?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The current study aimed to investigate the antioxidant, antibacterial, antidiabetic and antihemolytic potentials of heat-treated Petroselinum crispum, Trigonella-foenum graecum and Cymbopogon citratus. Ten min roasted and thirty min boiled fresh T. foenum-graecum and processed P. crispum revealed the highest DPPH scavenging activity (50% to 75% inhibition). All P. crispum samples displayed the highest antibacterial activity against K. pneumoniae and B. subtilis at 10, 20 and 30 min boiling and 20 min roasting and baking. The antidiabetic potency of herbs was increased upon 10 min roasting, while P. crispum was ineffective in reducing haemolysis. Pearson correlation analysis (PCA) displayed a positive relationship (r > 0.3) between phytochemicals and antioxidant activity and weak correlation (r < 0.3) with antidiabetic potentials. Gas chromatography-mass spectrometry (GCMS) indicated that some compounds disappeared on heating the herbs, while some compounds showed an increase in concentration. All-inclusive, this study endorses the application of roasted P. crispum and T. foenum-graecum against various diseases.
1. Introduction
Culinary herbs include fresh leaves, stems, or flowers of herbaceous plants that are ingested in various forms because of their properties, such as flavour enhancer and developing aroma or piquancy to foods and drinks [Citation1,Citation2]. Spices and herbs are considered the ‘heart of cooking’ in most parts of Asia as most of the dishes, such as curries and soups along with salads, are prepared using a combination of spices and medicinal herbs as ingredients [Citation3]. Quinic acid, malic acid, p-coumaric acid, hesperidin, rosmarinic acid, chlorogenic acid, and many other bioactive compounds exist in medicinal species, such as Stachys lavandulifolia, Marrubium astracanicum and Satureja boissieri, which contribute to their antimicrobial, immunomodulatory, antioxidant, and antidiabetic properties [Citation4–6]. Environmental stress and unhealthy lifestyle create imbalance in the free radicals that spontaneously generate in the human body and induce severe oxidative-stress-related diseases like cancer and diabetes [Citation7].
Pakistan is an agricultural country comprising remarkable natural resources and food species commonly used by the local community [Citation8]. Many edible plants are cultivated and consumed in Pakistan but being a developing country, more often faces issues in the purity and efficacy of these products [Citation9]. Recently, numerous fermentations, heating and drying treatments have been introduced to increase the nutritional quality and the antioxidant profile of medicinal herbs [Citation10,Citation11]. Petroselinum crispum and Trigonella-foenum graecum are the most important culinary herbs that are consumed in our daily lives. P. crispum mainly originated from Mediterranean regions, but now it is cultivated worldwide [Citation12]. Finely chopped leaves and stems of P. crispum are used as flavouring agent in soups, minces, and sauces [Citation1]. Besides being used in cooking, it also has diuretic potential and anticancer properties and prevents cardiovascular diseases [Citation13,Citation14].
T. foenum-graecum is a leguminous crop that consists of proteins (25–35%), fats (5.0–7.5%), dietary fibres (20–30%), and several minerals and vitamins [Citation15,Citation16]. Owing to the presence of different phytochemicals, these herbs exhibit antidiabetic, antioxidant, and immunomodulating activities [Citation17,Citation18]. Previously, ultrasound and microwave-assisted heating methods along with drying methods were applied to extract galactomannan from T. foenum-graecum and to ameliorate physico-chemical, rheological, and biological properties [Citation19,Citation20]. Similarly, Cymbopogon citratus is also an aromatic herb and is commonly known as ‘lemon grass’ due to its lemon-like odour, which is due to the presence of citral, a monoterpene [Citation21]. It is used as a taste enhancer in tea, juices, and other beverages [Citation2], and is also used to cure digestive problems, stomachache, high blood pressure, fever, cough, and other oxidative-stress-related ailments [Citation22,Citation23]. Different heating methods also enhance the nutritional and biological activities of C. citratus [Citation24].
Various studies have highlighted the efficacy along with the toxicity potential of many other culinary herbs that are cultivated in various geographical regions [Citation9,Citation14], but the effect of different cooking methods on bioactivities has not been investigated yet. Keeping this in view, the present study was designed to provide insight into the antioxidant, antibacterial, and antidiabetic potentials of fresh and processed samples of P. crispum (parsley), T. foenum-graecum (fenugreek), and C. citratus (lemon grass) by applying three different heat treatments i.e. roasting, baking, and boiling to them. Among these, 10 min roasted P. crispum showed the best bioactivities. Hence, it was further selected to ascertain its antihemolytic potential followed by the identification of various volatile compounds using the GC-MS method.
