct neural process during inhibitory control. Fifty-eight college-age students (mean age = 19.2 ± 1.0 years old) participated in this within-subjects, crossover, pretest-posttest design study. Participants engaged in a 20-minute bout of aerobic exercise condition and an active control condition on separate days in a counterbalanced order. The aerobic exercise condition consisted of walking/jogging on a treadmill at moderate intensity (approximately 70% of age-predicted maximum heart rate). The active control condition consisted of walking on the treadmill at 0.5 mph and 0% grade. Before and after both conditions, participants completed a flanker task, with concurrent EEG data collection. Despite shorter reaction times following aerobic exercise relative to following active control condition, neither aerobic exercise nor active control condition modulated midfrontal theta event-related synchronisation (which indexes control and behavioural monitoring process) or frontal alpha event-related desynchronisation (which reflects cognitive integration mechanism). Collectively, while acute aerobic exercise enhances inhibitory control performance at a behavioural level, these effects do not extend to the modulation of neural processes underlying behaviour adaptation and cognitive integration mechanisms.
Despite a robust body of literature demonstrating the beneficial effects of acute bouts of moderate-intensity aerobic exercise on cognitive control (Kao et al., Citation2022; Ludyga et al., Citation2016; Pontifex et al., Citation2019), a cognitive process involves in goal-oriented behaviours (Diamond, Citation2013), the underlying neural mechanisms remain underexplored. While investigations in this area have used event-related potentials (ERP) to investigate the effects of acute bouts of exercise on attention resources allocation (e.g., the P3 component from ERP) (Chu et al., Citation2015; Kao et al., Citation2022; Tsai et al., Citation2014, Citation2016), conflict resolution (e.g., the N450) (Chang et al., Citation2017; Hsieh et al., Citation2018), and motor preparation (e.g., the CNV) (Kamijo et al., Citation2004) during various cognitive control tasks relative to non-exercise control conditions, the time-domain averaging approach of ERPs may overlook valuable cognitive-related information within electroencephalography (EEG) data. Here, we used event-related EEG frequency dynamics, encompassing neural synchronisation and desynchronisation (a state of synchrony/desynchrony in a population of neurons in support of specific mental processes) at specific frequency bands relevant to events of interest (Klimesch, Citation1999; Makeig et al., Citation2004). Similar to ERPs, event-related frequency dynamics provide high temporal resolution; however, they operate through different underlying mechanisms. ERPs reflect the summation of event-induced post-synaptic activity from pyramidal neurons, whereas frequency dynamics reflect modulations of cortical oscillations within neural networks (Pfurtscheller & Lopes Da Silva, Citation1999). As such, event-related frequency dynamics could offer an additional perspective on the subtle neural changes induced by acute bouts of aerobic exercise.
Inhibitory control, a subdomain of cognitive control, involves the ability to suppress task-irrelevant goals and conflicting information/action to facilitate effective behavioural interactions with the environment (Diamond, Citation2013; Miyake et al., Citation2000). At a neural level, inhibitory control can be supported by the top-down regulatory mechanisms and behavioural adaptation mediated by the medial prefrontal cortex, the orbitofrontal cortex, and the anterior cingulate cortex (ACC) (Cavanagh & Frank, Citation2014). Specifically, the control function mediated by the medial-frontal circuit is reflected in modulations of midfrontal theta (4-7 Hz) oscillations (Cavanagh & Frank, Citation2014). Midfrontal theta serves as a neural marker for top-down control and monitoring processes, particularly when individuals perceive a need for enhanced control to adaptively adjust their behavioural response (Cohen & Donner, Citation2013; Duprez et al., Citation2020; Nigbur et al., Citation2012). Research has also indicated that midfrontal theta event-related synchronisation is increased after individuals commit a cognitive or behavioural error (Cavanagh et al., Citation2012). Collectively, increased midfrontal theta event-related synchronisations may indicate more effective cortical control over behaviour monitoring and adaptation processes.
In addition to midfrontal theta event-related synchronisation, alpha (∼8-13 Hz) event-related desynchronisation serves as an index of cognitive strategy control, e.g., the suppression of task-irrelevant cortical region during tasks with increased perception (Kolev et al., Citation1999), attention (Krause et al., Citation2000), memory (Klimesch, Citation1999), cognitive control (Klimesch, Citation1999; Krause et al., Citation2000), and sensorimotor activity (Rizzo et al., Citation2022). Alpha event-related desynchronisation is also associated with activity of thalamocortical networks involved in regulating sensory information and alertness (Klimesch, Citation1997). Increased frontal alpha event-related desynchronisation may indicate the implementation of a cognitive integration mechanism that organises sensory information and consciousness perception (Rizzo et al., Citation2022), supress task-irrelevant cortical regions, and enhances alertness. These cortical modulations may, in turn, facilitate further information processing.
Midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation play pivotal roles in supporting inhibitory control. However, research examining the effects of acute aerobic exercise on these EEG frequency dynamics during inhibitory control is still limited. Regarding midfrontal theta oscillations, two studies in young adults have reported conflicting findings (Ciria et al., Citation2018; Griggs et al., Citation2023). Specifically, study from Ciria et al. found no change in global theta oscillations during inhibitory control following either moderate – or light-intensity aerobic exercise (Ciria et al., Citation2018). However, this study has two notable limitations. First, it primarily focused on global theta oscillations using a data-driven approach, leaving the specific effects of aerobic exercise on theta oscillations over the medial-frontal region untested (Ciria et al., Citation2018); second, it lacked a valid control condition (e.g., non-exercise or active control condition), which increased the risks of practice or familiarity effects. On the other hand, study by Griggs et al. observed enhanced midfrontal theta event-related synchronisations during task component with lower inhibitory control demands (e.g., congruent trials during a flanker task) in young adults who engaged in vigorous-intensity aerobic exercise condition, compared to those who engaged in a non-exercise control condition (Griggs et al., Citation2023). The inconsistent findings between these two studies (Ciria et al., Citation2018; Griggs et al., Citation2023) may be attributed, at least in part, to differences in exercise intensity (e.g., light and moderate intensities in Ciria et al. study vs. vigorous intensity in Griggs et al. study) and selection of cortical regions (e.g., global theta oscillations in Ciria et al. study vs. frontal theta oscillations in Griggs et al. study). Regarding frontal alpha desynchronisation, existing literature also present conflicting findings. While a few studies in young adults reported no effects of acute aerobic exercise on global (Ciria et al., Citation2018) and frontal alpha desynchronisation (Griggs et al., Citation2023), Chang et al. study found increased frontal alpha desynchronisation following acute bouts of aerobic exercise in older adults (Chang et al., Citation2015). These contradictory results could be attributed to methodological heterogeneity across studies, such as participants’ age (Chang et al. study focused on older adults vs. Griggs et al. and Ciria et al. studies focused on young adults), selection of cortical regions (e.g., global alpha oscillations in Ciria et al. study vs. frontal alpha oscillations in Chang et al. and Griggs et al. studies), exercise intensity (e.g., vigorous intensity in Griggs et al. study vs. moderate intensity in Ciria et al. and Chang et al. studies). In addition, a notable methodological limitation shared by the aforementioned studies (Chang et al., Citation2015; Ciria et al., Citation2018; Griggs et al., Citation2023) is the absence of pre-test cognitive performance assessment. This omission makes it challenging to account for day-to-day variations in cognitive performance and limits the interpretation of “direction of change” in outcome measures induced by aerobic exercise (Pontifex et al., Citation2019). Without pre-test measures, conclusions regarding the effects of aerobic exercise on cognition are restricted to differences between experimental conditions/groups, rather than establishing a causal relationship between exercise and cognitive changes. Therefore, further research should address these methodological gaps to provide a clearer understanding of the effects of acute aerobic exercise on distinct neural processes during inhibitory control.
