Abstract
The present study aimed to investigate the neurotoxicity of iron oxide and silver NPs solely or combined. At the molecular level, the exposure to both NPs induced marked DNA fragmentation, downregulation of mtTFA, and upregulation of PGC-1α expression. Both NPs caused decline in acetylcholine esterase, norepinephrine, serotonin, dopamine, and antioxidants enzymes, while causing an increase in lipid peroxidation, nitric oxide, tumour suppressor gene p53, tumour necrosis factor-α, interleukin-6, acetylcholine, and norepinephrine. NPs exposure was associated with severe histologic changes in brain architecture. The effect of the combined exposure to both NPs was more pronounced than each one alone. This study showed that the mechanism of neurotoxicity may involve different pathways including changes in gene expression of mTFA and PGC-1α, induced DNA fragmentation, deregulated neurotransmitters, oxidative stress and disturbed cytokine production and tumour suppressor protein p53.
1. Introduction
Iron oxide nanoparticles (Fe2O3NPs) are one of the nanoparticles (NPs) which are widely used for various biomedical applications, including; targeted delivery and magnetic resonance imaging (MRI). In medical imaging, the application of Fe2O3NPs has increased the resolution range and sensitivity. Even though the significant benefits of Fe2O3NPs, it is important to detect the possible damaging effects on the cell. The toxicity of NPs could be mediated through the oxidative stress and inflammatory response [Citation1]. Singh et al. reported the in vivo oxidative damage induced by Fe2O3NPs. But, the mechanisms of toxicity of Fe2O3NPs exposure remain controversial [Citation2].
Silver nanoparticles (AgNPs) are one of the most nanomaterials used. It is widely used in many medical applications including; retinal therapies, drug delivery in cancer, constituents of dental alloys, burn-related infections, implant surfaces for treating the wound and catheters [Citation3]. Also, AgNPs are incorporated in textile and cosmetics, or used as coatings in various household goods [Citation4]. Due to the wide application of AgNPs in many daily life products, its safety is a growing concern. The generation of reactive oxygen species (ROS) might be involved in the AgNPs-induced toxicity [Citation5]. The diffusion of AgNPs into the cellular membranes may cause proliferation inhibition, proteins and nucleic acids damages, and mitochondrial dysfunction [Citation6]. Genotoxicity and cytotoxicity of AgNPs are evident in both in vitro and in vivo [Citation7]. AgNPs caused inhibition of new DNA synthesis, increased apoptosis, inhibition of cell proliferation, and sever morphological abnormalities [Citation8]. In vitro studies demonstrated that the exposure to AgNPs enhanced ROS production and caused p53-dependent apoptosis, mitochondria damage [Citation9].
So far, from the literature review, the researchers have focused on the toxicity of individual nanoparticles, but there are a few studies on the combination of more than one NPs. Also, the neurotoxicity of the combination of NPs is very little especially in vivo and at the molecular level in the brain. Therefore, the aims of the present study were to (i) identify the neurotoxicity of iron oxide nanoparticles (Fe2O3NPs) and silver nanoparticles (AgNPs) alone or in combination, (ii) assess the effect of Fe2O3NPs, AgNPs and their combination on different pathways that may involved in the neurotoxicity including, the expression of the gene participates in the control of mitochondrial biogenesis and functions namely; mitochondrial transcription factor A (mtTFA) and peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α), induction of cell death, disturbed neurotransmitters and cytokine production, oxidative stress and deregulated tumour suppressor protein p53.
2. Materials and Methods
2.1. Tested compounds and doses
Iron oxide nanoparticles (Fe2O3NPs), Nanopowder >50 nm particle size (TEM) and Silver nanoparticles (AgNPs), Nanopowder >100 nm particle size were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). The hydrodynamic size distribution of each nanoparticle in the aqueous nanoparticles solution was determined by the Dynamic Light Scattering (DLS) process using a Zetasizer Nano ZS from Malvern. The iron oxide nanoparticles and AgNPs were suspended in distilled water by sonication and orally administered to the rats at the dose of 5 and 50 mg/kg BW according to the previous studies of Szalay et al.[Citation10] and Sharma et al.[Citation11]; respectively.
2.2. Animals and experimental groups
Forty adult male Wistar rats weighing 160–170 g and 5–6 months of age were used in the present study. Animals were obtained from Faculty of Medicine, Alexandria University, Alexandria, Egypt. Animals were kept on basal diet and tap water which were provided ad libitum. After two weeks of acclimation, animals were divided into 4 equal groups, 10 rats each. Group 1 served as control, group 2 was administered orally with Fe2O3NPs (5 mg/kg BW; >50 nm), group 3 was treated orally with AgNPs (50 mg/kg BW; >100 nm) and group 4 was orally administered with the mixture of Fe2O3NPs with AgNPs. The doses of iron oxide nanoparticles and silver nanoparticles were treated every day for 79 days.
2.3. Blood samples collection and tissue preparations
At the end of the 79th day of the experimental period, all animals of each group were sacrificed by cervical dislocation. Blood samples were collected in test tubes containing heparin as an anticoagulant and placed immediately on ice. The blood samples were centrifuged at 860×g for 20 min for the separation of plasma. The plasma was kept at −80°C until analyses of the tested parameters. The brain was immediately removed, washed using chilled saline solution (0.9%), removed the adhering fat and connective tissues, and dissected out to obtain cerebral hemispheres. The cerebral hemispheres were divided into different parts; one part was used for DNA isolation for assessment of DNA fragmentation, second part was used for RNA isolation for gene expression analysis, another part was immersed immediately in formalin for histological analysis, the last part of brain was minced and homogenized (10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a Potter-Elvehjem type homogenizer. The homogenates were centrifuged at 10,000Xg for 20 min at 4°C, to pellet the cell debris and the supernatant was collected and stored at −80°C for the determination of tested parameters.
2.4. Body and brain weights
Initial and final body weights were recorded. The brain was immediately removed, washed using chilled saline solution (0.9%), removed the adhering fat and connective tissues, dried on tissue papers and weighed.
2.5. Quantitative analysis of brain gene expression of mitochondrial transcription factor A (mtTFA) and peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α) using quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from brain tissues using GF-1 Total RNA Extraction Kit (Vivantis, Malaysia). ViPrimePLUS One-Step Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Green Master Mix (Vivantis, Malaysia) was used for the relative quantitative determination of the gene expression of mtTFA [Citation12] and PGC-1α [Citation13] at mRNA level using β-actin as a reference gene. The primer sequences used are as follow: β-actin; F: 5′-AGCCATGTACGTAGCCATCC-3′ and R: 5′-CTCTCAGCTGTGGTGGTGAA-3′, PGC-1α; F: 5′-AAACTTGCTAGCGGTCCTCA-3′ and R: 5′-TGGCTGGTGCCAGTAAGAG-3′, and mTFA; F: 5′CCCTGGAAGCTTTCAGATACG-3′ and R: 5′-AATTGCAGCCATGTGGAGG-3′.
2.5. Assay of DNA fragmentation
DNA fragmentation, as a marker of cell death, was assayed according to the method of Miller et al.[Citation14].
2.6. ELISA measurements
Tumour suppressor gene p53, tumour necrosis factor- alpha (TNF-α) and interleukin-6 (IL-6) were assayed by using Enzyme-linked Immunosorbent Assay (ELISA) kits (MyBioSource, San Diego, USA) in brain tissue homogenates according to the manufacturer instructions. Dopamine and serotonin levels and norepinephrine hormone were determined by using a competitive inhibition enzyme immunoassay technique kits for the in vitro quantitative measurement from Cloud-Clone Corp. Houston, USA.
2.7. Markers of oxidative stress and antioxidant parameters
Malondialdehyde (MDA) as a lipid peroxidation index in brain homogenate was assayed as thiobarbituric acid-reactive substances (TBARS) which measured at 532 nm by using 2-thiobarbituric acid (2,6-dihydroxypyrimidine-2-thiol; TBA). TBARS were measured by the method of Tappel and Zalkin[Citation15]. Total antioxidant capacity (TAC) in brain homogenates was assayed by the method of Koracevic et al.[Citation16]. The level of nitric oxide (NO) was assayed by the method of Montgomery and Dymock[Citation17] in brain homogenate. Superoxide dismutase (SOD) activity was measured according to Mishra and Fridovich[Citation18]. SOD was determined and the assay procedure involves the inhibition of epinephrine auto-oxidation in an alkaline medium (pH 10.2) to adrenochrome, which is markedly inhibited by the presence of SOD. Epinephrine was added to the assay mixture, containing tissue supernatant and the change in extinction coefficient was followed at 480 nm in a Spectrophotometer. The activity of glutathione peroxidase (GPx) was assayed by the method of Chiu et al.[Citation19] in brain homogenate. Glutathione S-transferase (GST) activity was determined according to Habig et al.[Citation20]. GST catalyzes the conjugation reaction with glutathione in the first step of the mercapturic acid synthesis. The activity of GST was measured in brain homogenate and p-nitrobenzylchloride was used as the substrate. The absorbance was measured spectrophotometrically at 310 nm using UV-Double Beam Spectrophotometer. Catalase (CAT) activity was determined in brain homogenate using the Luck method involving the decomposition of hydrogen peroxide (Luck)[Citation21]. The CAT activity was measured spectrophotometrically at 240 nm by calculating the rate of degradation of H2O2, the substrate of the enzyme. Reduced glutathione (GSH) content was determined and the method utilized metaphosphoric acid for protein precipitation and5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) for colour development and its density was measured at 412 nm. GSH was determined according to the method of Jollow et al.[Citation22].
2.8. Histopathological examination
Brain specimens were obtained from rats, and immediately fixed in 10% formalin, and then treated with a conventional grade of alcohol and xylol, embedded in paraffin and sectioned at 4–6 μm thickness. The sections were stained with Haematoxylin and Eosin (H&E) stain for studying the histopathological changes (Drury et al.)[Citation23].
2.9. Statistical analysis
Results are reported as means ±SE. Statistical analysis for all studied parameters was performed using the general linear model (GLM) produced by Statistical Analysis Systems Institute (SAS). Duncan’s New Multiple Range Test was used to test the significance of the differences between means [Citation24]. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. DNA fragmentation
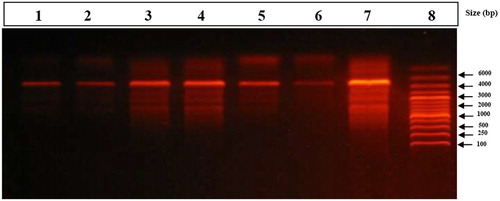
Figure shows the genomic DNA fragmentation in brain tissues of rats treated with Fe2O3NPs and AgNPs or their combination. There are very low or undetectable DNA laddering (DNA fragmentation) in the control rats (Lane 1 & 2). AgNPs alone (Lane 3 & 4) revealed DNA fragmentations which appear as DNA smearing. Fe2O3NPs alone (Lane 5 & 6) causes a mild degree of brain genomic DNA fragmentation. The combination group (Lane 7) showed massive DNA fragmentations which appear as DNA smearing compared to control group. DNA fragmentation was more pronounced in the combination group compared to AgNPs or Fe2O3NPs alone.
Figure 1. Stained agarose gel electrophoresis of genomic DNA of brain. Control group (Lane 1 & 2) showed very low or undetectable DNA laddering (DNA fragmentation) compared to DNA marker (Lane 8, 1 kb). Silver nanoparticles alone (Lane 3 & 4) revealed DNA fragmentations which appear as DNA smearing. Iron oxide nanoparticles (Lane 5 & 6) showed very low brain genomic DNA fragmentation. The combination group (Lane 7) showed massive DNA fragmentations which appear as DNA smearing compared to DNA marker (Lane 8).
3.2. Body and brain weights
As shown in Table , there is no significant difference in the initial body weight at the start of the experiment. Regarding the final body weight and weight gain during the experiment, the rats exposed to NPs are significantly lighter than with significantly lower weight gain and brain weight compared to the control rats. The rats co-exposed to both NPs showed the lowest final body and brain weights and least weight gain (Table )
Table 1. The initial and final body weight, weight gain and brain weights (grams) of male rats exposed to iron oxide nanoparticles (Fe2O3NPs) and/or silver nanoparticles (AgNPs).
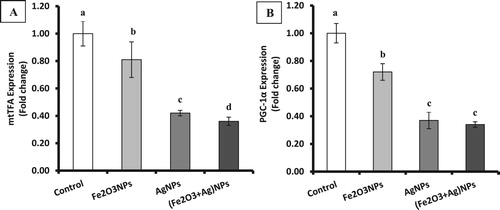
3.3. Brain expression of PGC-1α and mtTFA
The present results showed significant (P < 0.05) suppression of the brain gene expression of mitochondrial transcription factor A (mtTFA) in rats treated with iron oxide nanoparticles, silver nanoparticles or their combination by about 19%, 58% and 64% of control value, respectively (Figure ). Also, the expression of brain peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α) showed a significant decline in the rats treated with Fe2O3NPs, AgNPs or their combination by about 28%, 63% and 66% of control value, respectively. It was clear that the suppression of mtTFA and PGC-1α was more pronounced in AgNPs and combination groups compared with Fe2O3NPs group.
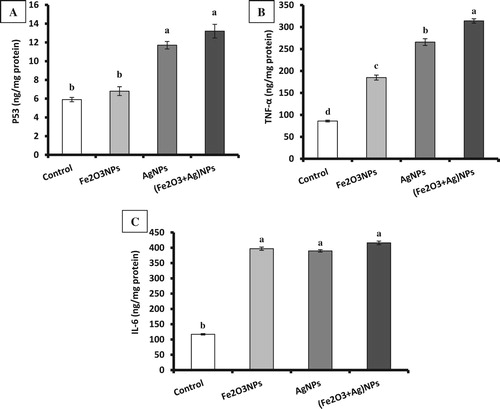
3.4. Plasma P53, TNF-α, and IL-6
Data showed that exposure of rats to Fe2O3NPs or AgNPs result in a significant increase in p53, TNF-α and IL-6 compared to the control group. The co-exposure of rats to Fe2O3NPs and AgNPs showed the more pronounced elevation of p53, TNF-α, and IL-6 compared to each one alone (Figure ).
3.5. Plasma and brain neurotransmitters
The mean values of plasma and brain acetylcholine (ACh), acetylcholine esterase (AChE), dopamine, serotonin and norepinephrine of male rats treated daily with Fe2O3NPs, AgNPs or their combination are presented in (Table ). Treatment with Fe2O3NPs, AgNPs, and their combination resulted in a significant decrease in plasma and brain AChE, dopamine and serotonin compared to control group. On the other hand, acetylcholine and norepinephrine were significantly increased. These results indicated that treatment with Fe2O3NPs, AgNPs, and their combination caused neurotoxicity via decreased AChE, dopamine and serotonin and increased acetylcholine and norepinephrine and this is more pronounced in the combination group compared to Fe2O3NPs and AgNPs alone (Table ).
Table 2. Brain and plasma activities of acetylcholine esterase (AchE) and levels of acetylcholine (Ach), norepinephrine (NE), sertonine, and dopamine in male rats exposed to iron oxide nanoparticles (Fe2O3NPs) and/or silver nanoparticles (AgNPs).
3.6. Oxidative stress parameters and antioxidants
Treatment with Fe2O3NPs, AgNPs, and their combination caused a significant decrease in the brain activities of antioxidant enzymes (SOD, CAT, GPx, GST) and decline in the brain levels of GSH and total antioxidant capacity (TAC); while the levels of TBARS and NO were significantly elevated compared to control group. The effect of the combination of Fe2O3NPs with AgNPs was more pronounced than each one alone (Table ).
Table 3. Brain levels of thiobarbituric acid-reactive substances (TBARS), nitric oxide (NO), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPX) and total antioxidant capacity (TAC) in male rats exposed to iron oxide nanoparticles (Fe2O3NPs) and/or silver nanoparticles (AgNPs).
3.7. Histopathological examination
Brain histology and quantitative analysis of male rats treated with Fe2O3NPs, AgNPs and the mixture of Fe2O3NPs and AgNPs was shown in Figure and Table . Microscopic examination of brain sections of control group reveals normal brain tissues formed of glial, pyramidal cells and purkinj’s fibres showing no pyknosis and no vacuolization. The brain section of Fe2O3NPs group revealed moderate neuronal degeneration, pyknosis of the nuclei with pericellular edema, dilation of blood vessels and vacuolization as compared to the control group. The brain sections of AgNPs nanoparticles revealed mild neuronal degeneration, pyknosis of the nuclei with pericellular edema, dilation of blood vessels and vacuolization as compared to the control group. The brain sections of the mixture of Fe2O3NPs and AgNPs revealed moderate to marked neuronal degeneration, pyknosis of the nuclei with pericellular edema, dilation of blood vessels and vacuolization as compared to control, Fe2O3NPs and AgNPs groups. It was pronounced that the histological alteration of the combination group was more than each of them alone.