2. Materials and methods
2.1. Plant collection
Fresh samples of three culinary herbs, viz. P. crispum (Acc. no. NK-134), T. foenum-graecum (Acc. no. 03660), and C. citratus (Acc. No. 129848), were collected from Hamza Nursery Farm, Rawalpindi and their accession numbers were obtained from Herbarium of Quaid-e-Azam University and National Agricultural Research Center, Islamabad. Similarly, processed samples of these herbs were taken for analyses from Central Store Department (CSD)Rawalpindi.
2.2. Heat treatments
Three heat treatments, namely roasting (150–180°C), baking (200°C), and boiling (100°C), were given to each herb for 10, 20 and 30 min, whereas untreated samples were used as a control. The detail of untreated and heat-treated samples along with their acronyms, is given in Table .
Table 1. Acronyms used for the selected culinary herbs at different time intervals of heat-treatments.
2.3. Preparation of aqueous extracts
After heat treatments, samples (1.25 g each) were placed in reagent bottles and remained water-logged in 25 mL of distilled water for four days at 25 ± 2°C. Thereafter, the mixture was filtered using a muslin cloth and a Whatman filter paper and then concentrated using a water bath at 50°C to yield crude extracts. Besides lipophilic compounds, culinary herbs are also rich in hydrophilic compounds such as phenols and flavonoids. Moreover, an aqueous medium is preferably used for cooking these herbs; hence, aqueous extracts of selected herbs were prepared to target water-loving therapeutic compounds.
2.4. Antioxidant activity
Working concentrations of 300 µg/mL were prepared from the stock solution of 50 mg/mL of each sample and used for antioxidant assays.
2.4.1. DPPH radical scavenging assay
1 mL of prepared sample was added to 2 mL of 0.1 mM methanolic solution of DPPH and then placed in darkness at room temperature for 30 min [Citation25]. DPPH solution without samples was taken as negative control, while ascorbic acid was used as a standard. Absorbance was measured at 517 nm against methanol as a blank, and inhibition percentage was calculated:
2.4.2. Total reducing power (TRP) assay
The plant sample (1 mL) was mixed with 0.2 M phosphate buffer (2 mL, pH 6.6) and 1% of potassium ferricyanide (2 mL) and incubated at 50°C for 20 min. After that, 10% trichloroacetic acid (2 mL) was added to stop the reaction, followed by centrifugation at 2000 rpm for 10 min. The supernatant (2 mL) was added in the distilled water (2 mL) and 0.1% FeCl3 (0.4 mL) and again incubated for 10 min. The formation of ferrous ions (Fe2+) was measured at 700 nm, and ascorbic acid was used as a standard, with all samples run in triplicates [Citation26].
2.5. Antibacterial assay
Enterococcus faecalis (JH-22), Bacillus subtilis (ATCC- 6633), Escherichia coli (ATCC-15224), and Klebsiella pneumoniae (ATCC-1705) were collected from Microbiology and Biotechnology Research Lab, Fatima Jinnah Women University, Rawalpindi, and the activities were executed by a well-diffusion method using cefixime as a positive control. A single bacterial colony was picked from the fresh culture and dissolved in autoclaved distilled water (500 µL) followed by shaking. Wells were made with the help of a borer in the agar plates, and then 10 µL of bacterial inoculum was poured into the wells after enriching the agar medium (0.3 g agar in 20 mL of distilled water) solidification. Fifty microliters of each extract was aseptically introduced into the well and left for incubation for 24 h at 37°C. Inhibition zones produced around the wells were measured in millimetres, and activity index was determined [Citation27]:
2.6. Antidiabetic assay
About 500 µL of plant samples (100 µg/mL in DMSO) was dissolved in a 500 µL of 20 mM sodium phosphate buffer (pH 6.8) containing 20 µL of amylase (0.5 mg/mL) and incubated for 10 min at 25°C. Then 500 µL of 1% starch solution was mixed in a 0.02 M sodium phosphate buffer (pH 6.9) and added into each tube. After 15 min, 1 mL of dinitrosalicyclic acid was added to terminate the reaction and placed in the water bath for 5 min. Then 10 mL of distilled water was added, and absorbance was taken at 540 nm [Citation28]. The percentage inhibition was calculated using the following formula:
2.7. Antihemolytic assay
For this, 5 mL of human blood was taken in EDTA vials, and erythrocytes were separated by centrifugation (5 min) at 1500 rpm. The supernatant was discarded, while the pellet was washed with 0.2 M of 3 mL phosphate-buffered saline (PBS) solution (pH 7.4). The process was repeated three times and then diluted with PBS to prepare 4% suspension. About 1 mL of erythrocyte suspension was added to each plant extract (1000 and 10,000 ppm) along with 0.5 mL of PBS and then left for incubation. After 20 min, 0.5 mL of H2O2 was added and centrifuged for 10 min. The absorbance of the supernatant was measured at 540 nm, and quercetin was taken as a standard with PBS [Citation29]. The study approval (#BEC-FBS-QAU2019-143) for using blood was obtained from the Bioethical Committee of Quaid-i-Azam University, Islamabad (Pakistan).