The current investigation was grounded in the well-documented modulatory effects of acute bouts of exercise on inhibitory control (Chang et al., Citation2015; Chu et al., Citation2015; Hsieh et al., Citation2018; Kao et al., Citation2017) as well as the functional significance of midfrontal theta synchronisation (Cavanagh & Frank, Citation2014) and frontal alpha desynchronisation (Klimesch, Citation1999) in supporting inhibitory control. We aimed to better understand how aerobic exercise affects distinct neural processes evoked during an inhibitory control task. Specifically, we tested how midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation are changed following an acute bout of moderate-intensity aerobic exercise in contrast to changes following an active control condition in young adults. The novelty of the current investigation lies in the exploration of two EEG time–frequency components indicative of distinct neural processes subserving inhibitory control process. By addressing methodological limitations of previous studies through a rigorous crossover, within-subjects, pretest-posttest design, we sought to enhance understanding of neural processes that may be altered by acute bouts of aerobic exercise and provide stronger causal inference. We believe that our investigation is practically relevant given that midfrontal theta and frontal alpha oscillations are closely related to various psychological and behaviour symptoms (e.g., anxiety, addiction, attention) (McLoughlin et al., Citation2022; Wang & Griskova-Bulanova, Citation2018). Building upon findings from previous studies (Chang et al., Citation2015; Griggs et al., Citation2023), we hypothesised that young adults would exhibit greater midfrontal theta event-related synchronisation, indicative of better behaviour monitoring and adaptation, as well as frontal alpha event-related desynchronisation, reflecting the implementation of cognitive integration mechanisms, during an inhibitory control task following an aerobic exercise condition relative to following an active control condition. We also hypothesised that such neural modulations would coincide with improved performance on an inhibitory control task following aerobic exercise relative to following an active control condition.
Method
Participants
The current investigation was a secondary analysis of data from McGowan et al. (Citation2019), with the novel aim of investigating midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation. The portion of the task performance data (i.e., response accuracy and mean reaction times) reported herein that overlap with the McGowan et al. study was presented only to better inform the neural oscillations findings. Analyses were conducted using the full sample of 58 college aged young adults (M = 19.2 ± 1.0 years, 32 females; 84.5% identified as White/Caucasian, 5.2% identified as Asian, 5.2% identified as Black/African American, 5.2% identified as multiracial) from Michigan State University. All participants reported being free of neurological disorders or physical disabilities, indicated normal or corrected-to-normal vision, and provided written informed consent. All experimental protocols were approved by the Institutional Review Board at Michigan State University and all methods were carried out in accordance with those protocols and relevant guidelines/regulations regarding the research with human participants. Demographic and fitness data for all participants are provided in .
Table 1. Participant demographic and fitness characteristics (mean ± SD).
Measures
Inhibitory control task
Inhibitory control was assessed using a letter version of the Eriksen flanker task (Chandler et al., Citation2021; Eriksen & Eriksen, Citation1974; McGowan et al., Citation2019; Moser et al., Citation2011). Participants were instructed to attend to and to respond as accurately as possible to a centrally presented letter nested among a lateral array of letters that were either congruent (e.g., “TTTTT” or “IIIII”) or incongruent (e.g., “IITII” or “TTITT”) with the centrally presented letter. Following completion of 80 practice trials to reduce the potential for learning related effects, participants completed 160 experimental trials grouped into two blocks of 80 trials. Within each block of trials congruency was equiprobable and participants were presented with perceptually similar letter pairs (e.g., pretest block 1: I – T, pretest block 2: U – V, posttest block 1: M – N, posttest block 2: E – F) and were instructed to respond by pressing the button assigned to the centrally presented target stimulus. To ensure a high degree of task difficulty, the button-letter assignments were reversed at the midpoint of each block (e.g., left button press for “T” through the first 40 trials of Block 1, then right button press for “T” through the last 40 trials of Block 1). Flanking letters were presented 55 ms prior to target letter onset, and all five letters remained on the screen for a subsequent 100 ms (for a total stimulus duration of 155 ms) with a response window of 1000 ms and a variable inter-trial interval of 2300, 2400, 2500, 2600, or 2700 ms. Stimuli were presented focally on a black background on an Asus VG248QE 144 Hz LCD monitor using PsychoPy (Peirce, Citation2009). Reaction time was quantified as the mean speed of responding following the onset of the stimulus only for correct trials while response accuracy was quantified as the proportion of correct responses relative to the number of trials administered. To ensure the integrity of the data, reaction times exceeding the upper bound and response accuracy below the lower bound of 3.5 times the interquartile range were identified as outliers and removed from analysis.
Neuroelectric activity assessment
EEG activity was recorded from 64 electrode sites (Fpz, Fz, FCz, Cz, CPz, Pz, POz, Oz, Fp1/2, F7/5/3/1/2/4/6/8, FT7/8, FC3/1/2/4, T7/8, C5/3/1/2/4/6, M1/2, TP7/8, CB1/2, P7/5/3/1/2/4/6/8, PO7/5/3/4/6/8, O1/2) arranged in an extended montage based on the International 10–10 system (Chatrian et al., Citation1985) using a Neuroscan Quik-Cap (Compumedics, Inc., Charlotte, NC). Recordings were referenced to averaged mastoids (M1, M2), with AFz serving as the ground electrode. Additional electrodes were placed above and below the left orbit and on the outer canthus of both eyes to monitor electrooculographic (EOG) activity with a bipolar recording. Continuous data were digitised at a sampling rate of 500 Hz with a DC to 70 Hz filter using a Neuroscan SynAmps RT amplifier. The EEG data were then imported into EEGLAB (Delorme & Makeig, Citation2004) and prepared for temporal ICA decomposition. Data > 2 s prior to the first event marker and 2 s after the final event marker were removed to restrict computation of ICA components to task-related activity. The continuous data were filtered using a 0.05 Hz high-pass Butterworth IIR filter to remove slow drifts (Chandler et al., Citation2019; Chandler et al., Citation2020; Gwizdala et al., Citation2021; Pontifex et al., Citation2017), and the mastoid electrodes were removed prior to ICA decomposition. ICA decomposition was performed using the extended infomax algorithm to extract sub-Gaussian components using the default settings called in the MATLAB implementation of this function in EEGLAB with the block size heuristic set at (floor[sqrt(EEG.pnts/3)]) (Chandler et al., Citation2019; Chandler et al., Citation2020; Gwizdala et al., Citation2021; Pontifex et al., Citation2017). Following ICA decomposition, the eyeblink artifact components were identified using the icablinkmetrics function (Logan et al., Citation2021; Pontifex et al., Citation2017; Raine et al., Citation2018) and the EEG data were reconstructed without the eyeblink artifact.
Following removal of the eye blink components, stimulus-locked epochs were created for correct trials from −1500 to 1500 ms around the stimulus which were baseline corrected using the entire sweep (Hsieh et al., Citation2020), and filtered using a zero-phase shift low-pass filter at 30 Hz. Trials with artifact exceeding ± 100 μV were rejected. To ensure the integrity of the signal, stimulus locked epochs were visually inspected blind to the experimental condition, time point, and congruency prior to computing mean waveforms (mean number of included trials: exercise pretest = 57.7 ± 12.1, exercise posttest = 55.2 ± 11.8, active control pretest = 55.7 ± 12.0, active control posttest = 55.9 ± 11.1). The EEGLAB toolbox was utilised to analyse the event-locked EEG responses. Data analysis and reporting of time–frequency processing comply with the latest recommendation and publication guidelines by Keil and colleagues (Keil et al., Citation2022). Oscillatory EEG power was computed by a Morlet-based wavelet transform with a width of 3 cycles of single trial data for a frequency band between 4–30 Hz using the newtimef() function in EEGLAB. Event-related spectral permutations were computed on the wavelet-transformed epochs at each time point and wavelet frequency to yield time-frequency maps. Oscillatory power (the magnitude of the analysed signal) was then averaged across accepted trials. The mean oscillatory power was rescaled by the baseline values from −300 to −100 ms relative to stimulus onset and taking the log10 transform of this quotient (dB) (dB power = 10 × 10 [power/baseline]), to allow a direct comparison of results of interest across frequencies. Mean oscillatory power was then collapsed across frontal electrodes (F1, FZ, F2, FC1, FCZ, FC2) to create a frontal region of interest. This pre-determine region of interest complies with the latest publication guidelines (Keil et al., Citation2022) and can be supported by several studies that also investigated modulations of midfrontal theta oscillations and frontal alpha oscillations (Cohen & Donner, Citation2013; Hsieh et al., Citation2020; Kao et al., Citation2020, Citation2021; van Noordt et al., Citation2022). The mean power from this region of interest in the time interval between 50 and 500 ms at 4–7 Hz was extracted as an index of frontal theta activity. The mean power from this region of interest in the time interval between 100 and 500 ms at 9–13 Hz was extracted as an index of frontal alpha activity. The frequency bands and time windows of midfrontal theta and frontal alpha were selected by inspecting the time–frequency plots (a) and by following procedures in previous relevant studies who also focused on midfrontal theta (Cohen & Donner, Citation2013; Hsieh et al., Citation2020; van Noordt et al., Citation2022) and frontal alpha oscillations (S. C. Kao et al., Citation2020, Citation2021).
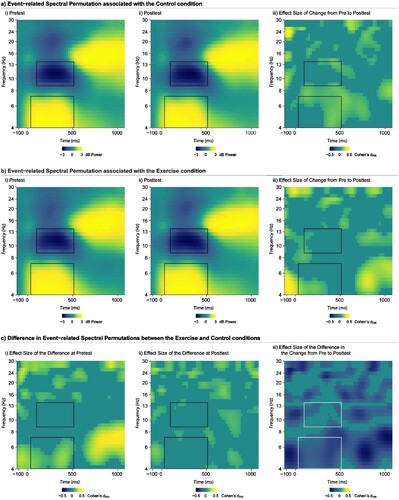
Figure 1. a) Plot of the change in event-related spectral permutation induced by the active control condition showing the activity at pretest (i), posttest (ii), and the statistical change (in Cohen’s dᵣₘ) from pretest to posttest (iii). b) Plot of the change in event-related spectral permutation induced by the aerobic exercise condition showing the activity at pretest (i), posttest (ii), and the statistical change from pretest to posttest (iii). c) Plot illustrating the statistical differences (in Cohen’s dᵣₘ) in event-related spectral permutation at pretest (i) and posttest (ii), alongside the statistical difference (in Cohen’s dᵣₘ) in change from pretest to posttest for the aerobic exercise relative to active control condition (iii). All plots illustrate data from the frontal region of interest collapsed across congruencies of the flanker task. Time-Frequency segments of interest are indicated by the bounding box. Theta synchronisation and alpha desynchronization are depicted as power increase between 50 and 500 ms at 4–7 Hz and power decrease between 100 and 500 ms at 9–13 Hz, respectively, relative to baseline (−300 to −100 ms).
Cardiorespiratory fitness assessment
Measures of relative peak oxygen consumption (ml/kg/min) were assessed using a computerised indirect calorimetry system (ParvoMedics True Max 2400) while participants ran or walked on a motor-driven treadmill (Trackmaster TMX425C). During the cardiorespiratory fitness assessment, participants exercised at a constant speed with 2.5% increases in grade every 2 minutes until the participant was no longer able to maintain the exercise intensity(American College of Sports Medicine, Citation2018). Measures of heart rate (Model: H7; Polar Electro, Kempele, Finland) and OMNI rating of perceived exertion (RPE) were recorded during the assessment. Consistent with previous investigations, attainment of maximal effort was evidenced by attainment of two of four criteria for reaching VO2max (Chandler et al., Citation2019; Chandler et al., Citation2020; Gwizdala et al., Citation2021; McGowan et al., Citation2019).
Procedure
Participants visited the laboratory on two separate days (mean days apart 7.9 ± 5.8 days; mean time of day difference, 2.3 ± 2.2 h). Prior to each experimental session, participants were instructed to abstain from exercise as well as alcohol/drug and caffeine consumption for 24 hours. To ensure that any observed effects were unrelated to the specific order in which participants received the experimental conditions, participants were randomly assigned into two different session orders (Day 1: exercise, Day 2: active control or Day 1: active control, Day 2: exercise) using a within-participants crossover pretest-posttest design (Gwizdala et al., Citation2021; Kao et al., Citation2023; McGowan et al., Citation2019). During each session, heart rate was measured at two-minute intervals throughout the experimental condition using a Polar HR monitor (Model H7, Polar Electro, Finland) alongside OMNI ratings of perceived exertion (Utter et al., Citation2002). As previously reported in McGowan et al. (Citation2019), the exercise experimental condition consisted of 20 min of exercise on a motor-driven treadmill at an intensity between 65 and 75% of age predicted maximum (205.8 − (0.685 ∗ Age)) (Robergs & Landwehr, Citation2002) heart rate (HR = 137.0 bpm [95% CI: 135.5–138.4]; % Heart Rate Reserve = 54.0% [95% CI: 51.4–56.5]). The active-control condition consisted of 20 min of walking on a motor-driven treadmill at the lowest possible speed and grade (0.5 mph and 0% grade; HR = 88.9 bpm [95% CI: 84.1–93.8]; % Heart Rate Reserve = 13.8% [95% CI: 10.2–17.4]). This use of an active control condition can be supported by studies that aim to minimise participants’ expectations to intervention and maximise internal validity of intervention (Weng et al., Citation2015). To reduce experimenter interaction and non-exercise related stimuli, participants watched an emotionally-neutral video (minutes 65–85 and 85–105; Wonders of the Universe) during the entire period of both experimental conditions. The inhibitory control task and concurrent neuroelectric activity assessment were conducted approximately 30 min prior to and 10 min following each experimental condition (see McGowan et al. (Citation2019) for more details characterising the experimental conditions). The 10-minute post-exercise recovery allowed participants’ heart rates returned to within 10% of baseline levels to minimise the confounding effects of exercise-induced hyperthermia and tachycardia (Pontifex et al., Citation2015). Eyes closed followed by eyes open EEG recording was conducted for 5 minutes each prior to the start of the Flanker task at both prettest and posttest. At the end of the second experimental session, participants completed the cardiorespiratory fitness assessment to ensure that the maximal exercise test did not induce potential changes in cognition.
Statistical analysis
All data analyses were performed in R Version 4 (R Core Team, Citation2019) utilising a familywise alpha level of p = 0.05. The analysis was conducted using a 2 (Mode: exercise, active control) × 2 (Time: pre-test, post-test) × 2 (Congruency: congruent, incongruent) univariate multi-level model including the random intercept for each participant. Additional random intercepts associated with participant by mode, participant by time, and participant by congruency interactions were considered but were not identified as statistically relevant. The multi-level model analysis was performed using the Rmimic package (Pontifex, Citation2022) which provides a standardised implementation wrapper around the lme4 (Bates et al., Citation2015), lmerTest (Kuznetsova et al., Citation2017), and emmeans (Lenth et al., Citation2017) packages in R (R Core Team, Citation2019) with Kenward-Roger degrees of freedom approximations and Benjamini-Hochberg false discovery rate control = 0.05 for post-hoc decompositions. Cohen’s f 2 and d with 95% confidence intervals were computed as standardised measures of effect size, using appropriate variance corrections for within-subject (drm) comparisons (Lakens, Citation2013). Given a sample size of 58 participants and beta of 0.20 (i.e., 80% power), the present research design theoretically had sufficient sensitivity to detect conventional t-test differences exceeding d = 0.37 (with a two-sided alpha) as computed using G*Power 3.1.2 (Faul et al., Citation2007).
Results
Behavioural performance
As the present investigation is a secondary analysis of data from McGowan et al. (Citation2019), a summary of the relevant behavioural findings is provided below. Reaction time was observed to be shorter at posttest (378.5 ± 55.3 ms) relative to pretest (388.2 ± 59.2), t (101) = 3.4, p = 0.001, drm = 0.25 [95% CI: 0.10–0.39], only in response to the exercise experimental condition. No such effect was observed for the active control condition (pretest: 385.7 ± 54.4 ms; posttest: 382.3 ± 55.4 ms), t (100) = 1.2, p = 0.2, drm = 0.09 [95% CI: −0.06–0.23]. Although a marginal Mode × Time interaction was observed for response accuracy, F (1, 220) = 3.9, p = 0.049, f 2’s = 0.05 [95% CI: 0.01–0.15], post-hoc decomposition of this interaction did not reveal any significant effects t’s(120) ≤ 1.4, p’s ≥ 0.2, drm’s ≤ 0.16 [95% CI: – 0.08–0.39].
Neuroelectric activity
Please see for time–frequency plots depicting oscillations of midfrontal theta synchronisation and frontal alpha de-synchronisation.
Midfrontal theta
Please see for a summary of F-test statistics in relation to midfrontal theta synchronisation. Analysis of midfrontal theta activity revealed a main effect of Congruency, such that congruent trials (2.2 ± 1.6 dB) exhibited lower power than incongruent trials (2.7 ± 1.4 dB), F(1, 377.1) = 20.8, p < 0.001, dᵣₘ = 0.37 [95% CI: 0.21–0.53]. No differences between aerobic exercise and active control or from pre – to post-test were observed, F’s (1, 377) ≤ 1.6, p’s ≥ 0.2, f 2’s ≤ 0.07 [95% CI: 0.0–0.21].
Table 2. Summary of fixed effects of the neuroelectric measures using Kenward-Roger degrees of freedom approximation (rounded to the nearest integer).
Frontal alpha
summarises F-test statistics in relation to frontal alpha desynchronisation. No differences in frontal alpha activity were observed between aerobic exercise and active control, between pretest and posttest, or between congruent and incongruent trials, F’s (1, 377) ≤ 0.8, p’s ≥ 0.36, f 2’s ≤ 0.15 [95% CI: 0.0–0.38].
Discussion
Our secondary analysis aimed to investigate how midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation during the performance of an inhibitory control task were influenced by moderate-intensity aerobic exercise compared to an active control condition in college-age adults. We observed shorter reaction times across both congruent and incongruent trials following aerobic exercise (as reported previously by McGowan et al., Citation2019). However, midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation remained unchanged following a single bout of moderate-intensity aerobic exercise or an active control condition. Although this finding was inconsistent with our a priori hypothesis, it provides insight into the spectrum of cognitive processes that underlie inhibitory control operations. Notably, our study presents two methodological advancements compared to relevant studies focusing on theta event-related synchronisation (Ciria et al., Citation2018; Griggs et al., Citation2023) and/or frontal alpha event-related desynchronisation (Chang et al., Citation2015; Griggs et al., Citation2023; Kao et al., Citation2020, Citation2021). We employed a robust randomised, within-participants, crossover pretest-posttest design and introduced a novel active control condition to mitigate potential confounds arising from day-to-day variations in cognition, expectation, and non-exercise stimuli (Pontifex et al., Citation2019). This approach allowed us to directly compare changes in neural indices of inhibitory control between aerobic exercise and active control conditions, addressing a gap in previous research.
Given that the Physical Activity Guidelines for Americans in 2018 specifically indicate that a single bout of aerobic exercise can lead to temporary enhancement in cognition and brain function (Erickson et al., Citation2019), an increasingly body of research has focused on the post-exercise enhancement in various cognitive domains, such as attention, executive function, and memory (Pontifex et al., Citation2019). Some studies have further indicated that acute enhancement in cognition induced by aerobic exercise can last for up to 80 minutes following exercise (Hung et al., Citation2013; Ludyga et al., Citation2019; Yu et al., Citation2020). In the context of inhibitory control, research has shown that acute bouts of moderate-intensity aerobic exercise can lead to improvements in either reaction times (Aly & Kojima, Citation2020; Griggs et al., Citation2023; Kao et al., Citation2017) or response accuracy (Griggs et al., Citation2023) during task components with varied inhibitory control demands. The observed shorter reaction times during both congruent and incongruent trials following aerobic exercise, without differences between task trials, suggest a general facilitative effect of acute moderate-intensity aerobic exercise on processing speed involving inhibitory control. However, two studies implementing vigorous aerobic exercise (Griggs et al., Citation2023) or high-intensity interval training (Kao et al., Citation2017) found greater exercise-induced enhancements in the performance of incongruent trials versus congruent trials, suggesting that the effects of acute exercise on inhibitory control might be intensity-dependent. Specifically, moderate-intensity aerobic exercise exerts a general facilitative effect whereas high-intensity aerobic exercise yields selective benefits to task components with increased inhibitory control demands.
While there has been considerable attention on the post-exercise enhancement of inhibitory control at a task level, our understanding of how acute bouts of aerobic exercise affect specific cognitive processes during inhibitory control tasks remains limited. Existing literature has primarily focused on the changes induced by acute moderate-intensity aerobic exercise in attention resources allocation (e.g., the P3 component from ERP) (Chu et al., Citation2015; Kao et al., Citation2022; Tsai et al., Citation2014, Citation2016), conflict resolution (e.g., the N450) (Chang et al., Citation2017; Hsieh et al., Citation2018), and motor preparation processes (e.g., the CNV) (Kamijo et al., Citation2004) during various cognitive control tasks. Our study goes further by examining EEG time–frequency oscillatory markers that reflect distinct neural processes. Midfrontal theta event-related synchronisation is indicative of modulations in the medial-frontal mediated top-down control processes, which affords better understanding of neural resources invested during the signalling, monitoring, and control of stimulus-response conflict in favour of subsequent behavioural adaptations (Cavanagh & Frank, Citation2014). Contrary to our hypothesis, our findings suggest that the control and behavioural monitoring processes indexed by midfrontal theta remain unchanged in response to a single bout of moderate-intensity aerobic exercise. This finding aligns with previous research investigating the effect of a bout of moderate-intensity aerobic exercise on theta oscillations during inhibitory control in young adults (Ciria et al., Citation2018) and school-age children (Hsieh et al., Citation2023; Kao et al., Citation2023). These converging findings imply that the control and behavioural monitoring mechanisms mediated by the medial-frontal cortical regions may not alter significantly in response to acute bouts of aerobic exercise at moderate intensity. Furthermore, our findings are supported by studies utilising the frontal N2 component from ERP. The frontal N2, elicited between 200 and 400 ms following stimulus onset, is maximal over the frontocentral region and is thought to be specifically related to the neural resources required to monitor and detect task-evoked conflicts between stimulus acquisition and response execution (Larson et al., Citation2014). Several studies in college-age students have indicated a null effect of acute moderate-intensity aerobic exercise (Bailey et al., Citation2021; Chang et al., Citation2017; Themanson & Hillman, Citation2006) on frontal N2 evoked during an inhibitory task. However, it is crucial to distinguish the cognitive implications of frontal N2 from midfrontal theta. While the frontal N2 is associated with conflict detection and monitoring, midfrontal theta plays a unique role in orchestrating online monitoring and behavioural adaptation by signalling and communicating with long-range task-relevant cortical areas (e.g., lateral prefrontal cortex, motor cortex, parietal cortex and somatosensory cortex) responsible for top-down sensorimotor decision-making, inhibition, attention modulations, and behaviour adaptation when cognitive control is needed (Cavanagh & Frank, Citation2014; Cohen & Donner, Citation2013; Duprez et al., Citation2020).
Contrary to our hypothesis, acute sessions of moderate-intensity aerobic exercise did not seem to affect frontal alpha event-related desynchronisation in young adults. Our findings are consistent with studies reporting no significant effects of moderate-intensity aerobic exercise on global alpha oscillations (Ciria et al., Citation2018). By integrating a pre-test cognitive assessment and employing a more precise electrode selection, our study provides stronger evidence supporting the null effect of moderate-intensity aerobic exercise on frontal alpha event-related desynchronisation in young adults. An alternative method for assessing neural activity related to inhibitory control is through pupillometry. Previous research has established a direct link between pupil dilation and activity of the locus-coeruleus (Joshi et al., Citation2016; Murphy et al., Citation2014), a cortical region with projections to the prefrontal cortex, thalamus, and hypothalamus that regulates sensory inputs, alertness, and arousal (Aston-Jones & Cohen, Citation2005). Intriguingly, two studies employing task-evoked pupil dilation as an indicator of arousal and alertness modulation also reported null effects of moderate-intensity aerobic exercise on task-evoked pupil dilation in college-age students (McGowan et al., Citation2019; Shigeta et al., Citation2021). These pupillometry findings somewhat support the null effects of moderate-intensity aerobic exercise on frontal alpha desynchronisation observed in our study. Taken together, these converging findings provide compelling evidence suggesting that the cognitive integration mechanism and alerting system may remain unaffected by acute sessions of moderate-intensity aerobic exercise in young adults. However, it is important to note that the effects of acute exercise on frontal alpha oscillations may be intensity dependent. For instance, a study by Kao et al. found that acute sessions of high-intensity interval exercise, but not moderate-intensity exercise, influenced frontal alpha desynchronisation during a working memory task in young adults (Kao et al., Citation2021). This potential intensity-dependent effect warrants further investigation, particularly in inhibitory control tasks.