Figure 4. Light micrograph of brain of male rats. Control group (a) showed normal glial. Iron oxide nanoparticles group (b) revealed pericellular edema, dilation of blood vessels, vacuolization and pyknosis of nuclei. Silver nanoparticles group (c) revealed mild neuronal degeneration and pyknotic nuclei, pericellular edema, dilation of blood vessels and neuronal vacuolization. Combination group of iron oxide nanoparticles and silver nanoparticles (d) revealed neuronal degeneration and pyknotic nuclei, pericellular edema, dilation of blood vessels and neuronal vacuolization (H & E; X 40).
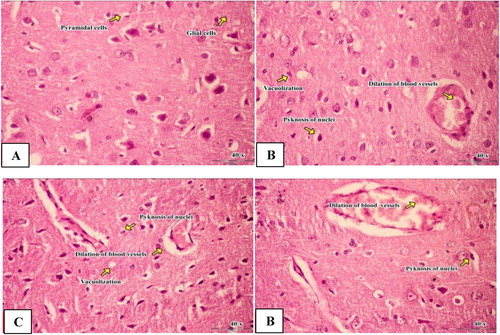
Table 4. Quantitative analysis of brain histology of male rats exposed to iron oxide nanoparticles (Fe2O3NPs) and/or silver nanoparticles (AgNPs).
4. Discussion
The neurotoxicity of Fe2O3NPs and AgNPs was ranging from the direct effects on antioxidant enzyme activities and on neurotransmitters and cytokines to the suppressive effects on the expression of genes encoding PGC-1α and mTFA proteins which controlling mitochondrial biogenesis and functions. Also, the neurotoxicity effects through changing the architecture of the tissues and the integrity of cellular genome by induction of cytotoxic effects. The brain is a highly metabolic tissue with intense demand for mitochondria especially for neurons in the central nervous system [Citation25]. Mitochondrial biogenesis plays an essential role in maintaining mitochondrial homeostasis to meet the physiological needs of neuronal cells. The factors regulating mitochondrial biogenesis include mitochondrial transcription factor A (mtTFA), which enhance the transcription and replication of mtDNA. The expression of mtTFA is regulated by peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α), the master regulator of mitochondrial biogenesis [Citation26].
In the present study, the decreased expression of mtTFA and PGC-1α in brain tissues of rats treated with AgNPs and Fe2O3NPs may indicate a decreased mitochondrial biogenesis and mtDNA replication and transcription that may lead to mitochondrial dysfunction. It was reported that the exposure of rats to AgNPs results in accumulation of the NPs in different organs including the brain and inducing blood–brain barrier and neuronal destruction [Citation27]. Also, in line with our data, AgNPs exposure leads to Ag+ accumulation in the rodent brain, altering the expression of genes involved in neuronal function in the neuronal cell line [Citation28]. Generally, most molecules cannot cross the blood–brain barriers (BBB), as BBB is a tight barrier to protect the brain from xenobiotics penetration. However, NPs with varying particle sizes can overcome BBB and enter into the brain, or enter into the brain through the nerve endings of the olfactory bulb [Citation29]. The suppressive effect of AgNPs on the brain gene expression of mtTFA and PGC-1a may postulate that these NPs have the ability to pass through BBB to reach neuron cells to cause a direct consequence of oxidative stress and increased free radicals and lipid peroxide which confirmed in this study [Citation30].
DNA fragmentation at the internucleosomal level is a hallmark feature of apoptotic cell death to produce a laddering pattern. In necrosis, after cell rupture, DNA fragmentation is a later phenomenon, endonucleases and proteases degraded the chromatin into a smear pattern instead of a ladder pattern since the histones are destroyed by the proteases and expose the entire length of DNA to the nucleases [Citation31]. It was clear from the obtained data that DNA fragmentation showed a mixed pattern of smearing and “laddering” of DNA fragments which might be due to the random DNA fragmentation process encountered with apoptosis. The present results clearly showed that silver NPs produce more prominent fragmentation of DNA than iron oxide NPs in the brain. The genotoxic effects of iron oxide NPs may result from the direct action of leached iron ions from iron oxide NPs or the indirect action of excessive ROS [Citation32]. These effects of iron oxide NPs on DNA can affect the DNA structure (oxidation of nucleotides, crosslinking, and strand breaks) as well as DNA replication and transcription (as evidenced in the present study).
