ABSTRACT
In the current study, experimental (UV–visible, Fourier transform infrared [FTIR], 1H-NMR and scanning electron microscope) and computational (UV–visible, FTIR, 1H-NMR, HOMO–LUMO, steric and geometric parameters) analyses of acephate, glyphosate, monocrotophos and phorate were performed for the first time. Computational studies were performed at the HF/6–311G(d,p) level of theory. It was found that experimental values of UV–visible, FTIR, 1H-NMR and geometric data were in very good agreement with the computational ones. The current study may assist future studies, like spectral analysis, pesticide(s) detection, surface behaviour and decomposition analysis of top selling titled pesticides of world market.
1. Introduction
Plant protection has become necessary to increase the food production due to increase of the worldwide growth of the human population [Citation1–3]. It is expected that the chemical pressure on the environment will further increase. Consequently, the uses of pesticides become a necessary part of agriculture, and their analysis is a prime most necessity of current time [Citation1–3]. To analyse these toxic compounds, either on their development stages or in the environmental compartments, it is obligatory to develop theoretical studies to assist the experimental studies. In literature, few studies have been reported to check the spectral, geometrical, toxicological and biodegradation aspects of contaminants/pesticides [Citation2–14].
Organophosphate pesticides (OPs) are the major class of pesticides, used as replacement of the toxic organochlorine pesticides in agricultural applications [Citation2,Citation3]. These are easily degradable, but more toxic than organochlorines [Citation2,Citation3,Citation15]. OPs inhibit the phosphorylation of acetylcholinesterase and exert primarily a cholinergic toxicity [Citation3]. From a health perspective, it is always risky to analyse the spectral, environmental and physicochemical properties of the toxic compounds including OPs [Citation2–4,Citation15,Citation16]. As per the review reports of Terry [Citation1] and Kumar et al. [Citation2], OPs are toxic to humans and non-target organisms, exposure of OPs has been associated with prolonged impairments in attention, memory and other domains of cognition, as well as chronic illnesses, where these symptoms are manifested (e.g. Gulf War Illness, Alzheimer’s disease) [Citation1,Citation2]. Maximum exposure of OPs and other chemicals is experienced during analysis, detection, formulation and applications in the agriculture. Computational studies may provide the advantage over traditional analysis and detection processes, and the same may allow to chemists/researchers/users to overcome the excess exposure of OPs and other chemicals.
In literature, few computational studies on OPs have been reported [Citation1–7]. Among these studies, maximum studies were reported on the herbicide, glyphosate. Ali et al. [Citation8] have reported an extensive ab initio Molecular Orbital calculations at the HF/3–21G(d) level to study the conformational energy surfaces of N-hydroxy-glyphosate and N-amino-glyphosate [Citation8]. Kaliannan et al. [Citation17] have reported fully optimized structure and rotational potential around the active site nitrogen (about the C–N and N–C bond) at the HF/3–21G(d) level [Citation17]. Zhang et al. [Citation18] have studied THz spectra of molecular and solid forms of acephate using first principles calculations based on the density functional method [Citation18]. A tandem mass spectrometry of methamidophos was performed by Rifai et al. [Citation19], which was supported by B3LYP/6–31G(d) calculations [Citation19].
As per our best information, the current study is the first detailed report on the experimental and computational analysis of titled molecules. Wide applications of titled molecules in the world market may make this study more significant [Citation20]. Future studies including the spectral analysis, pesticide(s) detection, surface behaviour and decomposition analysis of these pesticides may be acierated by this study.
In the current topic, we have performed the experimental and computational studies of acephate (1), glyphosate (2), monocrotophos (3) and phorate (4) (detailed information under supplementary Table S1). The specific objective of the current study was to perform experimental and computational study with the aim to evaluate and understand the structural, energetic and stability properties of titled pesticides 1–4.
2. Experimental
2.1. Material and instruments
All the pesticides were gifted by Gautmi Ltd, Andhra Pradesh, India, and having purity more than 95%. Ultraviolet–visible (UV–visible) experiments were performed on Shimadzu-1800s UV–visible spectrometer (cubed 1 cm length) in aqueous medium at different pH (4, 7 and 10) maintained by using HCl and NaOH dilute solutions. The wavelength-dependent molar absorptivity coefficient ε (M−1 cm−1) was obtained using Beer–Lambert’s law: A = log (I0/I) = ε × L × C, where A is the absorbance (dimensionless), I0 is the reference intensity taken with the cell filled with distilled water, I is the intensity with the cell filled with an aqueous solution of the studied pesticide, L is the path length of the two cells (cm) and C is the concentration of pesticide (in mol L−1).
Transmission Fourier transform infrared (FTIR) experiments were performed on a Shimadzu-8400. Molecules 1 and 2 are solid and these were analysed by taking 1.5 mg of the compound one by one in 200 mg KBr, mixed and crushed well. Then the KBr powder was pressed at a pressure of 10 tons into 16 mm diameter discs, using a Specac, UK die-press. A pure disc of potassium bromide (KBr) of 200 mg was used to measure the background spectrum. Next time, 200 mg KBr and 1.5 mg of the compound 1 or 2 were added and the above procedure was repeated. Two extra KBr discs of 200 mg each were used to analyse liquid samples 3 and 4. Drops of pure compounds 3 or 4 were placed on the surface of one of the thin KBr discs and allowed to evaporate in a vacuum oven. The final spectrum was obtained by taking wavenumber (cm−1) on the x-axis and transmittance (%) on the y-axis. As per our assumption, “Liquid drop on KBr disc” is a shortcut and time-saving method. To check the accuracy, the FTIR data of molecule 3 and 4 were compared with the National Institute of Standards and Technology (NIST) data.
Nuclear magnetic resonance (NMR) analyses were performed on an NMR spectrophotometer (Bruker Avance III, 400 MHz) by taking trimethylsilane as an internal reference. Compounds 1–4 were prepared in deuterated dimethyl methyl sulphoxide (d6−DMSO) in different sample tubes having 2 mL capacity. Approximately 2 mg of the solid compounds of molecules 1 and 2 was used and 500 µL of molecules 3 and 4 was used. The samples in tubes were allowed to settle down and soluble liqueurs poured into NMR sample analysis tubes. These NMR sample analysis tubes were caped well and put into the NMR instrument automated ballet. Finally, the NMR spectra were recorded in ppm values.
Scanning electron microscopy (SEM) was performed to check the surface topography and distribution of elemental composition on the surface. Different elements and surface topographies emit different quantities of electrons. SEM analysis was performed at SAIF Coachi Kerala, India, on the instrument, JEOL Model JSM-6390LV in the following conditions; resolution: 3 nm (Acc V 30 KV, WD 8 mm, SEI); 8 nm (Acc V 3.0 KV, WD 6 mm, SEI); 15 nm (Acc V 1.0 KV, WD 6 mm, SEI); Magnification: 10×, 100× and 1000× with Probe Current: 1 pA–1 mA.
2.2. Computational study
The computational studies of molecules 1–4 were performed using the Personal Computer-General Atomic and Molecular Electronic Structure System (PC-GAMESS) version 13.0 [Citation4–14]. The theoretical, steric parameters (stretch, bend, stretch-bend, torsion, non-1,4 VDW, 1,4 VDW, dipole/dipole and steric energy for frame), polarizability, infrared intensities (FTIR), UV–visible and 1H-NMR study of the titled pesticides in the ground state (a minimum energy state of molecule) were calculated. For the first time, computational analysis was performed using the Hartree Fork (HF) method with the 6–311++G(d,p) basis set through the GAMESS programme [Citation4–13], using Cambridge Software ChemBio3D Ultra 13.0.
To calculate the above-mentioned parameters, the executable programme file of GAMESS (using Cambridge Software ChemBio3D Ultra 13.0.) was run on PC. In first step, structures of molecules 1–4 were prepared by using Chemdraw [Citation7–13], and then converted into 3D structures. One by one, all the 3D structures were optimized into minimum energy structure by using the appropriate commands (calculations – GAMESS interface – set basis (HF-6–311++G(d,p)) – temperature (298°C) – energy minimization – run). In the next step, the desired parameters were calculated by using the appropriate commands (calculations – GAMESS interface – desired parameter, e.g. prediction of IR spectrum or UV spectrum or NMR spectrum or polarization or dipole moment – set basis (HF-6–311++G(d,p)) – temperature (298°C) – run). The steric parameters were optimized through MM2 computational optimization. Finally, output files of all the parameters were obtained (calculations – GAMESS interface – view output files). All the qualitative and quantitative studies were done on the basis of output files obtained after complete optimizations.
3. Results and discussion
Structurally, OPs are pentavalent phosphorus-containing organic compounds that possess three different substituents including P9O/S moiety to fill the valences on the phosphorus atom [Citation1–3]. Considering per configurational and conformational arrangement aspects, all the molecules have different arrangements around the P atom with different energy values [Citation1–3,Citation13,Citation14,Citation17–19]. In this study, the analysis of single conformer of each molecule was performed which has a minimum energy value because only the stable or minimum energy value molecule can provide accurate data [Citation9–14,Citation17–19]. The structural view (top, side and front view) of minimum energy structures as well HOMO–LUMO and dipole views of 1–4 are shown in the supplementary figure (S2). To make the study more effective and interesting, a relationship between molecules 1–4 was established on the basis of structures of titled molecules (Supplementary S2), i.e. “for glyphosate, two moieties are OH, for monocrotophos these two moieties are –OCH3, for phorate these moieties are –OEt, and for acephate these moieties are –OCH3 and –SCH3”.
3.1. Effect of pH variation on titled molecules
The effect of pH on molecules 1–4 was observed experimentally. The changes in absorbance were measured at 235 nm for 1, 215 nm for 2, 330 nm for 3 and 260 nm for 4 using a concentration of 0.01 M (where M means molar) (Supplementary figure S3). Concentration was taken as a dependent factor over variable absorption maxima (λmax) of molecules 1–4. The variability in the absorption maxima (235 nm for 1, 215 nm for 2, 330 nm for 3 and 260 nm for 4) was decided to find out the significant results at different pH values. The absolute values of molar absorptivity coefficients at these wavelengths were determined at different pH values 4, 7 and 10 by using the relationship A = ε × L × C, where L is the cuvette length (1 cm). The calculated values of molar absorptivity (ε) were as shown in supplementary figure S3; for molecule 1 at 235 nm, 96.5 ± 0.22 M−1 cm−1 at pH 4, 122.6 ± 0.31 M−1 cm−1 at pH 7 and 233.0 ± 0.42 M−1 cm−1 at pH 10. For molecule 2, the calculated values of molar absorptivity (ε) were as at 215 nm, 24.1 ± 0.08 M−1 cm−1 at pH 4, 29.2 ± 0.12 M−1 cm−1 at pH 7 and 148.0 ± 0.34 M−1 cm−1 at pH 10. Similarly, for molecule 3, the calculated values of molar absorptivity (ε) were as at 330 nm, 181.7 ± 0.23 M−1 cm−1 at pH 4, 188.0 ± 0.29 M−1 cm−1 at pH 7 and 199.9 ± 0.37 M−1 cm−1 at pH 10. Finally, for molecule 4, the calculated values of molar absorptivity (ε) were as at 260 nm, 147.8 ± 0.21 M−1 cm−1 at pH 4, 160.1 ± 0.26 M−1 cm−1 at pH 7 and 162.9 ± 0.29 M−1 cm−1 at pH 10. Overall, it was found that the 0.01 M solution of molecule 3 has the highest values of molar absorptivity (ε) from pH 4 to 10, followed by 4, 1 and 2 (Supplementary figure S3).
Compared to molar absorptivity (ε) values at pH 4 with pH values at 7 and 10, there were 27% and 141% increase observed for molecule 1, 21% and 514% increase observed for molecule 2, 3% and 10% increase observed for molecule 3, and 8% and 11% increase observed for molecule 4. In terms of percentage increase with an increase in pH values, molecule 2 has shown excellent increase, followed by 1, 4 and 3. Among the titled molecules 1–4, at pH 4, the order of percentage change among the molar absorptivity (ε) was 3 > 4 > 1 > 2, at pH 7, the order of molar absorptivity (ε) was 1 > 2 > 4 > 3 and at pH 10, the order of molar absorptivity (ε) was 2 > 1 > 4 > 3. At pH 10, all molecules show greater molar absorptivity (ε) followed by pH 7 and 4. Interestingly, there were drastic changes in absorbances or molar absorptivity (ε) observed in case of molecules 1 and 2 at higher pH 10 (Supplementary figure S3).
To find out the reason behind drastic changes with pH, we have compared literature studies with the current study. It was found that at pH 4 molecule 1 is in cationic form (at N atom) due to the acidic environment of the solution (). As the pH increases to 7, the molecule starts to attain its free or original state (lone pairs with every hetro atom like N and O, and especially C9O group which acts at the chromophoric group allowing π − π* and n − π* transitions). At pH 10, it was observed that molecule 1 was involved in H-bonding leading to the formation of six-membered ring(s) and increases the conjugation. As a result of conjugation, increase in the π − π* transition was observed, which leads to the higher molar absorptivity (ε) () [Citation21–27]. A similar fact was applicable for molecule 2 and molecule 3 due to the formation of five- and four-membered chelation rings through H-bonding. In case of molecule 4, there was almost stationary change in molar absorptivity (ε) with an increase in pH due to non-conjugation and non-chelation ring nature.
Figure 1. Most probable mechanism of H-bonding of studied pesticides at different pH: acephate (molecule 1) and glyphosate (molecule 2).
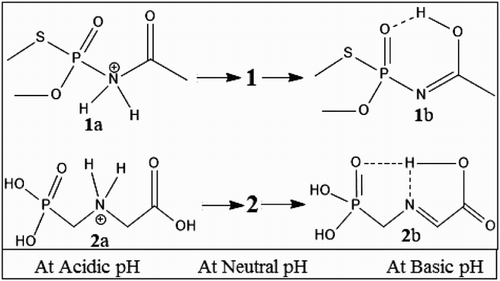
Recent studies have revealed that hydrolysis of molecules 1–3 is very slow in water, and half life increases up to tenfold [Citation28–31]. The slow decomposition was attributed to the formation of the six-membered ring through H association (). From the above discussion, the observed order of stability was as; 2 > 1 >> 3. The fact behind this stability order was the formation of kinetically and thermally stable five-member ring by 2, followed by 1 (six-membered less stable, i.e. kinetic stability only) and 3 (four member not stable, i.e. kinetically and thermally non-stable). As per Baeyer Strain theory, four-membered ring is non-stable due to of angle strain.
Moreover, the acid dissociation constant (pKa values) has a direct relationship with pH values, so pKa values can play a significant role in decomposition (thermally, chemically and biochemically), persistence and reactivity of molecules [Citation28–33]. The pKa values depend upon the number of dissociated hydrogen ions, and it is directly proportional to the stability of the molecule (ΔG = 2.303 RT pKa). The reported pKa value of 1 is 6.54 (at nitrogen), 15.73 (at nitrogen) for 3 and −0.58 (at OH near P), 2.95 (at OH of COOH), 8.37 (at OH near P) and 6.34 (at nitrogen) for 2. At acidic pH, the protonated form predominates, while at a higher pH these molecules (1–3) exist in the anionic form. Hence, the electrostatic interaction between the positively charged surface and protonated favours adsorption and increases their removal.
3.2. Experimental cum computational comparative spectroscopic analysis
In spectroscopic analysis, very good agreement between computational and experimental data was observed. The negotiable differences were observed because theoretical calculations were performed in the gaseous phase, while the experimental results were obtained in the solid/liquid phase [Citation32–38]. Excellent results were obtained for UV–visible analysis, where negligible difference was observed between the computational and experimental analysis. The observed difference was 3 nm for molecule 1, 4 nm for molecule 2, 2 nm for molecule 3 and 1 nm for molecule 4 ( and Supplementary figure S4).
Table 1. Details of observed and optimized vibrational frequencies, UV plots and 1H-NMR analysis of titled molecules 1–4.
The comparative FTIR analyses showed that the difference between the computational and experimental analysis was between 71–10 cm−1 {(v(N–H); 71, v(C9O); 50, δ(N–H); 63, v(N–C); 10, v(P9O); 45, δ(C–O); 14 and δ(N–C); 50)} for molecule 1, 56–2 cm−1 {(v(N–H); 25, v(C9O); 56, δ(N–H); 51, v(N–C); 19, v(P9O); 4, δ(C–O); 2 and δ(N–C); 5)} for molecule 2, 22–2 cm−1 {(v(N–H); 19, v(C=O); 2, δ(N–H); 22, v(N–C); 3, v(P9O); 7, δ(C–O); 12 and δ(N–C); 6)} for molecule 3 and 17–3 cm−1 {(v(P9S); 8, v(P–O); 17, δ(C–S); 3, v(C–O); 11 and δ(C–S); 5)} for molecule 4 ( and Supplementary figure S5).
The major differences in the C9O and N–H frequencies of molecules 1 and 2 were observed, which was greater than 40 cm−1. In addition to the above-mentioned fact (computational data in the gaseous phase and experimental in the solid/liquid phase), the other most effective reason behind it was the H-bonding [Citation21–24]. H-bonding was not counted or optimized in computational analysis. Due to the presence of H-bonding, the experimental frequencies were observed at lower wave numbers as compared to computational ones. The higher difference (>20 cm−1) was observed at the N–H frequencies of molecules 3. It was due to the involvement of N–H in resonance with C9O [Citation34–37]. There was less than 20 cm−1 difference observed in the P–O frequency molecule 4. It may be due to the average frequency of two free rotating P–O bonds having different environment.
In addition to FTIR analysis, “Liquid drop on KBr disc”, a shortcut and time-saving method was performed. To check the accuracy, the FTIR data of molecule 3 and 4 were compared with the NIST data (Supplementary figures S6 and S7). It observed that both the spectrums were very similar to each other at observable FTIR bands. Hence, we can apply this method to the FTIR analysis of toxic liquid chemicals including pesticides. This method can avoid consumption of the time to change the liquid sample holder and exposure of toxic pesticide(s). Same time, this method is environment friendly because the liquid sample holder needs two to three drops of sample but one drop is sufficient on the KBr disc. Most importantly, this practice is allowed to avoid the addition of two extra drops of the toxic compound into the environment.
The observable difference between the computational and experimental analysis by 1H-NMR analysis was 0.21–0.03 ppm {(–O–CH3; 0.04), (–CO–CH3; 0.21) and (–S–CH3; 0.03)} for molecule 1, 0.10–0.39 ppm {(–OH; 0.10), (–CH2–CO; 0.39) and (–P–CH2–; 0.30)} for molecule 2, 0.53–0.00 ppm {(–NH–; 0.10), (9CH–; 0.06), (–O–CH3; 0.03), (9C–CH3; 0.53) and (–NH–CH3; 0.00)} for molecule 3 and 0.63–0.02 ppm {(–O–CH2–; 0.32), (S–CH2–S; 0.27), (–S–CH2–; 0.63), (O–CH2–CH3; 0.02) and (S–CH2–CH3; 0.06)} for molecule 4 ( and Supplementary figures S8 and S9). The reasons behind it were very much similar as discussed in the UV and FTIR section. The additional fact was the influence of solvent with molecule(s) [Citation33–38].
3.3. Chemical reactivity
Reactivity of any molecule, especially of the agrochemicals is always an interesting point of chemical study. Based on the HOMO–LUMO energy difference, the order of reactivity was as 4 (5.84 eV) > 2 (10.31 eV) ∼ 3 (10.46 eV) > 1 (12.59 eV). HOMO–LUMO analysis has shown that these molecules are chemically less reactive at RT because all these molecules have a energy gap of more than 1.30 eV [Citation13,Citation14,Citation39]. Often, in organic chemistry, we like to know the “partial” charge on a carbon atom to see if it might be a good nucleophile or the site for electrophilic attack. The charge density is important as these are the electrons most likely to be involved in the chemical reactivity [Citation11–14,Citation18]. The exact order of charge densities at phosphorus (P) atom of titles molecules was 3 (2.094) > 2 (1.970) > 1 (1.857) > 4 (1.797) (). Similarly, the order of charge density at oxygen or sulphur of P9O/P9S was 3 (−1.213) > 1 (−1.187) > 2 (−1.17) > 4 (−0.961) ().
Table 2. Calculated Huckel charged densities of titled molecules.
Huckel charge densities have been considered as useful quantities to illustrate the charge distributions of molecules [Citation2]. Charge distributions can give the information about how the molecules interact with another molecule. The current study has shown that P atoms of all titled pesticides suspected for nucleophilic attack due to positive charge densities and oxygen or sulphur directly attached to the P atom acted as a nucleophile [Citation1–3,Citation40]. This information may help the decomposition mechanism designing processes under acidic (electrophilic or cationic attack (metal ions)) and basic (nucleophilic attack) conditions [Citation40]. The hydrolysis or decomposition of any molecule by biological microorganisms (bacteria, enzyme, fungi, etc.) depends upon the electrophilic and nucleophilic attacks too. The most suspected bonds for the chemical decomposition of titled molecules are P–N bond in acephate, C–N bond glyphosate, P–O bond in monocrotophos and P–S bond in phorate [Citation28–31]. During the bio-decomposition, the most suspected bonds are C–N bond in acephate and glyphosate, P–O bond in monocrotophos and P–S bond in phorate [Citation41–46]. Final products of all processes are phosphate oxides, ammonia and carbon dioxide [Citation28–33].
3.4. Molecular stability of molecules on the basis of computational study
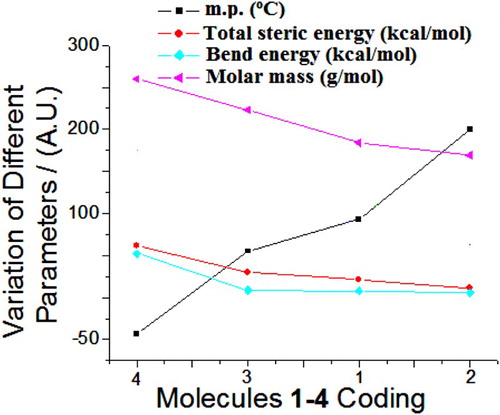
The steric energy parameters (stretch, bend, stretch-bend, torsion, non-1,4 VDW, 1,4 VDW, dipole/dipole and steric energy for frame) of molecules 1–4 were analysed computationally. The exact order of steric energy (kcal/mol) for molecules was as 4 >> 3 > 1 > 2 ( and ). A reciprocal order was analysed with melting points of molecules (). has revealed that molar mass and size of molecules can increase the steric parameters.
Table 3. Calculated steric parameters of molecules 1–4.
3.5. Polarizabilities and the prediction of the biodegradation
Bio-decomposition of the agrochemical has been considered as a huge task. For the last 50 years, it has become a mammoth problem for scientific communities. Computational optimizations of any molecule may assist chemists to design the biodegradation pathway(s) of the agrochemicals. In recent studies, few authors have reported the biodegradation of chemicals on the basis of polarizabilities optimization of molecules [Citation4–12]. In the current study, out of four molecules 1–4, three molecules 1–3 are highly water soluble, so the risk of leaching into the aquatic environment is high if these are not quickly decomposed. The persistence of these toxic molecules in soils is a threat for soil fertility as they may inhibit the growth of beneficial microorganisms of soils [Citation24–31,Citation41–50]. So, parameters including the dipole moment and polarizability of any molecule can play the decisive role for its biodegradation including toxic agrochemicals [Citation47–49].
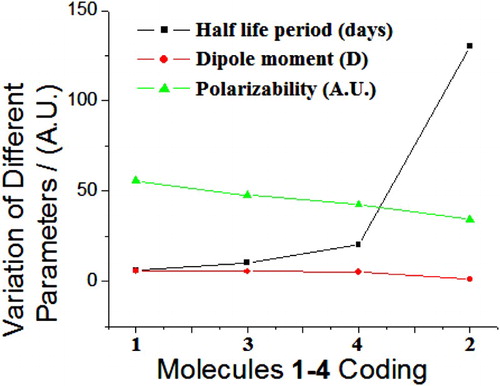
The average half life period of 1 on soil was 0.5–6 days, 5–197 days for 2, 2–10 days for 3 and 3–20 days for 4. These half lives periods of titled molecules were varying reciprocally with computational parameters namely dipole moment and polarizability (). The computed averaged static dipole polarizability and dipole moment of molecules 1–4 were calculated. The component of the polarizability tensor optimized along the z-axis, i.e. long molecular axis. The values of averaged polarizabilities depend upon the positions of substituents bound to the moiety [Citation5–11]. The average polarizability (⟨α⟩) was calculated as per the formula reported in the literature [Citation5–11], i.e. ⟨α⟩ = (αxx + αyy + αzz)/3. On the basis of average polarizability and dipole moment, the order of bio-decomposition was as 1 > 3 >> 4 > 2. This order was in excellent agreement with the experimental decomposition as mentioned in the literature [Citation41–50] ().
In recent studies, it has been analysed that under the recommended dose-level nitrogen containing molecules like glyphosate, monocrotophos and acephate stimulate the soil microbial growth of nitrification bacteria as well as soil fungi [Citation41–46]. Glyphosate breakdown provided N to microbes for microbial activity. But high doses, long-term or repeated use inhibits the count of total fungi, Acremonium strictum, Penicillium glabrum and Aspergillus fumigatus significantly [Citation46]. On the other hand, the presence of phorate at a recommended level significantly decreased dinitrogen fixation [Citation45,Citation46], numbers of Staphylococcus, Micrococcus, Fusarium, Humicola and Rhizopus [Citation46]. Phorate and glyphosate persisted in the rhizospheric soils, the difference is that glyphosate is non-toxic to microorganisms while phorate cause toxicity in short life time [Citation41–50]. The large-scale use of pesticides in crop management systems emphasizes the need to understand their effects on soil microbial communities. The pesticides may change the soil environment due to toxicity to soil microorganisms. The relationships of different structures of pesticides on the growth of various groups of soil microorganisms are not easily predicted. Some pesticides stimulate the growth of microorganisms, but other pesticides have depressive effects or no effects on microorganisms. So, the prediction of biodegradation through the theoretical studies may assist the experimental studies which are also a demand of time.
3.6. Geometric parameters optimization and comparison with literature
The geometric parameters (bond length and bond angles) were performed on the crystalline molecules only. To check the crystallinity, SEM analysis was performed of molecules 1–4. SEM analysis showed that molecules 1 and 2 have a rectangular cube and ice cube like morphology and both were crystalline in nature [Citation51–53]. The morphology (noncrystalline) of 3 and 4 was similar to each other, but quite different form 1 and 2 (Figure S10). Here, bond length and bond angles of the molecules 1 and 2 were optimized and compared with the literature [Citation51]. The optimized theoretical geometric parameters of glyphosate were found to be in good agreement with the corresponding experimental literature data () [Citation5–9]. In literature, not a single study on geometric parameters of molecules 1 is reported till date, so the current study may assist the future study like single crystal analysis of molecule 1 (). All the results on geometric parameters (bond length and bond angles) of crystalline molecules 1 and 2 are tabulated in .
Table 4. Calculated geometric parameters of acephate (molecule 1) and glyphosate (molecule 2) and comparison with literature data of glyphosate.
4. Environmental applicability
These studies are very important, especially with environmental concern and real-world analysis applications. For example, the detection of glyphosate by UV–visible spectrophotometric methods which are reliable and belongs to real-time analysis, interactions of pesticides with the soils and their adsorption on clay and humates [Citation41–50,Citation54–63]. These studies are the foundation of detection of various contaminants and their metabolites through the chromatographic methods in water, soils, fruits, crops, vegetables and other samples [Citation41–46,Citation50–56,Citation64]. The derivatization of glyphosate, acephate and monocrotophos at NH through various molecules (Sanger’s reagent) may be detectable in real time [Citation53–55,Citation64]. In literature, the analysis of the glyphosate has been mentioned, where derivatization done was analysed by UV–visible and HPLC methods only [Citation53–55,Citation64]. These studies are applicable to all the molecules containing secondary amine [Citation60–63]. After the derivatization, UV–visible analysis can be used to detect these molecules from various compartments of the environment [Citation54–63]. In our recent study, we have found that acephate and glyphosate were detectable by UV–visible, FTIR, mass and NMR studies [Citation60,Citation63]. Most importantly, these new derivatives have multiple bioactivities with different stability factors as mentioned in the current study also [Citation60,Citation63]. Glyphosate derivative was prepared after the computational studies which have shown easy biodegradation and low toxicity [Citation63]. Moreover, the detection of pesticides and their metabolites through the spectroscopic methods is still an unexposed area, especially of OPs including the titled molecules, except glyphosate [Citation51–54,Citation64]. At the same time, computational or theoretical analysis may fill the gap, especially to analyse the properties which are experimentally tough to perform. Hence, the calculations of such parameters become equally important or necessity of time achieved through the computational optimization.