Of note, our task and EEG findings suggest that modulations in the behaviour adaptation process and cognitive integration mechanism do not appear to contribute to acute exercise induced improvements in inhibitory control. The dissociation between improvement in task performance and unaltered EEG oscillations following acute exercise implies that neither behaviour adaptation nor cognitive integration mechanism are the primary neural mechanisms responsible for the effects of acute moderate-intensity aerobic exercise on inhibitory control. Instead, other neural processes, such as attention resource allocation (indicated by the P3 component from ERP) and conflict resolution (indexed by the N2 component from ERP), may serve as more tangible markers. Indeed, preliminary data indicates that alterations in the P3 and N2 components are associated with improved inhibitory control following acute moderate-intensity aerobic exercise (Hsieh et al., Citation2018; Yu et al., Citation2020).
In conclusion, the current study aimed to examine the effects of acute aerobic exercise on midfrontal theta event-related synchronisation and frontal alpha event-related desynchronisation during inhibitory control in young adults. Contrary to our expectations, neither the control and behavioural monitoring processes, as indicated by midfrontal theta oscillations, nor the cognitive integration mechanisms, as reflected by frontal alpha oscillations, were modulated by acute moderate-intensity aerobic exercise. This is despite the observed enhancement in processing speed related to inhibitory control following aerobic exercise. Our findings provide valuable insights into the neural processes possibly involved in the effects of acute aerobic exercise on inhibitory control. However, there is still a need for further research to examine how these cognitive processes respond to different exercise doses, modalities, and intensities. Addressing these gaps in knowledge will enhance our understanding of the impact of exercise on cognitive function.
CRediT authorship contribution statement
Shu-Shih Hsieh: Conceptualisation, Formal Analysis, Writing – Original Draft, Writing – Review & Editing.
Amanda L. McGowan: Methodology, Software, Validation, Investigation, Data Curation, Writing – Review & Editing, Supervision, Project Administration.
Madison C. Chandler: Investigation, Writing – Review & Editing.
Matthew B. Pontifex: Methodology, Software, Validation, Formal Analysis, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualisation, Supervision, Project Administration.
Acknowledgments
We thank the participants who participated in the study and the many undergraduate research assistants who assisted with data collection.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [SSH], upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aly, M., & Kojima, H. (2020). Acute moderate-intensity exercise generally enhances neural resources related to perceptual and cognitive processes: A randomized controlled ERP study. Mental Health and Physical Activity, 19, 100363. https://doi.org/10.1016/j.mhpa.2020.100363
- American College of Sports Medicine. (2018). ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins.
- Aston-Jones, G., & Cohen, J. D. (2005). Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. Journal of Comparative Neurology, 493(1), 99–110. https://doi.org/10.1002/cne.20723
- Bailey, B. W., Muir, A. M., Bartholomew, C. L., Christensen, W. F., Carbine, K. A., Marsh, H., LaCouture, H., McCutcheon, C., & Larson, M. J. (2021). The impact of exercise intensity on neurophysiological indices of food-related inhibitory control and cognitive control: A randomized crossover event-related potential (ERP) study. NeuroImage, 237, 118162. https://doi.org/10.1016/j.neuroimage.2021.118162
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
- Cavanagh, J. F., & Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421. https://doi.org/10.1016/j.tics.2014.04.012
- Cavanagh, J. F., Zambrano-Vazquez, L., & Allen, J. J. B. (2012). Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology, 49(2), 220–238. https://doi.org/10.1111/j.1469-8986.2011.01293.x
- Chandler, M. C., McGowan, A. L., Brascamp, J. W., & Pontifex, M. B. (2021). Phasic activity of the locus-coeruleus is not a mediator of the relationship between fitness and inhibition in college-aged adults. International Journal of Psychophysiology, 165, 1–7. https://doi.org/10.1016/j.ijpsycho.2021.03.007
- Chandler, M. C., McGowan, A. L., Burles, F., Mathewson, K. E., Scavuzzo, C. J., & Pontifex, M. B. (2020a). Aerobic fitness unrelated to acquisition of spatial relational memory in college-aged adults. Journal of Sport & Exercise Psychology, 42(6), 472–479. https://doi.org/10.1123/jsep.2020-0004
- Chandler, M. C., McGowan, A. L., Ferguson, D. P., & Pontifex, M. B. (2020b). Carbohydrate mouth rinse has no effects on behavioral or neuroelectric indices of cognition. International Journal of Psychophysiology, 151, 49–58. https://doi.org/10.1016/j.ijpsycho.2020.02.012
- Chandler, M. C., McGowan, A. L., Payne, B. R., Hampton Wray, A., & Pontifex, M. B. (2019). Aerobic fitness relates to differential attentional but not language-related cognitive processes. Brain and Language, 198, 104681. https://doi.org/10.1016/j.bandl.2019.104681
- Chang, Y. K., Alderman, B. L., Chu, C. H., Wang, C. C., Song, T. F., & Chen, F. T. (2017). Acute exercise has a general facilitative effect on cognitive function: A combined ERP temporal dynamics and BDNF study. Psychophysiology, 54(2), 289–300. https://doi.org/10.1111/psyp.12784
- Chang, Y. K., Chu, C. H., Wang, C. C., Song, T. F., & Wei, G. X. (2015). Effect of acute exercise and cardiovascular fitness on cognitive function: An event-related cortical desynchronization study. Psychophysiology, 52(3), 342–351. https://doi.org/10.1111/psyp.12364
- Chatrian, G. E., Lettich, E., & Nelson, P. L. (1985). Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. American Journal of EEG Technology, 25(2), 83–92. https://doi.org/10.1080/00029238.1985.11080163
- Chu, C. H., Alderman, B. L., Wei, G. X., & Chang, Y. K. (2015). Effects of acute aerobic exercise on motor response inhibition: An ERP study using the stop-signal task. Journal of Sport and Health Science, 4(1), 73–81. https://doi.org/10.1016/j.jshs.2014.12.002
- Ciria, L. F., Perakakis, P., Luque-Casado, A., & Sanabria, D. (2018). Physical exercise increases overall brain oscillatory activity but does not influence inhibitory control in young adults. NeuroImage, 181, 203–210. https://doi.org/10.1016/j.neuroimage.2018.07.009
- Cohen, M. X., & Donner, T. H. (2013). Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. Journal of Neurophysiology, 110(12), 2752–2763. https://doi.org/10.1152/jn.00479.2013
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
- Duprez, J., Gulbinaite, R., & Cohen, M. X. (2020). Midfrontal theta phase coordinates behaviorally relevant brain computations during cognitive control. NeuroImage, 207, 116340. https://doi.org/10.1016/j.neuroimage.2019.116340
- Erickson, K. I., Hillman, C., Stillman, C. M., Ballard, R. M., Bloodgood, B., Conroy, D. E., Macko, R., Marquez, D. X., Petruzzello, S. J., & Powell, K. E. (2019). Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Medicine & Science in Sports & Exercise, 51(6), 1242–1251. https://doi.org/10.1249/MSS.0000000000001936
- Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. https://doi.org/10.3758/BF03203267
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Griggs, M. A., Parr, B., Vandegrift, N. S., & Jelsone-Swain, L. (2023). The effect of acute exercise on attentional control and theta power in young adults. Experimental Brain Research, 241(10), 2509–2520. https://doi.org/10.1007/s00221-023-06660-3
- Gwizdala, K. L., Ferguson, D. P., Kovan, J., Novak, V., & Pontifex, M. B. (2021). Placebo controlled phase II clinical trial: Safety and efficacy of combining intranasal insulin & acute exercise. Metabolic Brain Disease, 36(6), 1289–1303. https://doi.org/10.1007/s11011-021-00727-2
- Hsieh, S. S., Chueh, T. Y., Morris, T. P., Kao, S. C., Westfall, D. R., Raine, L. B., Hopman, R. J., Pontifex, M. B., Castelli, D. M., Kramer, A. F., & Hillman, C. H. (2020). Greater childhood cardiorespiratory fitness is associated with better top-down cognitive control: A midfrontal theta oscillation study. Psychophysiology, 57(12), e13678. https://doi.org/10.1111/psyp.13678
- Hsieh, S. S., Huang, C. J., Wu, C. T., Chang, Y. K., & Hung, T. M. (2018). Acute exercise facilitates the N450 inhibition marker and P3 attention marker during stroop test in young and older adults. Journal of Clinical Medicine, 7(11), https://doi.org/10.3390/jcm7110391
- Hsieh, S. S., Kao, S. C., Raine, L. B., Lloyd, K. M., Pontifex, M. B., & Hillman, C. H. (2023). Acute bouts of aerobic exercise do not modulate task-evoked midfrontal theta oscillations in school-age children. Journal of Cognitive Enhancement, 8(1-2), 9–20. https://doi.org/10.1007/s41465-023-00281-y
- Hung, T. M., Tsai, C. L., Chen, F. T., Wang, C. C., & Chang, Y. K. (2013). The immediate and sustained effects of acute exercise on planning aspect of executive function. Psychology of Sport and Exercise, 14(5), 728–736. https://doi.org/10.1016/j.psychsport.2013.05.004
- Joshi, S., Li, Y., Kalwani, R. M., & Gold, J. I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89(1), 221–234. https://doi.org/10.1016/j.neuron.2015.11.028
- Kamijo, K., Nishihira, Y., Hatta, A., Kaneda, T., Kida, T., Higashiura, T., & Kuroiwa, K. (2004). Changes in arousal level by differential exercise intensity. Clinical Neurophysiology, 115(12), 2693–2698. https://doi.org/10.1016/j.clinph.2004.06.016
- Kao, S. C., Baumgartner, N., Noh, K., Wang, C. H., & Schmitt, S. (2023). Acute effects of intense interval versus aerobic exercise on children’s behavioral and neuroelectric measures of inhibitory control. Journal of Science and Medicine in Sport, 26(6), 316–321. https://doi.org/10.1016/j.jsams.2023.05.003
- Kao, S. C., Chen, F. T., Moreau, D., Drollette, E. S., Amireault, S., Chu, C. H., & Chang, Y. K. (2022). Acute effects of exercise engagement on neurocognitive function: A systematic review and meta-analysis on P3 amplitude and latency. International Review of Sport and Exercise Psychology, https://doi.org/10.1080/1750984X.2022.2155488
- Kao, S. C., Wang, C. H., & Hillman, C. H. (2020). Acute effects of aerobic exercise on response variability and neuroelectric indices during a serial n-back task. Brain and Cognition, 138, 105508. https://doi.org/10.1016/j.bandc.2019.105508
- Kao, S. C., Wang, C. H., Kamijo, K., Khan, N., & Hillman, C. (2021). Acute effects of highly intense interval and moderate continuous exercise on the modulation of neural oscillation during working memory. International Journal of Psychophysiology, 160, 10–17. https://doi.org/10.1016/j.ijpsycho.2020.12.003
- Kao, S. C., Westfall, D. R., Soneson, J., Gurd, B., & Hillman, C. H. (2017). Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology, 54(9), 1335–1345. https://doi.org/10.1111/psyp.12889
- Keil, A., Bernat, E. M., Cohen, M. X., Ding, M., Fabiani, M., Gratton, G., Kappenman, E. S., Maris, E., Mathewson, K. E., Ward, R. T., & Weisz, N. (2022). Recommendations and publication guidelines for studies using frequency domain and time-frequency domain analyses of neural time series. Psychophysiology, 59(5), e14052. https://doi.org/10.1111/psyp.14052
- Klimesch, W. (1997). EEG-alpha rhythms and memory processes. International Journal of Psychophysiology, 26(1-3), 319–340. https://doi.org/10.1016/S0167-8760(97)00773-3
- Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2-3), 169–195. https://doi.org/10.1016/S0165-0173(98)00056-3
- Kolev, V., Yordanova, J., Schürmann, M., & Baţar, E. (1999). Event-related alpha oscillations in task processing. Clinical Neurophysiology, 110(10), 1784–1792. https://doi.org/10.1016/S1388-2457(99)00105-4
- Krause, C. M., Èki, L. S., Koivisto, M., Saarela, C., Èggqvist, A. H., Laine, M., Èma, H., & Èinen, È. (2000). The effects of memory load on event-related EEG desynchronization and synchronization. Clinical Neurophysiology, 111(11), 2071–2078. www.elsevier.com/locate/clinph.
- Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. https://doi.org/10.18637/jss.v082.i13
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. https://doi.org/10.3389/fpsyg.2013.00863
- Larson, M. J., Clayson, P. E., & Clawson, A. (2014). Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology, 93(3), 283–297. https://doi.org/10.1016/j.ijpsycho.2014.06.007
- Lenth, R., Love, J., & Herve, M. (2017). emmeans: Estimated marginal means, aka least-squares means. https://github.com/rvlenth/emmeans.