The present study showed that AgNPs and Fe2O3NPs treatment caused inhibition in the activities of antioxidant enzymes, reduced the levels of glutathione and increased lipid peroxidation level in the brain tissues which may imply enhanced generation of ROS. These ROS are highly reactive and directly involved in the oxidative stress to cellular proteins and DNA [Citation33], and induction of necrotic and/or apoptotic cell death [Citation2]. Another important target of AgNP is the mitochondrial electron transport chain, the inhibition of complex II and IV by AgNPs caused accumulation of electrons which escape and directly react with oxygen to form the superoxide anion radical [Citation34]. It was documented that, the release of free iron ion as a result of lysosomal degradation of iron oxide NPs affects the iron homeostasis [Citation35]. The free iron is then stored in ferritin and hemosiderin causing iron overload which triggers the production of the ROS through catalyzing Fenton reaction when the iron storage capacity of these proteins is exceeded [Citation36].
Nanoparticles may affect the behaviour as it caused a decrease in the levels of neurotransmitters like Dopamine (DA) in exposed animals [Citation37]. The present results reported that used nanoparticles cause disruptions in the brain neurotransmitter. Effect of AgNPs on neurotransmitters may be due to reduced the amplitude of voltage-gated ionized sodium current, which may result in a decrease in intracellular Na+ concentration as a result of decreased Na+ influx, which plays a key role in the transport of neurotransmitters including serotonin, norepinephrine, and dopamine [Citation38]. This result is in agreement with our results which showed a decrease in neurotransmitters and increase in noradrenaline concentration.
Nanoparticles exposure stimulates the cells to secrete higher levels of the pro-inflammatory cytokine, TNF-α. Overproduction of ROS activates cytokines and upregulates IL-6 and TNF-α as indicators of pro-inflammatory signalling processes as a counter-reaction to oxidative stress [Citation39]. The present study showed that iron oxide NPs, AgNPs and their combination caused a significant increase in cytokines (IL-6, P53 and TNF-α) in brain tissues. As stated above the toxicity of NPs was associated with increasing intracellular ROS levels which resultant oxidative stress and may triggers the pro-inflammatory mediators in both in vivo and in vitro [Citation40]. It is well known that, the oxidative stress causes the activation of principal cascades of inflammation such as the phosphoinositide 3-kinase (PI3-K), mitogen-activated protein kinase (MAPK), and NF-κB pathways [Citation41]. Derstjerna et al. reported that AgNPs were found localized in the developing CNS which may interfere with the complex events responsible for normal CNS development [Citation42]. Also, exposure to AgNPs caused cell death, disturbed cell proliferation, inflammatory response, and oxidative stress mechanisms. AgNPs are able to pass the intact BBB causing oxidative stress associated with typical pathological signs such as inflammation. Camilla et al. found histological alteration in brain section after exposure to AgNPs. The study indicated a neuronal degeneration with dilation of blood vessels and pericellular edema [Citation43].
5. Conclusion
The present study showed that Fe2O3NPs and AgNPs alone or in combination caused neurotoxicity through epigenetic changes in the gene expression of mtTFA, and PGC-1α that may result in mitochondrial dysfunctional which accelerate the generation of ROS and oxidative stress. These effects are associated with impaired antioxidant defense systems and disturbed cytokines production and accelerated cell death through apoptotic and necrotic cell death as indicated by the pattern of DNA fragmentation. Also, changes in neurotransmitters and histology in the brain were found. This study found also that concomitant exposure of both the NPs caused more damage to the brain when compared with the individual NP alone.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Abdelsalam Abdalla Abuzreda http://orcid.org/0000-0002-6179-5703
Maher Abd EL-Nabi Kamel http://orcid.org/0000-0002-6791-9850
References
- Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomedicine. 2015;10:1727–1741.
- Singh N, Jenkins GJ, Asadi R, et al. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1(1):5358.
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12.
- Wei LY, Lu JR, Xu HZ, et al. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20:595–601.
- AshaRani PV, Low Kah Mun G, Hande MP, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–290.
- Roy R, Kumar S, Tripathi A, et al. Interactive threats of nanoparticles to the biological system. Immunol Lett. 2010;158(1):79–87.