2.8. Phytochemical analysis
Quantitative tests were performed to determine the phenolic, flavonoid and alkaloid contents in the extracts of selected herbs. Total phenolic content (TPC) was evaluated using Folin–ciocalteu reagent, and results were articulated using a calibration curve obtained from the standard gallic acid [Citation30]. Similarly, total flavonoid contents (TFC) were determined by the Aluminium chloride colorimetric method using quercetin as a standard [Citation31]. Moreover, total alkaloid contents were measured by following the standard protocol of Shamsa et al. [Citation32], and atropine was used as a standard. All experiments were carried out three times, and mean values were calculated.
2.9. Gas chromatography mass spectrometry (GCMS) analysis
P. crispum sample (10 g) was mixed in 100 mL of ultrapure distilled water, and then hydrodistillation (40 min) was performed using the microwave. About 60 µL of oil obtained at the end was used for analysis. Agilent technologies (GC7890B and MS5977A) instrument equipped with DB-5MS fused capillary column with 30 × 0.25 mm ID and 0.25 µm film thickness were used for the GCMS experiment. The starting temperature was set at 50°C for 1 min, which was increased up to 250°C at the rate of 5°C/min with a 5-min hold. The flow rate of carrier gas (helium) was 1.22 mL/ min, and 1 µL of the sample was injected in the split mode (10:1) with 82.7 kPa pressure in the column. The injector temperature was maintained at 250°C, and the ion-source temperature was set at 280°C. The mass spectrum was obtained by electron ionization (70 eV), and the detector was operated at 35–500 mass scan (m/z). Total GC running time was 34 min, and the compounds were identified using the database of NIST and WILEY [Citation33,Citation34]. The total per cent of compounds was assessed to determine the total volatile compounds present in each sample.
2.10. Statistical analysis
Results were expressed as mean ± standard deviation from the triplicate experiments. Two-way ANOVA was performed, and the Least Significant Difference (LSD) was determined using statistics 8.1 software. The correlation between phytochemical contents and bioactivities was determined using Pearson’s Correlation Coefficient Test via Microsoft Excel, 2010.
3. Results
3.1. Antioxidant activity
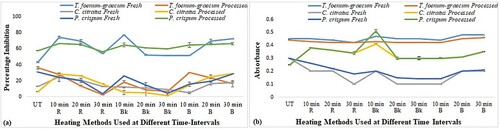
Heat-treated samples of fresh T. foenum-graecum and processed P. crispum revealed the highest activity ranging from 50% to 77% inhibition. Among different heat treatments, 10 min roasting was found to be very effective in most of the samples, followed by 30 min boiled and 10 min baked samples. Comparison made among different herbs indicated that 10 min baked (77.3% inhibition) and 10 min roasted (74.3% inhibition) T. foenum-graecum possessed the highest DPPH activity (Figure (a)), while processed P. crispum showed the highest reducing potential at 10 min baking (Figure (b)). Overall, results were statistically significant (p < 0.05) as determined by two-way ANOVA.
3.2. Antibacterial assay
K. pneumoniae and B. subtilis were the most susceptible microorganisms as fresh boiled herbs produced significant inhibition zones (≥0.8 activity index) against them. Only FPR30 and PCBk20 revealed 0.5% activity against E. coli, indicating that E. coli is the most resistant bacterial strain. PCR30 (5 ± 0.2 mm), FTR20 (14 mm ± 0.1 mm) and FTR10 (16 ± 0.4 mm) displayed significant activities against E. coli and E. faecalis. Similarly, FPU (17 ± 2.1 mm) and FPB10 (15 ± 0.2 mm) displayed the highest activity against K. pneumoniae, and PPB10 depicted the highest (17 ± 2.8 mm) activity against B. subtilis, respectively. All-inclusive, fresh and processed samples of P. crispum revealed the highest (≥15 mm inhibition zones) antibacterial activity (Table ).
Table 2. