?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The current study focuses on optimizing, characterizing, and purifying a thermostable amylase from thermophilic bacteria Geobacillus icigianus (BITSNS038). The amylase was produced optimally at 18–24 h of incubation in the presence of starch and tryptone at 7.5 and 3.0 g/L, respectively (pH 7.0, 70°C, and 150 rpm). Km, Vmax values for starch were 2.17 mg/mL and 4.16 U/mL respectively. The enzyme showed excellent thermal stability at 70°C, retaining 62.5% residual activity after 8 h and a reduced deactivation rate constant (kd) of 0.001. When tested for its efficacy in starch hydrolysis, it showed 34.5% hydrolysis of corn starch slurry and antibiofilm activity, but no antimicrobial activity was noticed. The ultrafiltration led to partial purification of amylase showed 2.67-fold purification with a molecular weight in the range of 45 and 66 kDa. This is probably the first-ever report on a thermostable amylase from G. icigianus and its extensive potential uses.
1. Introduction
Amylases are glycoside hydrolases that cleave α-1, 4-glycosidic linkages of polysaccharides such as starch to liberate various simple sugars like maltose and glucose [Citation1]. Amylases are among the oldest and pivotal industrial biocatalysts, comprising approximately 30% of the global enzyme market. It finds major commercial applications in the starch processing industry (starch liquefaction and saccharification), baking, pulp, and paper industry, fruit juice clarification, detergents, desizing of textiles, and distilling industries [Citation2,Citation3]. Apart from conventional usage, amylases have been lately explored as an effective antibiofilm agent, thus paving the way towards developing biofilm infection treatment strategies [Citation4,Citation5]. Also, the recent fabrication of amylase-based bioconjugates has marked advancement towards robust biocatalysis in pharmaceutical applications [Citation6].
When the processes in starch industries are to be considered, the high thermostability of amylase has always been imperative. It is because these industrial processes have a requirement of high temperature such as hydrolysis of gelatinized starch (60°C–80°C); liquefaction and saccharification of starch (95°C–110°C); production of dextrose syrup (90°C–100°C) etc [Citation7–10]. To date, scientists have acquired very few maltooligosaccharide-forming amylases (MFAses) for the industrial production of maltooligosaccharides. Moreover, the low thermostability of the MFAs makes them inappropriate for the industrial process of sugar production from starch at high temperatures [Citation11]. However, with the aid of a thermostable enzyme, starch processing can be carried out at relatively lower temperatures, such as starch slurry processing and saccharification, which is carried out at a comparatively lower temperature of 50°C–60°C and pH around 6.5 [Citation12]. Much better results were obtained when starch hydrolysis was carried out at 60°C–70°C by thermostable α-amylases [Citation13]. Several bacterial species produce thermostable amylases; however, the usage of thermophiles has few added advantages to the process. They are great candidates for metabolic engineering and the production of highly stable industrial products. The growth and production at high temperatures limit the scope of contamination, thereby simplifying the industrial processes and making them economically viable. Also, the saccharification processes are carried out at high temperatures, and the use of thermophiles as a thermostable amylase source makes them an ideal candidate in many industrial applications as they provide a broad temperature range and possess high thermal stability. This also has an additional advantage in recombinant development as the sudden variation of temperatures does not easily affect the thermophiles [Citation14,Citation15]. Therefore, screening microorganisms with the ability to secrete novel amylases with enhanced stability at extreme temperatures is necessary [Citation16]. Another aspect is to find out the production of amylase under optimized conditions. Apart from the conventional method of One Factor at A Time (OFAT), factorial designs have been used to optimize the production of amylase nano powder [Citation17]. Thermostability, pH stability, and long-term stability are significant desired characteristics for the amylases to be potentially applicable in various processing industries [Citation18]. Hence, more inputs are being given towards the search for new strains with the desired properties [Citation19]. The genera Geobacillus have always been of biotechnological interest due to its ability to secrete thermostable enzymes [Citation20], and its potential is still to be studied. In the context of this background, the present work was focused on optimizing various bioprocess conditions for enhanced production of amylase from Geobacillus strain and kinetic and thermostability characterization of crude amylase enzyme. The efficiency of amylase in the process of starch saccharification and its antimicrobial and antibiofilm properties was also checked.
2. Materials and methods
2.1 Microorganism, medium, and culture conditions
The novel amylase-producing bacterial strain (BITSNS038) was isolated from a mud sample of hot spring Surajkund, Jharkhand, India and was identified as Geobacillus icigianus by 16S rRNA analysis [Citation21]. A loopful of active culture was used to inoculate 50 mL of production medium and cultivated at 70°C, pH 7.0, and 150 rpm. The amylase production medium consisted of (g/L): Yeast extract 3, Tryptone 3, NaCl 1, MgSO4·7H2O 0.2, and K2HPO4 1 [Citation22]. The medium was supplemented with 1% starch as an inducer. The control flasks contained all medium constituents except the inoculum were kept under the same experimental conditions.
2.2 Study of growth profiling for amylase production
A study on growth profiling for enzyme synthesis was investigated up to 72 h of incubation under agitated conditions. One milliliter of the sample from the enzyme production medium was drawn aseptically at different time intervals (0–72 h) and checked for the colony-forming unit (CFU/mL as growth). Cell biomass has been reported in terms of total viable cell count as CFU/mL. Total viable cell count was determined by serial dilution and plate counting method [Citation22]. 0.05 mL of different inoculum dilutions were spread plated on nutrient agar plates and incubated for 24 h at 70°C. Henceforth, the microbial concentration of the inoculum was determined in CFU/mL. The rest of the sample was processed by centrifuging at 4°C, 10,000 rpm for 10 min. Cell-free supernatant was analyzed for amylase assay.
2.3 Determination of amylase activity
The activity of amylase produced by Geobacillus icigianus was estimated by determining the starch hydrolysis rate. Standard amylase activity assay protocol was followed where 0.5 mL of the suitably diluted enzyme was added to a reaction mixture containing 0.5 mL of the substrate (1% (w/v) starch in 0.1 M sodium phosphate buffer, pH 7.0) and incubated at 70°C for 30 min. The reaction was stopped by centrifuging the reaction mixture and adding 500 μL of alkaline copper tartrate reagent to the supernatant of the reaction mixture. It was boiled for 10 min, and then 500 μL of arsenomolybdate reagent was added to it. The optical density of the reaction product (maltose) was measured at 620 nm, and the amount of reducing sugar was estimated to a standard curve of maltose using the Nelson-Somogyi method [Citation23,Citation24]. One unit of amylase activity is shown as a unit (U) defined as a microgram of maltose released/mL/min under the standard conditions.
2.4 Optimization of culture conditions using one factor at a time (OFAT) approach
2.4.1 Effect of pH and different carbon sources on amylase production
The pH of the medium was varied in the range 4.0–10.0. Un-inoculated media was taken as control. The amount of starch concentration was fixed as 10 g/L. In order to study the effect of different carbon sources, the starch in the production medium was substituted by glucose, maltose, lactose, sucrose, xylose, fructose, glycerol, corn starch in the concentration of 10 g/L. The incubation conditions were 70°C, 150 rpm for 18–24 h.
2.4.2 Effect of starch and tryptone concentration on amylase production
To optimize starch concentration on amylase production, its concentration in the production medium was varied from 5.0–12.5 g/L, maintaining other medium constituents constant. Inoculated media without starch was used as a control. The medium was incubated at 70°C, pH 7.0, 150 rpm for 18–24 h, and cell-free supernatant was used for amylase assay. Similarly, tryptone concentration was varied from 1.0–5.0 g/L under the same operating conditions, and its effect on the amylase production was determined.
2.4.3 Study on kinetic modeling for biomass and product formation
Evaluation of cell growth and product formation was done and simulated with the predicted data of amylase yield by G.icigianus. The kinetics of G.icigianus was studied using a logistic model, i.e. Velhurst-Pearl equation was applied to determine biomass (X) (in terms of viable cells as CFU/mL) and enzyme activity/product (P) formation [Citation25].
2.5 Characterization of crude amylase
2.5.1 Effect of pH on enzyme activity
To determine the effect of different pH on amylase activity, the reaction mixture was incubated at 60°C and 70°C for 30 min using pH values in the range from 5.0 to 9.0. Different buffer solutions with variation in pH prepared for amylase assay were: sodium acetate (pH 5.0 and 6.0), sodium phosphate (pH 7.0), and Tris-HCl (pH 8.0 and 9.0) all at 0.1 M.
2.5.2 Effect of temperature and thermostability studies
Effect of different temperatures on amylase activity was performed by incubating the reaction mixture for 30 min at different temperatures of 30°C, 40°C, 50°C, 60°C, 70°C, and 80°C keeping other parameters constant, and the enzyme activity was recorded. The thermal stability was also assayed at 60°C and 70°C, respectively, for different time intervals (0–9 h), keeping other parameters constant. After the incubation, the enzyme activity was evaluated. Thermostability parameters, i.e. deactivation rate constant (kd) and half-life (t1/2) were determined.
2.5.3 Study of kinetic properties of amylase
For the kinetic study, increasing starch concentration from 0.1% to 1% (w/v) was mixed with a fixed enzyme concentration, and the reaction mixture was kept for incubation at optimal conditions (pH 7.0, 30 min, 70°C) followed by enzyme assay. The kinetic parameters, Km and Vmax for amylase was determined by constructing the Lineweaver–Burk plot.
2.6 Statistical analysis
All the experiments were carried out in triplicates for the significance of the data. The product and biomass formation trends were subjected to analysis of variance (ANOVA) as a single factor with an alpha value of 0.05 (95% confidence). The pH variation, carbon sources, tryptone, starch concentration, the effect of different temperatures on amylase activity were subjected to single-factor ANOVA analysis, whereas the effect of pH on amylase activity was analyzed via 2way ANOVA using Graphpad Prism 7.0.
2.7 Determination of α and β amylase from crude supernatant
To determine the type of amylase in crude supernatant, the reaction mixture was incubated for 30 min at 70°C and pH 6.9 for α amylase activity and pH 4.8 for β amylase activity, respectively. The assay was performed according to the method described by Bernfeld [Citation26].
2.8 Applications based studies of amylase
Studies related to the applications of the crude amylase were also evaluated for its saccharification, antimicrobial, and antibiofilm activity.
2.9 Saccharification assay of corn starch
The amylase produced was tested for starch hydrolysis. Slurry of 10% (w/v) corn starch was prepared in 0.1 M sodium phosphate buffer (pH 7.0). Later, 0.5 mL of this slurry was taken as substrate and treated with 0.5 mL of the enzyme at 70°C. The reaction mixture was incubated for different time intervals (1–3 h) for observing the process of starch saccharification [Citation27].
The % saccharification of starch was calculated using the following formula:
(1)
(1)
where H1 is the amount of reducing sugar in the supernatant after enzymatic hydrolysis and H0 is raw starch before the reaction [Citation28].
2.10 Antimicrobial activity of amylase
Using the agar well diffusion method, the crude and partially purified amylase were tested for their efficacy as an antibacterial and antifungal agent. The amylase antibacterial activity was determined against the Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) and Gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli). Similarly, antifungal activity was tested against common plant pathogens such as Sclerotium sp. and Rhizoctonia sp. The 6-mm wells were punched in the plates (nutrient agar plates and potato dextrose agar plates) using a sterile borer. An amount of 100 µL of the enzyme was loaded into the punctured wells. Plates were incubated at 37°C, 24 h for antibacterial activity, and at 28°C, 5–6 days for antifungal activity. Inhibitory observations were made after that.
2.11 Antibiofilm activity of amylase
Antibiofilm activity of amylase was determined against the following biofilm-forming bacteria: Bacillus subtilis, Staphylococcus aureus, and Bacillus sp. (50°C isolates). The 6-mm wells were punched in the nutrient agar plates using a sterile borer. 40 µL of the sample was loaded into the wells, and the plates were incubated at 37°C and 50°C (for Bacillus sp.) for 24–48 h. Observations were made after that for the presence or absence of antibiofilm activity.
2.12 Determination of protein content
Protein content in the amylase was quantified using the Bradford method. Bradford reagent was added to the samples and incubated at room temperature for 10 min. Absorbance was taken at 595 nm using a UV-Vis spectrophotometer. A standard curve of BSA (Bovine Serum Albumin) was plotted, and the protein concentration was determined in the sample.
2.13 Partial purification and molecular weight determination of amylase
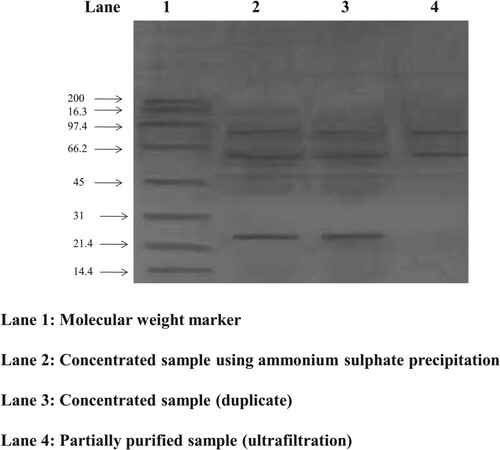
Partial purification of amylase was carried out using a combination of ammonium sulfate precipitation (80%–95% saturation) and buffer exchange with a membrane of 10 kDa (Ultra-filtration) followed by estimation of amylase and protein concentration. The molecular weight of the enzyme was determined using SDS-PAGE. 10% of the SDS gel was prepared according to the standard protocol (Laemmli, 1970), and the samples were loaded onto the wells [Citation1,Citation29]. The molecular weight of the enzyme was determined against a standard pre-stained marker of the range 10–200 kDa. The gel was run against 100 V for 60 min. Coomassie Brilliant Blue (CBB, R-250) staining was performed with 0.4% of CBB in 10% of glacial acetic acid and 40% of methanol in de-ionized water.
3. Results and discussion
Our previous study, Soy et al. [Citation21], isolation and preliminary study on enzyme screening, revealed that the novel isolate G. icigianus (BITSNS038) is thermophilic and amylolytic. Amylase activity without optimization of production conditions was observed as 0.81 U/mL.
3.1 Evaluation of growth kinetic profile of Geobacillus icigianus (BITSNS038)
The bacteria were cultivated in an amylase production medium supplemented with 1% (w/v) starch, and the kinetic growth profile for the production of both cell biomass and amylase were analyzed at different time intervals and shown in Figure .
Figure 1. Growth kinetic profile for amylase production and cell biomass by strain G. icigianus BITSNS038. Results represented as Mean ± standard deviation of triplicates.
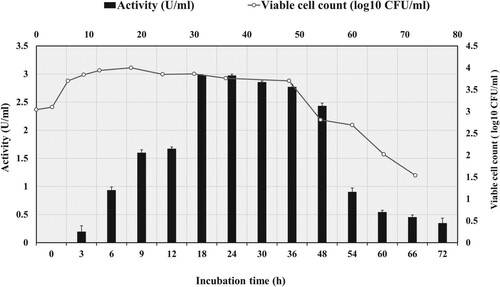
It was observed from Figure that amylase activity increased with incubation time, and maximum amylase (2.983 U/mL) production took place after 18 h, beyond which there was no further increase in amylase activity. Although there was a gradual decline in amylase activity after 24 h, the production was more or less stable between 18 and 48 h. Thus, the incubation period of 24 h was optimized and used for other optimization processes. On the further increase of incubation time, however, there was no increment in amylase production, perhaps due to cells entering the decline phase, which might be due to exhaustion of nutrients or release of toxic by-products in the production medium later stage. The lowest amylase activity (0.349 U/mL) was observed at 72 h.
It was also observed that maximum cell growth occurred after 18 h of growth profiling. Biomass has been reported in terms of total viable cell count as logCFU/mL. The growth profile (Figure ) indicated that the production of amylase enzyme was growth associated [Citation30]. In general, extracellular α-amylases from microbial sources are secreted as primary metabolites, and their production is growth associated [Citation31,Citation32].
In the present study, maximum amylase production was achieved at 18 h, suggesting the rapid production of amylase compared to the previous reports on amylase production from Geobacillus sp. nov as 24 h at 70°C, G. thermoparaffinivorans(CB-13) 36 h at 60°C, and G. thermoleovorans 24 h at 65°C respectively [Citation27,Citation33,Citation34].
3.2 Evaluation of kinetic parameters
The current study has undertaken an unstructured, dynamic model for thermostable amylase production and biomass formation by G. icigianus. A non-structured kinetic model comprises a substrate (S), biomass (X), and product (enzyme) formation (P). Microbial growth is represented by a sigmoidal curve which Monod or logistic models can illustrate.
3.3 Study of biomass
Biomass formation was quantified at different time intervals in the amylase production medium. The maximum specific growth rate (µmax) was determined using Velhurst-Pearl Logistic equation for microbial growth analysis.
(2)
(2)
where Xm is maximum biomass production. μmax was determined during the exponential phase and calculated from the plot between ln (X/X0) and t.
Integrating (2) with X = X0 and t = 0, the following equation is achieved.
(3)
(3)
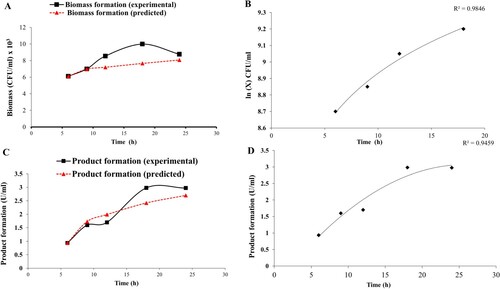
By putting the values in Equation (3), the predicted values were obtained. Experimental and predicted values were then analyzed. The pattern observed revealed that predicted and the experimental values were in good coherence (Figure (A)). A high R2 value of 0.984 (Figure (B)) further confirmed the suitability of the model.
Figure 2. (A) Comparative graph showing experimental and predicted values of biomass formation of G. icigianus (B) Plot ln(X) vs. t showing variation of biomass formation in batch culture during the exponential phase of bacterial growth curve (C) Comparative graph showing experimental and predicted values of product formation (amylase activity) by G. icigianus (D) Regression analysis for product formation at t = 0, i.e. beginning of exponential phase
3.4 Study of product formation
Product formation (enzyme activity) was evaluated using a logistic equation, which in its integrated form is as follows
(4)
(4)
where Pm is the maximum product formed, P0 is the initial product formed in the exponential phase of bacterial growth, and µPm is the maximum specific product formation rate.
The required values were incorporated in the equation, and the predicted value of product formation was determined. From the observed trend, it was evident that both predicted and experimental responses were in good accord with significant precision and reliability (Figure (C)). The R2 value of 0.945 suggested accuracy and confirmed that the logistic model befitted accurately (Figure (D)).
Various kinetic parameters namely µmax (0.041/h), Xm (9.99 × 103 CFU/mL), and µPm (0.122/h) recorded are tabulated in Table .
Table 1. Optimization condition achieved for each parameter after applying OFAT.
Thus, the studies confirmed that the Velhurst-Pearl equation was the most fitting equation that adequately explained both cell growth and product formation.
3.5 Optimization of culture conditions
Amylase is an important industrial enzyme with numerous applications. To meet the demands, maximum production of amylase is a necessity. Moreover, amylase production by microbes is significantly affected by physicochemical parameters of the medium [Citation35]. Hence, optimization of the production process is critical not only for enhanced production but also economically viable. In the present study, we have used the classical OFAT approach to optimize process conditions in thermostable amylase production.
3.6 Effect of pH on amylase production
Among all physicochemical factors, the pH of the growth medium plays a pivotal role in enzyme production [Citation36]. Thus, the effect of varying pH in enzyme production medium on amylase synthesis was investigated, and the result is depicted in Figure (A).
Figure 3. (A) Effect of different pH on amylase production. (B) Effect of different carbon sources on amylase production. (C) Effect of different concentrations of starch as carbon source on amylase production. (D) Effect of different concentrations of tryptone as nitrogen source on amylase production. Results represented as Mean ± standard deviation of triplicates. Statistical data represented as Punnet chart where ns represents no significant difference.
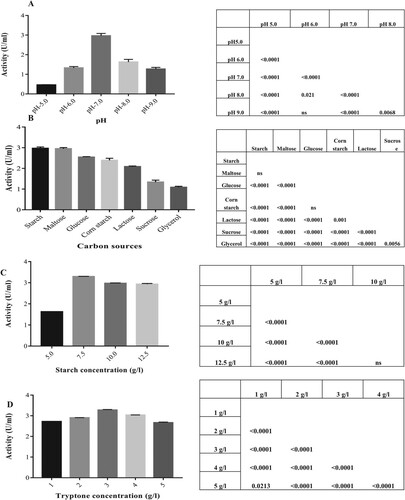
The study on optimization of pH showed that a pH value of 7.0 in the production medium was most suitable for maximum amylase production (2.962 U/mL) from G. icigianus. There was no growth in the medium of pH 4.0 and 10.0. Every enzyme has its pH optima, at which bonds within enzyme are influenced by H+ and OH− ions resulting in enzyme active site most complementary to the substrate and hence, enhanced reaction. A shift in pH value from optima results in inactive sites, which is least complementary to the substrate, resulting in a decline in the activity [Citation37]. Many studies on α-amylase production from Bacillus strains have pH optima as 6.0–7.0 for growth and amylase secretion [Citation16,Citation38], which further validates our result where maximum activity was obtained at neutral pH. Similar observations have also been reported by Acer et al. [Citation39]; El-Kady et al. [Citation40].
3.7 Effect of different carbon sources on amylase production
The effect of different carbon sources in enzyme production medium on amylase synthesis was investigated, and the result is depicted in Figure (B).
Optimizing various carbon sources showed that starch in the production medium was the most suitable carbon source for maximum amylase production (2.97 U/mL) from G. icigianus as producing microorganisms. Slightly less activity was observed in maltose (2.95 U/mL) and glucose (2.54 U/mL) than starch [Citation41,Citation42]. It was also observed that in comparison to starch, corn starch, lactose, and glycerol supported low amylase activity (Figure (B)). No growth was observed in the case of fructose and xylose. Results obtained were in accordance with previous reports [Citation40,Citation43] which showed maximum yield of amylase with soluble starch and then from maltose. Narang and Satyanarayana obtained similar optimum results, where the strain utilized starch, glucose, maltose, lactose, and maltodextrin [Citation44]. Several studies also state that carbon source preferred for enhanced amylase production is rice flour, starch, maltose, glucose, and fructose [Citation45–47]. Thus, the present strain can utilize whole carbon sources for amylase production, having starch as a preference. Starch is a ubiquitous carbon source as compared to maltose, thus, asserting economic production of amylase.
3.8 Effect of starch and tryptone concentration on amylase production
The effect of varying starch concentrations in enzyme production medium on amylase secretion was investigated, and the result is depicted in Figure (C).
The study of optimization of starch concentration showed that 7.5 g/L of starch in the production medium was most suitable for inducing maximum amylase production (3.29 U/mL) from G. icigianus (Figure (C)). On increasing the starch concentration up to 1% and beyond, a gradual reduction in the enzyme production was observed, exhibiting 2.92 U/mL of amylase activity which was constant even on further increment in starch (12.5 g/L).
Usually, amylase production is inducible in nature [Citation32], although few α-amylase productions having constitutive nature exist [Citation48]. According to the results obtained (Figure (C)), there was a significant increment in enzyme synthesis with increasing starch concentration (0.5%–0.75%). It justifies the inductive nature of amylase production from the strain. Moreover, G. icigianus could synthesize amylase only in the presence of starch in the fermentation medium (compared with control), which further validated the inducible nature of amylase production. However, higher starch concentration did not enhance the production; instead, there was a decline, and then a constant trend was observed in the activity (1% and above). It might be due to the rapid hydrolyzation of starch leading to the yield of maltose and glucose, known for feedback inhibition/catabolite repression. Another reason could be the high viscosity of fermentation broth due to the increased concentration of starch. It might have led to the interference of dissolved O2 transfer, resulting in decreased growth and lower amylase activity.
Similarly, the effect of varying concentrations of nitrogen source (tryptone) in fermentation medium on amylase production was studied Figure (D).
The study showed that 3 g/L of tryptone was optimum to enhance amylase production (3.28 U/mL). Ozdemir and co-workers reported lower amylase production (1.06 U/mL) using 10 g/L tryptone, while Afrisham and co-researchers reported high amylase production using ammonium chloride (inorganic nitrogen source) [Citation49,Citation50].
Thus, from the optimization results, it was observed that amylase activity of G. icigianus was elevated from 0.81 U/mL (non-optimized condition) to 3.29 U/mL; almost a 4-fold increment was achieved. Kikani and co-workers [Citation51] reported optimization of thermostable amylase production using response surface methodology; a 3-fold increment was reported. However, in the current report, 4.03-fold elevations in thermostable amylase activity have been achieved after OFAT optimization only with crude enzyme, giving an upper hand to the present report. Ravindran et al. [Citation52] reported a 1.3-fold increase in thermostable amylase activity post-optimization, significantly less than presented in the current report.
Table summarizes the optimum conditions achieved for the maximum production of amylase from G. icigianus.
3.9 Biochemical characterization of crude amylase
3.9.1 Effect of temperature on amylase activity
The effect of different temperatures on amylase activity was ascertained by incubating an enzyme–substrate reaction mixture at different temperatures. The results showed that activity increased sharply with an increase in incubation temperature up to 70°C, recordings a maximum activity of 3.29 U/mL (Figure (A)).
Figure 4. (A) Effect of different temperatures on amylase activity. Statistical data represented as Punnet chart where ns represents no significant difference (B) Effect of pH on amylase activity (C) Study on thermal stability of amylase at 60°C and 70°C. Results represented as Mean ± standard deviation of triplicates (D) Plot of ln (At/A0) vs. t for the determination of deactivation rate constant (Kd) (E) Lineweaver-Burk plot for the determination of Km and Vmax (F) Determination of α and β amylase in crude supernatant. Results represented as Mean ± standard deviation of triplicates.
Further increase in incubation temperature above optimal decreased the enzyme catalysis, which may be due to the denaturation of the enzyme. At 80°C, 48% amylase was active. From (Figure (A)), it was also observed that the amylase is active at a broad range of temperature (50°C–70°C). Maximum enzymes from hyperthermophilic sources have optimal activity at temperatures close to the host organism growth temperature, usually (70°C–125°C) [Citation53]. It further validates our study since G. icigianus showed optimum growth temperature at 70°C and exhibited optimum amylase activity at 70°C. This leads to conclusive evidence of the excellent thermostability of the enzyme. Similar studies by Ravindran et al. [Citation52] and Finore et al. [Citation54] have been reported on amylase activity with temperature optima of 70°C from B. stearothermophilus and Geobacillus thermoleovorans subsp. stromboliens is subsp. nov. respectively.
3.9.2 Effect of pH on amylase activity
Various factors govern enzyme catalysis. One such factor is reaction medium pH influencing the biocatalysis process. Thus, every enzyme has an optimum pH for maximum activity. The pH activity profile (5.0-9.0) of amylase secreted from the selected strain is shown in Figure (B).
The observation of Figure (B) revealed that the amylase secreted by G. icigianus exhibited a broad range of activity in both acidic and alkaline pH. At 60°C, the enzyme was stable at pH (5.0–7.0), and there was a gradual decline at alkaline pH. Comparatively, at 70°C gradual increase in amylase activity was observed with an increase in pH, which dropped rapidly at pH 8.0 and 9.0. The enzyme was more stable at pH 5.0–7.0 at both temperatures. Maximum activity of 3.23 and 3.32 U/mL was obtained at pH 7.0 and temperatures of 60°C and 70°C, respectively. The results obtained were consistent with previous reports [Citation55,Citation56]. However, thermostable amylase activity has also been reported at 65°C and pH 9.0 [Citation57].
3.10 Study on the thermal stability of amylase
The thermal stability of amylase was evaluated at 60°C and 70°C, respectively, as shown in Figure (C). In this process, pre-incubation of the crude enzyme was carried out for 6 h at 60°C and 8 h at 70°C, respectively. Posteriorly pre-incubation, residual amylase activity was determined. The original enzyme activity at (t = 0 min) was taken as 100%.
It is evident from Figure (C) that the enzyme was indeed stable for a longer duration at both temperatures. At 70°C, amylase almost retained its original activity for 120 min (99.52% activity retained). Although there was a drop in the stability trend with further incubation, the enzyme showed stability and retained 62.5% residual activity after 480 min. At 60°C, amylase could retain its original activity for only 15 min after that declining trend observed with 60.72% residual activity after 360 min (Figure (C)). The enhanced thermostability at 70°C might be due to heat-induced accessory proteins contributing towards enzyme stability which got inactivated at a lower temperature of 60°C. Another reason could be the presence of peptidase in crude enzymes, causing hydrolysis of amylases and compromising final amylolytic activity. Maybe peptidases are active at 60°C, and they are denatured at 70°C. Thus, our crude amylase possesses good thermostability features at 70°C compared to other reported studies on amylase. Acer et al. [Citation39] and Özdemir and co-workers [Citation58] reported 60% (60°C–120 min) and 62% (50°C–720 min) residual activity respectively. Another study by Fincan and Enez [Citation59] reported 50% residual activity (70°C–120 min, pH7.0) of amylase from G. stearothermophilus. In contrast, Du et al. [Citation10] reported residual activity of 86.87% at 65°C and 79.34% at 70°C, respectively, after 42 h.
3.11 Determination of thermostability attributes kd and t1/2 of amylase
As discussed previously, the amylase exhibited better thermostability at 70°C as; hence, thermostability parameters kd and t1/2 werefurther calculated and analyzed to determine its practicability and adaptability.
The thermal deactivation of the enzyme is a first-order unimolecular irreversible reaction [Citation60]. The kinetic decay in enzyme activity is expressed by an exponential equation given as
(5)
(5)
where At is activity measured at time t of heat treatment, A0 is initial activity at t = 0 min, and kd is deactivation rate constant.
kd was determined from the plot of ln[At/A0] vs. time (min) as shown in Figure (D). Moreover, t1/2 of the enzyme is inversely proportional to kd.
Thus,
(6)
(6)
From the plot ln[At/A0] vs. time (min) (Figure (D)), the slope value kd was determined as 0.001, and t1/2 calculated was 11 h 55 min. The values of kd and t1/2 have been tabulated in Table . The enzyme's improved half-life (11 h 55 min) reflected a lesser deactivation rate constant (0.001). It can be ascertained that the amylase from G.icigianus is considerably stable at a high temperature of 70°C, indicating its inherent structural stability. Ait Kaki El-Hadef El-Okki et al. [Citation61] reported amylase stability up to a temperature of 90°C. However, in terms of half-life, only around 165 min and 105 min of half-life were observed at temperatures 80°C and 90°C, respectively. Gandhi et al. [Citation62] reported t1/2 of 88 min at 60°C of a purified recombinant amylase which is much lesser than the present report. In another study, Kikani et al. [Citation51] reported t1/2 of 13 h at 70°C from A. rupiensis TS-4 at 70°C.
Table 2. Kinetic and thermostability parameters evaluated experimentally.
3.12 Determination of kinetic parameters of amylase
The effect of varying concentrations of a substrate on amylase activity from G. icigianus was investigated. The Km and Vmax were calculated for starch as substrate by Lineweaver–Burk plot (Figure (E)). The observed Km and Vmax values were 2.17 mg/mL and 4.16 U/mL. The values have been tabulated in Table .
3.13 The activity of α and β amylase in crude supernatant
The activity of α and β amylase from G. icigianus was assayed using crude supernatant. The activity of α amylase (3.1 U/mL) was more as compared to β amylase (1.96 U/mL) as observed in Figure (F).
3.14 Hydrolysis of corn starch slurry by crude amylase
The enzymatic saccharification of raw starch without heating has gained importance in recent years [Citation63]. Amylases that can hydrolyze starch at high temperature and low pH are profoundly required in starch saccharification processes for various syrups and bioethanol production [Citation64]. It is because of the viscosity issue, which is persistent in starch hydrolysis and can only be mitigated by operating the process at an elevated temperature [Citation51].
To determine the feasibility of amylase produced from G. icigianus in the starch saccharification process, corn starch slurry 10% (w/v) was treated with crude amylase at 70°C for a different time interval (1–3 h). Mimicking the industrial conditions, the incubation period was not extended beyond 3 h though the starch slurry concentration was taken as 10% instead of 30% (w/v). The maximum degree of hydrolysis for corn starch was calculated as 34.5% after 2 h, and the degree of hydrolysis was almost stable even on further incubation (Figure ).
Figure 5. %Hydrolysis of corn starch. Results represented as Mean ± standard deviation of triplicates.
From the above result, it can be concluded that the crude amylase of G. icigianus exhibited efficient hydrolysis as compared to previous studies [Citation65,Citation66], which report hydrolysis of 36.7% at 50°C and 30.8% at 65°C respectively after 4 h using purified amylase. Contrasting results were reported by Sudan et al. [Citation27], where 40.1% of corn starch hydrolysis was obtained using purified enzyme at 80°C, for 2 h. In the industrial process, the actual pH of the starch slurry is 4.5 [Citation28]. It is well documented that the gelatinization of most of the starch takes place at (60°C–80°C) [Citation67]. Thus, amylase from the Geobacillus strain could be a potential candidate since it exhibited better hydrolytic activity at 70°C. It is stable at a pH range of 5.0–7.0 and temperature of (50°C–70°C). Its application will eliminate the gelatinization step, thus reducing increased energy consumption and the overall cost of the saccharification process.
3.15 Antimicrobial and antibiofilm activity of amylase
The results of antimicrobial activity of crude and partially purified amylase can be depicted from the figures (Figures and ). The non-formation of the inhibition zone against the bacterial and fungal strains suggests that the amylase from G. icigianus does not have bactericidal and fungicidal properties. Kalpana and co-workers [Citation68] have also reported similar findings. A report from Elamary and Salem suggested that purified amylase had no significant bactericidal or antibacterial properties [Citation69]. Similarly, there have been reports of antifungal activity of amylase, but no report seems to address the antifungal activity of thermostable amylase [Citation70]. Contrastingly, Ahmad and co-workers suggest that alpha-amylase-assisted titanium dioxide nanoparticles have good bactericidal properties [Citation71].
Figure 6. Agar well diffusion assay revealing the lack of antibacterial activity of amylase enzyme on (A) E. coli (B) P. aeruginosa (C) B. subtilis and (D) S. aureus. Wells E and C contained 100 µL of partially purified and crude amylase enzyme, respectively.
Figure 7. Agar well diffusion assay depicting no antifungal activity of amylase on (A) Sclerotium sp. and (B) Rhizoctonia sp.
Biofilms are the layers of exopolysaccharides that protect the bacteria from thermal shock, antibiotics, and enzyme degradation. The development of biofilms creates complex issues in industrial processes and medical procedures, which has developed a need for compounds responsible for the degradation of these biofilms, i.e. antibiofilm properties. In the case of antibiofilm activity, it is evident from the figure (Figure ) that amylase has a significant antibiofilm activity which is in accord with the previous reports [Citation68,Citation69,Citation72]. However, the reports do not discuss the antibiofilm activity of thermostable amylase, which provides an upper hand to the current report. It is also evident from the results (Figure ) that the amylase could disrupt the EPS synthesis of biofilm-forming bacteria.
Figure 8. Antibiofilm activity of partially purified and crude amylase enzyme against two strains of EPS producing Bacillus sp. designated as (A) BITSNS010 (B) BITSNS018.
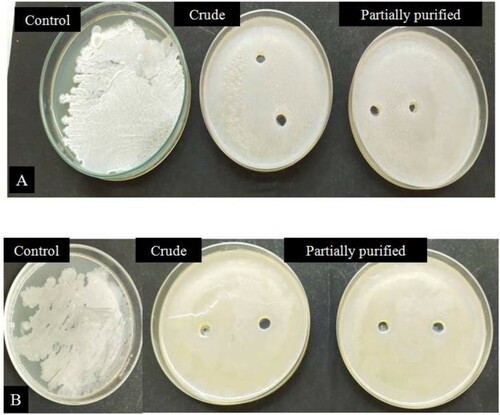
EPS assists in the formation of biofilm matrix and serves as a protective barrier sheathing bacterial cells [Citation5]. It is therefore vital to disintegrate EPS, and only then the antimicrobial agents will be able to penetrate and disrupt the biofilm formation hence targeting pathogenesis. Recently, enzymes have demonstrated efficient degradation of the EPS of the biofilms [Citation73]. Therefore, they can serve as potent biofilm inhibitors. Also, since the significant antibiofilm activity was observed with crude amylase and partially purified amylase (Figure ), it can be expected that antibiofilm activity will increase by several folds once the enzyme is completely purified.
3.16 Partial purification and SDS-PAGE analysis of amylase
The SDS-PAGE of the partially purified enzyme from G. icigianus is represented in Figure ; the prominent band can be observed between 45 and 66 kDa compared to the standard protein marker. The activity profiling of different purification steps revealed a 2.7 folds purification of amylase (Table ). The result has been consistent with the earlier reports on the purification of amylase [Citation69]. Most amylases also showed a mass of about 44 kDa during the purification process [Citation74].
Table 3. Partial purification of amylase from G. icigianus (BITSNS038).
Researchers have documented several reports on thermostable amylase from thermophiles, its characteristics, and applications, but this is probably the first report on the production and characterization of thermostable amylase from G. icigianus. A few attributes of our amylase makes it unique from other reported studies (Table ).
Table 4. The comparison of the present research reports with the present work on amylase along with their applications.
Aladejana and co-workers reported thermostable alpha-amylase from Bacillus subtilis with pH and temperature optima as 8.0°C and 60°C, respectively [Citation77]. More thermotolerant amylase with optimum activity at 70°C and pH 7.0 has been reported in the present work. Amylase from Aeribacillus pallidus was reported by Timilsina et al. [Citation75], and they utilized the purified enzyme for the liquefaction of starch from algal biomass. The optimum activity was achieved at 70°C and pH 7.0; the results were in agreement with the present work. Fincan and co-workers reported thermostable alpha-amylase from Bacillus licheniformis showing highest production at 36 h and pH 6.0. The purified enzyme was used for raw starch treatment [Citation13]. However, in the present work, antibiofilm activity was studied with the partially purified enzyme giving an advantage to the report. Thermostable alpha-amylase from Geobacillus sp. with optimum activity at 60°C and pH 7.0 was reported [Citation78]. The results were in good agreement with the present work (Table ).
4. Conclusion
There has always been a need for robust amylase as the industrial processes pose several limitations such as thermostability, degradation profile, and activity. The present work has targeted some of the issues such as thermostability, efficacy in saccharification, and antibiofilm activity of amylase. The study is the first-ever report on the amylase production from Geobacillus icigianus, which is thermostable (>70°C). The enzyme showed significant activity even at the temperatures of 80°C and 90°C. The amylase exhibited promising antibiofilm and saccharification activity. A saccharification activity of 34.5% was observed against corn starch slurry. It was also able to degrade EPS formation by biofilm-forming bacteria at 50°C. All these findings are indicative of the potential of amylase as a commercial enzyme. The ammonium sulfate precipitation and subsequent purification by dialysis led to the purification of the enzyme by 2.67-fold. However, the optimum conditions achieved (Starch 7.5 g/L, Tryptone 3 g/L, pH 7.0, incubation period 18–24 h) needs to be optimized at the reactor level for scale-up and further studies on amylase nanoparticles conjugates to target a broader range of biofilm-forming pathogenic microbes.
Acknowledgements
The authors acknowledge the Department of Biotechnology, Government of India, for providing DBT-JRF fellowship to Snehi Soy. The authors acknowledge Dr. Pragya Prakash, Application Scientist, Thermofisher Scientific, and Bishwajit Singh Kapoor, Research Scholar, National Institute of Technology, Durgapur, for valuable suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Paul JS, Beliya E, Tiwari S, et al. Production of biocatalyst α-amylase from agro-waste ‘rice bran’ by using Bacillus tequilensis TB5 and standardizing its production process. Biocatal Agric Biotechnol. 2020;26:101648.
- Pinjari AB, Kotari V. Characterization of extracellular amylase from Bacillus sp. strain RU1. J Appl Biol Biotechnol. 2018;6:29–34.
- Vaikundamoorthy R, Rajendran R, Selvaraju A, et al. Development of thermostable amylase enzyme from Bacillus cereus for potential antibiofilm activity. Bioorg Chem. 2018;77:494–506.
- Ashok C, Palanimuthu D, Selvadurai SD, et al. An apodictic review on recent approaches in enzyme technology. Biointerface Res Appl Chem. 2021;12:3446–3471.
- Jee SC, Kim M, Sung JS, et al. Efficient biofilms eradication by enzymatic-cocktail of pancreatic protease type-I and bacterial α-amylase. Polymers. 2020;12:3032.
- Abdel-Mageed HM, Radwan RA, AbuelEzz NZ, et al. Bioconjugation as a smart immobilization approach for α-amylase enzyme using stimuli-responsive Eudragit-L100 polymer: a robust biocatalyst for applications in pharmaceutical industry. Artif Cell Nanomed Biotechnol. 2019;47:2361–2368.
- Gomes I, Gomes J, Steiner W. Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: production and partial characterization. Bioresour Technol. 2003;90:207–214.
- Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresour Technol. 2003;89:17–34.
- Turner P, Mamo G, Karlsson EN. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact. 2007;6:9.
- Du R, Song Q, Zhang Q, et al. Purification and characterization of novel thermostable and Ca-independent α-amylase produced by Bacillus amyloliquefaciens BH072. Int J Biol Macromol. 2018;115:1151–1156.
- Pan S, Gu Z, Ding N, et al. Calcium and sodium ions synergistically enhance the thermostability of a maltooligosaccharide-forming amylase from Bacillus stearothermophilus STB04. Food Chem. 2019;283:170–176.
- Wu X, Wang Y, Tong B, et al. Purification and biochemical characterization of a thermostable and acid-stable alpha-amylase from Bacillus licheniformis B4-423. Int J Biol Macromol. 2018;109:329–337.
- Fincan SA, Özdemir S, Karakaya A, et al. Purification and characterization of thermostable α-amylase produced from Bacillus licheniformis So-B3 and its potential in hydrolyzing raw starch. Life Sci. 2021;264:118639.
- Zeldes BM, Keller MW, Loder AJ, et al. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front Microbiol. 2015;6:1209.
- Kherouf M, Habbeche A, Benamia F, et al. Statistical optimization of a novel extracellular alkaline and thermostable amylase production from thermophilic Actinomadura keratinilytica sp. Cpt29 and its potential application in detergent industry. Biocatal Agric Biotechnol. 2021;35:102068.
- Gupta R, Gigras P, Mohapatra H, et al. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38:1599–1616.
- Abdel-Mageed HM, Fouad SA, Teaima MH, et al. Optimization of nano spray drying parameters for production of α-amylase nanopowder for biotheraputic applications using factorial design. Dry Technol. 2019;37:2152–2160.
- Dheeran P, Kumar S, Jaiswal YK, et al. Characterization of hyperthermostable α-amylase from Geobacillus sp. IIPTN. Appl Microbiol Biotechnol. 2010;86:1857–1866.
- Ghorbel RE, Maktouf S, Massoud EB, et al. New thermostable amylase from Bacillus cohnii US147 with a broad pH applicability. Appl Biochem Biotechnol. 2009;157:50–60.
- Lebre PH, Aliyu H, De Maayer P, et al. In silico characterization of the global Geobacillus and Parageobacillus secretome. Microb Cell Fact. 2018;17:156.
- Soy S, Nigam VK, Sharma SR. Cellulolytic, amylolytic and xylanolytic potential of thermophilic isolates of Surajkund hot spring. J Biosci. 2019;44:124.
- Fossi BT, Tavea F, Fontem LA, et al. Microbial interactions for enhancement of α-amylase production by Bacillus amyloliquefaciens 04BBA15 and Lactobacillus fermentum 04BBA19. Biotechnol Rep. 2014;4:99–106.
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380.
- Shao Y, Lin AH. Improvement in the quantification of reducing sugars by miniaturizing the Somogyi-Nelson assay using a microtiter plate. Food Chem. 2018;240:898–903.
- Prakash P, Singh HR, Jha SK. Production, purification and kinetic characterization of glutaminase free anti-leukemic L-asparaginase with low endotoxin level from novel soil isolate. Prep Biochem Biotech. 2019;22:1–2.
- Bernfeld P. Amylase alpha and beta. Methods Enzymol. 1955;1:149–158.
- Sudan SK, Kumar N, Kaur I, et al. Production, purification and characterization of raw starch hydrolyzing thermostable acidic α-amylase from hot springs, India. Int J Biol Macromol. 2018;117:831–839.
- Mehta D, Satyanarayana T. Biochemical and molecular characterization of recombinant acidic and thermostable raw-starch hydrolysing α-amylase from an extreme thermophile Geobacillus thermoleovorans. J Mol Catal B Enzym. 2013;85–86:229–238.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Santos EO, Martins MLL. Effect of the medium composition on formation of amylase by Bacillus sp. Braz Arch Biol Technol. 2003;46:129–134.
- Passos ML, Ribeiro CP. Innovation in food engineering: new techniques and products. Boca Raton, FL: Polish Society of Microbiologists; 2009.
- Abou MD, El-Sayed AK, El-Fallal AA, et al. Production and partial characterization of high molecular weight extracellular α-amylase from Thermoactinomyces vulgaris isolated from Egyptian soil. Pol J Microbiol. 2011;60:65–71.
- Ulya M, Oesman F, Iqbalsyah TM. Low molecular weight alkaline thermostable α-amylase from Geobacillussp. nov. Heliyon. 2019;5:e02171.
- Quiñonez DE, Llanos AZ, Cotrina DC, et al. Producción de amilasas de Geobacillus themoparaffinivorans (CB-13) aisladas de los géiseres de candarave, tacna. Cienc Desarro. 2019;24:38–44.
- Sen SK, Dora TK, Bandyopadhyay B, et al. Thermostable alpha-amylase enzyme production from hot spring isolates Alcaligenes faecalis SSB17 – statistical optimization. Biocatal Agric Biotechnol. 2014;3:218–226.
- Ashger M, Asad MJ, Rahman SU, et al. A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J Food Eng. 2007;79:950–955.
- Demirkan E, Sevgi T, Başkurt M. Optimization of physical factors affecting the production of the α-amylase from a newly isolated Bacillus sp. M10 strain. Karaelmas Sci Eng J. 2017;7:23–30.
- Ravindar DJ, Elangovan N. Molecular identification of amylase producing Bacillus subtilis and detection of optimal conditions. J Pharm Res. 2013;6:426–430.
- Acer Ö, Bekler FM, Pirinççioğlu H, et al. Purification and characterization of thermostable and detergent-stable alpha-amylase from Anoxybacillus sp. AH1. Food Technol Biotechnol. 2016;54:70–77.
- El-Kady EM, Asker MS, Hassanein MS, et al. Optimization, production, and partial purification of thermostable α-amylase produced by marine bacterium Bacillus sp. NRC12017. IJPCR. 2017;9:558–570.
- Morkeberg R, Carlsen M, Nielsen J. Induction and repression of α-amylase production in batch and continuous cultures of Aspergillus oryzae. Microbiology. 1995;141:2449–2454.
- Ouattara HG, Reverchon S, Niamke SL, et al. Regulation of the synthesis of pulp degrading enzymes in Bacillus isolated from cocoa fermentation. Food Microbiol. 2017;63:255–262.
- Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, et al. Solid culturing of Bacillus amyloliquefaciens for α-amylase production. Food Technol Biotechnol. 2006;44:269–274.
- Narang S, Satyanarayana T. Thermostable alpha-amylase production by an extreme thermophile bacillus thermooleovorans. Lett Appl Microbiol. 2001;32:31–35.
- Prakash B, Vidyasagar M, Madhukumar MS, et al. Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem. 2009;44:210–215.
- Sharma A, Satyanarayana T. High maltose-forming, Ca2+-independent and acid-stable α-amylase from a novel acidophilic bacterium, Bacillus acidicola. Biotechnol Lett. 2010;32:1503–1507.
- Rahmati P, Sajedi RH, Zamani P, et al. Allosteric properties of Geobacillus maltogenic amylase. Enzym Microb Technol. 2017;96:36–41.
- Rao JLUM, Satyanarayana T. Improving production of hyperthermostable and high maltose-forming α-amylase by an extreme thermophile Geobacillus thermoleovorans using response surface methodology and its applications. Bioresour Technol. 2007;98:345–352.
- Afrisham S, Badoei-Dalfard A, Namaki-Shoushtari A, et al. Characterization of a thermostable, CaCl2-activated and raw-starch hydrolyzing alpha-amylase from Bacillus licheniformis AT70: production under solid-state fermentation by utilizing agricultural wastes. J Mol Catal B: Enzym. 2016;132:98–106.
- Ozdemir SC, Cihan AC, Kilic T, et al. Optimization of thermostable alpha-amylase production from Geobacillus sp. D413. J Microbiol Biotechnol Food Sci. 2016;6:689–694.
- Kikani BA, Kourien S, Rathod U. Stability and thermodynamic attributes of starch hydrolyzing α-amylase of Anoxybacillus rupiensis TS-4. Starch-Stärke. 2020;72:1900105.
- Ravindran R, Williams GA, Jaiswal AK. Evaluation of brewer's spent grain hydrolysate as a substrate for production of thermostable α-amylase by Bacillus stearothermophilus. Bioresour Technol Rep. 2019;5:141–149.
- Vieille C, Zeikus GJ. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 2001;65:1–43.
- Finore I, Kasavi C, Poli A, et al. Purification, biochemical characterization and gene sequencing of a thermostable raw starch digesting α-amylase from Geobacillus thermoleovorans subsp. stromboliensis subsp. nov. World J Microbiol Biotechnol. 2011;27:2425–2433.
- Mollania N, Khajeh K, Hosseinkhani S, et al. Purification and characterization of a thermostable phytate resistant α-amylase from Geobacillus sp. LH8. Int J Biol Macromol. 2010;46:27–36.
- Noshadi N, Mohammadi M, Najafpour GD, et al. Thermostable α-amylase from lignocellulosic residues using Bacillus amyloliquefaciens. Trans B Appl. 2017;30:1110–1117.
- Simair AA, Qureshi AS, Khushk I, et al. Production and partial characterization of α-amylase enzyme from Bacillus sp. BCC 01-50 and potential applications. Biomed Res Int. 2017;2017:1–9.
- Özdemir S, Okumus V, Ulutas MS, et al. Production and characterization of thermostable α-amylase from thermophilic Anoxybacillus flavithermus sp. nov. SO-19. Starch-Stärke. 2016;68:1244–1253.
- Fincan SA, Enez B. Production, purification, and characterization of thermostable α-amylase from thermophilic Geobacillus stearothermophilus. Starch-Stärke. 2014;66:182–189.
- Aymard C, Belarbi A. Kinetics of thermal deactivation of enzymes: a simple three parameters phenomenological model can describe the decay of enzyme activity, irrespectively of the mechanism. Enzyme Microb Technol. 2000;27:612–618.
- Ait Kaki El-Hadef El-Okki A, Gagaoua M, Bennamoun L, et al. Statistical optimization of thermostable α-amylase production by a newly isolated Rhizopus oryzae strain FSIS4 using decommissioned dates. Waste Biomass Valor. 2017;8:2017–2027.
- Gandhi S, Salleh AB, Rahman RN, et al. Expression and characterization of Geobacillus stearothermophilus SR74 recombinant α-amylase in Pichia pastoris. Biomed Res Int. 2015;2015:1–9.
- Sun H, Zhao P, Ge X, et al. Recent advances in microbial raw starch degrading enzymes. Appl Biochem Biotechnol. 2010;160:988–1003.
- Božić N, Lončar N, MŠ S, et al. Raw starch degrading α-amylases: an unsolved riddle. Amylase. 2017;1:12–25.
- Özdemir S, Fincan SA, Karakaya A, et al. A novel raw starch hydrolyzing thermostable α-amylase produced by newly isolated Bacillus mojavensis SO-10: purification, characterization and usage in starch industries. Braz Arch Biotechnol. 2018;61:e18160399.
- Zhang L, Yin H, Zhao Q, et al. High alkaline activity of a thermostable α-amylase (cyclomaltodextrinase) from thermoacidophilic Alicyclobacillus isolate. Ann Microbiol. 2018;68:881–888.
- Copeland L, Blazek J, Salman H, et al. Form and functionality of starch. Food Hydrocoll. 2009;23:1527–1534.
- Kalpana BJ, Aarthy S, Pandian SK. Antibiofilm activity of α-amylase from Bacillus subtilis S8-18 against biofilm-forming human bacterial pathogens. Appl Biochem Biotechnol. 2012;167:1778–1794.
- Elamary R, Salem WM. Optimizing and purifying extracellular amylase from soil bacteria to inhibit clinical biofilm-forming bacteria. PeerJ. 2020;8:e10288.
- Sapkota S, Khadka S, Gautam A, et al. Screening and optimization of thermo-tolerant Bacillus sp. for amylase production and antifungal activity. JIST. 2019;24:47–56.
- Ahmad R, Mohsin M, Ahmad T, et al. Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. J Hazard Mater. 2015;283:171–177.
- Lahiri D, Nag M, Sarkar T, et al. Antibiofilm activity of α-amylase from Bacillus subtilis and prediction of the optimized conditions for biofilm removal by response surface methodology (RSM) and artificial neural network (ANN). Appl Biochem Biotechnol. 2021;193:1853–1872.
- Saggu SK, Jha G, Mishra PC. Enzymatic degradation of biofilm by metalloprotease from Microbacterium sp. SKS10. Front Bioeng Biotechnol. 2019;7:192.
- Anand J, Ramamoorthy K, Muthukumar G, et al. Production and partial purification of α-amylase producing Streptomyces sp. SNAJSM6 isolated from seaweed Sargassum myriocystum J. Agardh. Indian J Geo Mar Sci. 2019;48:1245–1251.
- Timilsina PM, Pandey GR, Shrestha A, et al. Purification and characterization of a noble thermostable algal starch liquefying alpha-amylase from Aeribacillus pallidus BTPS-2 isolated from geothermal spring of Nepal. Biotechnol Rep. 2020;28:e00551.
- Matpan Bekler F, Güven K, Gül Güven R. Purification and characterization of novel α-amylase from Anoxybacillus ayderensis FMB1. Biocatal Biotransfor. 2021;39:322–332.
- Aladejana OM, Oyedeji O, Omoboye O, et al. Production, purification and characterization of thermostable alpha amylase from Bacillus subtilis Y25 isolated from decaying yam (Dioscorea rotundata) tuber. Not Sci Biol. 2020;12:154–171.
- Burhanoğlu T, Sürmeli Y, Şanlı-Mohamed G. Identification and characterization of novel thermostable α-amylase from Geobacillus sp. GS33. Int J Biol Macromol. 2020;164:578–585.