- Mathias FT, Romano RM, Kizys MM, et al. Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology. 2015;9(1):64–70.
- Miethling-Graff R, Rumpker R, Richter M, et al. Exposure to silver nanoparticles induces size- and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol in Vitro. 2014;28(7):1280–1289.
- Shi J, Sun X, Lin Y, et al. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials. 2014;35(24):6657–6666.
- Szalay B, Tátrai E, Nyírő G, et al. Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments. J Appl Toxicol. 2012;32(6):446–453.
- Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145(1):83–96.
- Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281(1):324–333.
- Li L, Pan R, Li R, et al. Mitochondrial biogenesis and peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity. Diabetes. 2011;60(1):157–167.
- Miller SA, Dykes DD, Polesky HFRN. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215.
- Tappel AL, Zalkin H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch Biochem Biophys. 1959;80:333–336.
- Koracevic D, Koracevic G, Djordjevic V, et al. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361.
- Montgomery HAC, Dymock JF. The determination of nitrate in water. Analyst. 1961;86:414–416.
- Mishra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Chiu DTY, Stults FH, Tappel AL. Purification and properties of rat lung soluble glutathione peroxidase. Biochimica et Biophysical Acta. 1976;445:558–566.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139.
- Luck HC. In: Bergmeyer MV, editor. Method of enzymatic analysis. Verlag chemic. 1974. Academic Press, New, 885.
- Jollow DJ, Michell JR, Zampaglionic N, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169.
- Drury RA, Wallington EA, Carleton S. Histological techniques. 5th ed. London (UK): Oxford University Press; 1980, 241–242.
- Duncan DB. Multiple ranges and multiple F tests. Biometrics. 1955;11(1):1–42.
- Wang B, Fengr W, Zhu Yun M, et al. Neurotoxicity of low-dose repeatedly intranasal instillation of nano- and submicron-sized ferric oxide particles in mice. J Nanopart Res. 2009;11(1):41–53.
- Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124.
- Tang JL, Xiong L, Wang S, et al. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol. 2009;9(8):4924–4932.
- Wijnhoven SW, Peijnenburg WJ, Herberts CA, et al. Nanosilver- a review of available data and knowledge gaps in humanand environmental risk assessment. Nanotoxicol. 2009;3:109–138.
- Koziara JM, Lockman PR, Allen DD, et al. The blood–brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6:2712–2735.
- Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG, Slikker W Jr. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci. 2010;118:160–170.
- Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996;10(5):587–597.
- Mesarosova M, Kozics K, Babelova A, et al. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol Lett. 2014;226:303–313.
- Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells gene. 2004;337:1–13.
- Ribeiro MP, Santos AE, Custódio JB. Mitochondria: the gateway for tamoxifen-induced liver injury. Toxicology. 2014;323:10–18.
- Voinov MA, Pagán JO, Morrison E, Smirnova TI, Smirnov AI. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc. 2011;133:35–41.
- Valdiglesias V, Kilic G, Costa C, et al. Effects of iron oxide nanoparticles: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ Mol Mutagen. 2015;56:125–148.
- Siddiqi NJ, Abdelhalim MA, El-Ansary AK, et al. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflammation. 2012;9:123.
- Safari M, Arbabi Bidgoli S, Rezayat SM. Differential neurotoxic effects of silver nanoparticles: A review with special emphasis on potential biomarkers. Nanomed J. 2015;3(2):83–94.
- Jia'en-Li J, Muralikrishnan S, Ng CT, et al. Nanoparticle-induced pulmonary toxicity. Exp Biol Med. 2010;235:1025–1033.
- Roco MC. National nanotechnology initiative, past, present, future. In: Handbook on nanoscience, engineering and technology. 2nd ed. Oxford (UK): Taylor and Francis Group; 2007, p. 3–26.
- Poljak-Blaži M, Jaganjac M, Žarković N. Cell oxidative stress: risk of metal nanoparticles. London (UK); New York (NY): CRC Press Taylor; 2010.
- Derstjerna ES, Johansson F, Klefbohm B, et al. Gold- and silver nanoparticles affect the growth characteristics of human embryonic neural precursor cells. PLOS ONE. 2013;8(3):e58211.
- Camilla R, Silvia B, Simona A, et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol. 2016;13:12.