5. Conclusion
The main aim of the study to provide all spectroscopic, morphological and computational analysis of widely used OP is achieved. The computational study (in gas-phase, at T = 298.15 K) of titled molecules has been obtained and compared with experimental data. The computational data reported in this work are in good agreement with those determined experimentally, giving enhanced support to the values calculated in this work for the properties without experimental data available in the literature, and suggesting that they can be used in future compilations of various parameters of compounds 1–4. Spectral studies, structures, charge polarization and HOMO and LUMO maps of the compounds 1–4 provided an enhanced knowledge about the reactivity, biological activities and bio-decomposition of these compounds. The slight differences observed between the optimized and experimental values result from the fact that the theoretical calculations were performed for the product in the gaseous phase, while the experimental results were obtained from the solid phase. Hopefully, the current study may assist studies like spectral analysis, pesticide(s) detection in total environment (soil–water–air), surface behaviour and decomposition analysis of top selling titled pesticides of world market. At the same time, computational or theoretical analysis may fill the gap, especially to analyse the properties which are experimentally tough to perform.
6. Safety
Acephate, glyphosate, monocrotophos and phorate are organophosphates that inhibit the activity of cholinesterase. Direct contact with these should be avoided. The work performed with these pesticides in the open should take place in a fume hood using gloves and eye protection.
Supplementry_material.doc
Download MS Word (833.5 KB)Acknowledgements
The authors would like to acknowledge Gautmi Ltd (Andhra Pradesh) for gift samples. SAIF Punjab University, Chandigarh and STIC Cochin are highly acknowledged for their instrumental facilities. The authors would like to acknowledge Director General of CCRAS (New Delhi), for his motivation and kind support. Also, RARIDD, Gwalior, is highly acknowledged for library facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Terry AV, Jr. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Therap. 2012;134:355–365. doi: 10.1016/j.pharmthera.2012.03.001
- Kumar V, Upadhyay N, Wasit AB, et al. Spectroscopic methods for the detection of organophosphate pesticides – a preview. Curr World Environ. 2013;8:313–318. doi: 10.12944/CWE.8.2.19
- Platte F, Michael Heise H. Substance identification based on transmission THz spectra using library search. J Mol Str. 2014;1073:3–9. doi: 10.1016/j.molstruc.2013.12.065
- Kumar V, Upadhyay N, Singh S, et al. Thin-layer chromatography: comparative estimation of soil’s atrazine. Curr World Environ. 2013;8:469–472. doi: 10.12944/CWE.8.3.17
- Gholivand K, Valmoozi AAE, Mahzouni HR, et al. Molecular docking and QSAR studies: noncovalent interaction between acephate analogous and the receptor site of human acetylcholinesterase. J Agric Food Chem. 2013;61:6776–6785. doi: 10.1021/jf401092h
- Long DA. The polarizability and hiperpolarizability tensors. In: Kiefer W, Long DA, editors. Non-linear Raman spectroscopy and its chemical applications. Dordrecht: Reidel; 1982. p. 99–112.
- Ostojic BD, Stankovic B, Ðor-devic DS. Theoretical study of the molecular properties of dimethyl anthracenes as properties for the prediction of their biodegradation and mutagenicity. Chemosphere. 2014;111:144–150. doi: 10.1016/j.chemosphere.2014.03.067
- Ali MMN, Kaliannan P, Venuvanalingam P. Ab initio computational modeling of glyphosate analogs: conformational perspective. Str Chem. 2005;16:491–506. doi: 10.1007/s10224-005-4615-8
- Schmidt MW, Baldridge KK, Boatz JA. General atomic and molecular electronic structure system. J Comput Chem. 1993;14:1347–1363. doi: 10.1002/jcc.540141112
- Young DC. Computational chemistry – a practical guide for applying techniques to real-world problems (electronics). New York (NY): Wiley; 2001.
- Esperdy K, Shillady DD. Simulated infrared spectra of Ho(III) and Gd(III) chlorides and carboxylate complexes using effective core potentials in GAMESS. J Chem Inf Comput Sci. 2007;41:1547–1552. doi: 10.1021/ci010057k
- Kumar V, Upadhyay N. Computational chemistry: a preview of density functional theory. Wilkes100-ICCS. Jalandhar: Elsevier; 2013. p. 504–510.
- Karabacak M, Kose E, Atac A, et al. Experimental (FT-IR, FT-Raman, UV–Vis, 1H and 13C NMR) and computational (density functional theory) studies on 3-bromophenylboronic acid. J Mol Str. 2014;1076:358–372. doi: 10.1016/j.molstruc.2014.07.058
- Pathak SK, Haress NG, El-Emam AA, et al. Structural, spectroscopic (FT-IR, FT-Raman and UV) studies, HOMO–LUMO, NBO, NLO analysis and reactivity descriptors of 2,3 difluoroaniline and 2,4-difluoroaniline. J Mol Str. 2014;1074:457–466. doi: 10.1016/j.molstruc.2014.06.036
- Mastrantonio GE, Erben MF, Della Védova CO. On the conformational behavior of O,O-dimethyl phosphamidothioate (S=P(OCH3)2NH2). J Mol Str. 2005;3:107–113. doi: 10.1016/j.molstruc.2004.09.014
- Prasad R, Upadhyay N, Kumar V. Simultaneous determination of seven carbamate pesticide residues in gram, wheat, lentil, soybean, fenugreek leaves and apple matrices. Microchem J. 2013;111:91–96. doi: 10.1016/j.microc.2012.12.014
- Kaliannan P, Mohamed Naseer Ali M, Seethalakshmi T, et al. Electronic structure and conformation of glyphosate: an ab initio MO study. J Mol Str. 2002;618:117–125. doi: 10.1016/S0166-1280(02)00467-0
- Zhang Y, Peng XH, Chen Y, et al. A first principle study of terahertz (THz) spectra of acephate. Chem Phys Lett. 2008;452:59–66. doi: 10.1016/j.cplett.2007.11.102
- Rifai A, Bourcier S, Jaber F, et al. Structures and dissociation mechanisms of protonated and electron ionized methamidophos. Int J Mass Spectro. 2013;340:7–15. doi: 10.1016/j.ijms.2013.02.003
- Grube A, Donaldson D, Kiely T, et al. Pesticide industry sales and usage: 2006 and 2007 market estimates. Washington (DC): United States Environmental Protection Agency; 2011; http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf.
- Kannappan R, Tooke DM, Spek AL, et al. An alternating chain of spider-like tris(peptides) stabilized by stacking and by N–H⋯N and N–H⋯O=C hydrogen bonding. J Mol Str. 2005;751:55–59. doi: 10.1016/j.molstruc.2005.04.043
- Zhou Y, Zheng YZ, Sun HY, et al. Hydrogen bonding interactions in ethanol and acetonitrile binary system: a near and mid-infrared spectroscopic study. J Mol Str. 2014;1069:251–257. doi: 10.1016/j.molstruc.2014.02.027
- Rawat P, Singh RN. Evaluation of molecular assembly, spectroscopic interpretation, intra-/inter molecular hydrogen bonding and chemical reactivity of two pyrrole precursors. J Mol Str. 2014;1075:462–470. doi: 10.1016/j.molstruc.2014.07.012
- Afzali R, Vakili M, Nekoei A-R, et al. Intramolecular hydrogen bonding and vibrational assignment of 1,1,1-trifluoro-5,5-dimethyl-2,4-hexanedione. J Mol Str. 2014;1076:262–271. doi: 10.1016/j.molstruc.2014.07.059
- Singh N, Khan IM, Ahmad A, et al. Preparation, spectral investigation and spectrophotometric studies of proton transfer complex of 2,2′-bipyridine with 3,5-dinitrobenzoic acid in various polar solvents. J Mol Str. 2014;1066:74–85. doi: 10.1016/j.molstruc.2014.02.017
- Singh N, Ahmad A. Synthesis and spectrophotometric studies of charge transfer complexes of p-nitroaniline with benzoic acid in different polar solvents. J Mol Str. 2014;1074:408–415. doi: 10.1016/j.molstruc.2014.05.076
- Kumar V, Upadhyay N, Kumar V, et al. Interactions of atrazine with transition metal ions in aqueous media: experimental and computational approach. 3Biotech. 2015;5:791–798.
- Das AC, Chakravarty A, Sen G, et al. A comparative study on the dissipation and microbial metabolism of organophosphate and carbamate insecticides in orchaqualf and fluvaquent soils of West Bengal. Chemosphere. 2005;58:579–584. doi: 10.1016/j.chemosphere.2004.07.007
- Kumar V, Upadhyay N, Kumar V, et al. A review on sample preparation and chromatographic determination of acephate and methamidophos in different samples. Arab J Chem. 2015;8:624–631. doi: 10.1016/j.arabjc.2014.12.007
- Kumar V, Upadhyay N, Kumar V, et al. Environmental exposure and health risks of the insecticide monocrotophos – a review. J Bio Env Sci. 2014;5:111–120.
- Ku Y, Lin HS, Wang W, et al. Decomposition of phorate in aqueous solution by ozonation. J Environ Sci Health B. 2009;42:143–149. doi: 10.1080/03601230601123268
- Kumar V, Manhas A. Designing syntheses characterization computational study and biological activities of silver-phenothiazine metal complex. J Mol Str. 2015;1099:135–140. doi: 10.1016/j.molstruc.2015.06.055
- Singh RN, Rawat P, Sahu S. A mixed experimental and DFT study on ethyl 4-[3-(4-dimethylamino-phenyl)-acryloyl]-3,5-dimethyl-1H-pyrrole-2-carboxylate. J Mol Str. 2014;1066:99–107. doi: 10.1016/j.molstruc.2014.02.041
- El-Sheshtawy HS, Baker AMA. Synthesis, structural, theoretical studies and biological activities of 3-(arylamino)-2-phenyl-1H-inden-1-one derivative. J Mol Str. 2014;1067:225–232. doi: 10.1016/j.molstruc.2014.03.042
- Mayer H, Kuckuk R, Heimlich F, et al. Spectroscopic (FT-IR, FT-Raman and NMR) and computational studies on 3-methoxyaniline. J Mol Str. 2014;1057:176–188.
- Kumar V, Chawla M, Cavallo L, et al. Complexation of trichlorosalicylic acid with alkaline and first row transition metals as a switch for their antibacterial activity. Inorganica Chim Acta. 2018;469:379–386. doi: 10.1016/j.ica.2017.08.064
- Singh RN, Rawat P, Sahu S. Synthesis, characterization and computational study on ethyl 4-(3-Furan-2yl-acryloyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate. J Mol Str. 2014;1076:437–445. doi: 10.1016/j.molstruc.2014.07.074
- Sudha S, Sundaraganesan N, Vanchinathan K, et al. Spectroscopic (FTIR, FT-Raman, NMR and UV) and molecular structure investigations of 1,5-diphenylpenta-1,4-dien-3-one: a combined experimental and theoretical study. J Mol Str. 2012;1030:191–203. doi: 10.1016/j.molstruc.2012.04.030
- Aihara J. Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A. 1999;103:7487–7495. doi: 10.1021/jp990092i
- Herschlag D, Jencks WP. Decreasing reactivity with increasing nucleophile basicity. The effect of solvation on β-nuc. for phosphoryl transfer to amines. J Am Chem Soc. 1986;108:479–483. doi: 10.1021/ja00285a010
- Kim K, Tsay OG, Atwood DA, et al. Destruction and detection of chemical warfare agents. Chem Rev. 2011;111:5345–5403. doi: 10.1021/cr100193y
- Lo CC. Effect of pesticides on soil microbial community. J Environ Sci Health Part B. 2010;45:348–359. doi: 10.1080/03601231003799804
- Lancaster SH, Hollister EB, Senseman SA, et al. Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate. Pest Manag Sci. 2010;66:59–64. doi: 10.1002/ps.1831
- Abdel-Mallek AY, Abdel-Kader MIA, Shonkeir AMA. Effect of glyphosate on fungal population, respiration and the decay of some organic matters in Egyptian soil. Microbiol Res. 1994;149:69–73. doi: 10.1016/S0944-5013(11)80139-4
- Gonzalez-Lopez J, Martinez-Toledo MV, Rodelas B, et al. Studies on the effects of the insecticides phorate and malathion on soil microorganisms. Environ Toxicol Chem. 1993;12:1209–1214. doi: 10.1002/etc.5620120709
- Singh BK. Organophosphorus-degrading bacteria: ecology and industrial applications. Nature Rev. 2009;7:156–164.
- Singh S, Singh N, Kumar V, et al. Toxicity monitoring and biodegradation of the fungicide carbendazim. Environ Chem Letters. 2016;14:317–329. doi: 10.1007/s10311-016-0566-2
- Singh S, Kumar V, Chauhan A, et al. Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett. 2017;16:1–27.
- Kumar V, Singh S, Singh J, et al. Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol. 2015;94:807–815. doi: 10.1007/s00128-015-1523-7
- Majid A, Adam A, Refat MS, et al. Spectral, thermal, XRD and SEM studies of charge-transfer complexation of hexamethylenediamine and three types of acceptors: π-, σ- and vacant orbital acceptors that include quinol, picric acid, bromine, iodine, SnCl4 and ZnCl2 acceptors. J Mol Str. 2013;1051:144–163. doi: 10.1016/j.molstruc.2013.08.006
- Sanches EA, Soares JC, Mafud AC, et al. Structural characterization of chloride salt of conducting polyaniline obtained by XRD, SAXD, SAXS and SEM. J Mol Str. 2013;1036:121–126. doi: 10.1016/j.molstruc.2012.09.084
- Krawczykt H, Bartczak TJ. New crystalline polymorphic form of glyphosate: synthesis, crystal and molecular structures of n-(phosphon-o-methyl) glycine. Phosphorus Sulfur Silicon. 1993;82:117–125. doi: 10.1080/10426509308047415
- Qian K, Tang T, Shi T, et al. Residue determination of glyphosate in environmental water samples with high-performance liquid chromatography and UV detection after derivatization with 4-chloro-3,5-dinitrobenzotrifluoride. Anal Chimica Acta. 2009;635:222–226. doi: 10.1016/j.aca.2009.01.022
- Lundgren LN. A new method for the determination of glyphosate and (Aminomethy1)phosphonic acid residues in soils. J Agric Food Chem. 1986;34:3232–3240. doi: 10.1021/jf00069a041
- Rastegarzadeh S, Pourreza N, Larki A. Dispersive liquid-liquid microextraction of thiram followed by microvolume UV-vis spectrophotometric determination. Spectrochim Acta A Mol Biomol Spectrosc. 2013;114:46–50. doi: 10.1016/j.saa.2013.05.020
- Schnurer Y, Persson P, Nilsson M, et al. Effects of surface sorption on microbial degradation of glyphosate. Environ Sci Technol. 2006;40:4145–4150. doi: 10.1021/es0523744
- Piccolo A, Celano G. Hydrogen bonding interactions between the herbicide glyphosate and water-soluble humic sub-stances. Environ Toxicol Chem. 1984;13:1737–1741. doi: 10.1002/etc.5620131104
- Rani R, Juwarkar A. Adsorption of phorate, an organophosphorus pesticide, on vertisol. Arch Environ Contam Toxicol. 2010;58:927–934. doi: 10.1007/s00244-009-9424-6
- Mazzei P, Piccolo A. Quantitative evaluation of noncovalent interactions between glyphosate and dissolved humic substances by NMR spectroscopy. Environ Sci Technol. 2012;46:5939–5946. doi: 10.1021/es300265a
- Kumar V, Kumar V, Kaur S, et al. Unexpected formation of N-phenyl-thiophosphorohydrazidic acid O,S-dimethyl ester from acephate: chemical, biotechnical and computational study. 3 Biotech. 2016;6:1–11. doi: 10.1007/s13205-015-0313-6
- Singh S, Kumar V, Upadhyay N, et al. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech. 2017;7:262. doi:10.1007/s13205-017-0900-9.
- Kaur S, Kumar V, Chawla M, et al. Pesticides curbing soil fertility: effect of complexation of free metal ions. Front Chem. 2017;5:1–9. doi: 10.3389/fchem.2017.00043
- Kumar V, Singh S, Singh R, et al. Design, synthesis, and characterization of 2,2-bis(2,4-dinitrophenyl)-2-(phosphonatomethylamino)acetate as a herbicidal and biological active agent. J Chem Biol. 2017;11:179–190. doi: 10.1007/s12154-017-0174-z
- Qian K, Tang T, Shi T, et al. Solid-phase extraction and residue determination of glyphosate in apple by ion-pairing reverse-phase liquid chromatography with pre-column derivatization. J Sep Sci. 2009;32:2394–2400. doi: 10.1002/jssc.200900118