- Logan, N. E., Raine, L. B., Drollette, E. S., Castelli, D. M., Khan, N. A., Kramer, A. F., & Hillman, C. H. (2021). The differential relationship of an afterschool physical activity intervention on brain function and cognition in children with obesity and their normal weight peers. Pediatric Obesity, 16(2), e12708. https://doi.org/10.1111/ijpo.12708
- Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E., & Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology, 53(11), 1611–1626. https://doi.org/10.1111/psyp.12736
- Ludyga, S., Pühse, U., Lucchi, S., Marti, J., & Gerber, M. (2019). Immediate and sustained effects of intermittent exercise on inhibitory control and task-related heart rate variability in adolescents. Journal of Science and Medicine in Sport, 22(1), 96–100. https://doi.org/10.1016/j.jsams.2018.05.027
- Makeig, S., Debener, S., Onton, J., & Delorme, A. (2004). Mining event-related brain dynamics. Trends in Cognitive Sciences, 8(5), 204–210. https://doi.org/10.1016/j.tics.2004.03.008
- McGowan, A. L., Chandler, M. C., Brascamp, J. W., & Pontifex, M. B. (2019). Pupillometric indices of locus-coeruleus activation are not modulated following single bouts of exercise. International Journal of Psychophysiology, 140, 41–52. https://doi.org/10.1016/j.ijpsycho.2019.04.004
- McLoughlin, G., Gyurkovics, M., Palmer, J., & Makeig, S. (2022). Midfrontal theta activity in psychiatric illness: An index of cognitive vulnerabilities across disorders. Biological Psychiatry, 91(2), 173–182. https://doi.org/10.1016/j.biopsych.2021.08.020
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
- Moser, J. S., Schroder, H. S., Heeter, C., Moran, T. P., & Lee, Y.-H. (2011). Mind your errors. Psychological Science, 22(12), 1484–1489. https://doi.org/10.1177/0956797611419520
- Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H., & Balsters, J. H. (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35(8), 4140–4154. https://doi.org/10.1002/hbm.22466
- Nigbur, R., Cohen, M. X., Ridderinkhof, K. R., & Stürmer, B. (2012). Theta dynamics reveal domain-specific control over stimulus and response conflict. Journal of Cognitive Neuroscience, 24(5), 1264–1274. https://doi.org/10.1162/jocn_a_00128
- Peirce, J. W. (2009). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2, 10. https://doi.org/10.3389/neuro.11.010.2008
- Pfurtscheller, G., & Lopes Da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110(11), 1842–1857. https://doi.org/10.1016/S1388-2457(99)00141-8
- Pontifex, M. B. (2022). Rmimic. https://github.com/mattpontifex/Rmimic.
- Pontifex, M. B., Gwizdala, K. L., Parks, A. C., Billinger, M., & Brunner, C. (2017a). Variability of ICA decomposition may impact EEG signals when used to remove eyeblink artifacts. Psychophysiology, 54(3), 386–398. https://doi.org/10.1111/psyp.12804
- Pontifex, M. B., McGowan, A. L., Chandler, M. C., Gwizdala, K. L., Parks, A. C., Fenn, K., & Kamijo, K. (2019). A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychology of Sport and Exercise, 40, 1–22. https://doi.org/10.1016/j.psychsport.2018.08.015
- Pontifex, M. B., Miskovic, V., & Laszlo, S. (2017b). Evaluating the efficacy of fully automated approaches for the selection of eyeblink ICA components. Psychophysiology, 54(5), 780–791. https://doi.org/10.1111/psyp.12827
- Pontifex, M. B., Parks, A. C., Henning, D. A., & Kamijo, K. (2015). Single bouts of exercise selectively sustain attentional processes. Psychophysiology, 52(5), 618–625. https://doi.org/10.1111/psyp.12395
- Raine, L. B., Kao, S.-C., Pindus, D., Westfall, D. R., Shigeta, T. T., Logan, N., Cadenas-Sanchez, C., Li, J., Drollette, E. S., Pontifex, M. B., Khan, N. A., Kramer, A. F., & Hillman, C. H. (2018). A large-scale reanalysis of childhood fitness and inhibitory control. Journal of Cognitive Enhancement, 2(2), 170–192. https://doi.org/10.1007/s41465-018-0070-7
- R Core Team. (2019). R: a language and environment for statistical computing. http://www.R-project.org/.
- Rizzo, M., Petrini, L., Del Percio, C., Lopez, S., Arendt-Nielsen, L., & Babiloni, C. (2022). Mirror visual feedback during unilateral finger movements is related to the desynchronization of cortical electroencephalographic somatomotor alpha rhythms. Psychophysiology, 59(12), e14116. https://doi.org/10.1111/psyp.14116
- Robergs, R. A., & Landwehr, R. (2002). The surprising history of the" HRmax= 220-age" equation. Journal of Exercise Physiology Online, 5(2), 1–10.
- Shigeta, T. T., Morris, T. P., Henry, D. H., Kucyi, A., Bex, P., Kramer, A. F., & Hillman, C. H. (2021). Acute exercise effects on inhibitory control and the pupillary response in young adults. International Journal of Psychophysiology, 170, 218–228. https://doi.org/10.1016/j.ijpsycho.2021.08.006
- Shvartz, E., & Reibold, R. C. (1990). Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviation, Space, and Environmental Medicine, 61(1), 3–11.
- Themanson, J. R., & Hillman, C. H. (2006). Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience, 141(2), 757–767. https://doi.org/10.1016/j.neuroscience.2006.04.004
- Tsai, C. L., Chen, F. C., Pan, C. Y., Wang, C. H., Huang, T. H., & Chen, T. C. (2014). Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology, 41, 121–131. https://doi.org/10.1016/j.psyneuen.2013.12.014
- Tsai, C. L., Pan, C. Y., Chen, F. C., Wang, C. H., & Chou, F. Y. (2016). Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Experimental Physiology, 101(7), 836–850. https://doi.org/10.1113/EP085682
- Utter, A. C., Robertson, R. J., Nieman, D. C., & Kang, J. (2002). Children’s OMNI Scale of Perceived Exertion: Walking/running evaluation. Medicine & Science in Sports & Exercise, 34(1), 139–144. https://doi.org/10.1097/00005768-200201000-00021
- van Noordt, S., Heffer, T., & Willoughby, T. (2022). A developmental examination of medial frontal theta dynamics and inhibitory control. NeuroImage, 246, 118765. https://doi.org/10.1016/j.neuroimage.2021.118765
- Wang, G. Y., & Griskova-Bulanova, I. (2018). Electrophysiological activity is associated with vulnerability of Internet addiction in non-clinical population. Addictive Behaviors, 84, 33–39. https://doi.org/10.1016/j.addbeh.2018.03.025
- Weng, T. B., Pierce, G. L., Darling, W. G., & Voss, M. W. (2015). Differential effects of acute exercise on distinct aspects of executive function. Medicine & Science in Sports & Exercise, 47(7), 1460–1469. https://doi.org/10.1249/MSS.0000000000000542
- Yu, C. L., Hsieh, S. S., Chueh, T. Y., Huang, C. J., Hillman, C. H., & Hung, T. M. (2020). The effects of acute aerobic exercise on inhibitory control and resting state heart rate variability in children with ADHD. Scientific Reports, 10(1), 19958. https://doi.org/10.1038/s41598-020-76859-9
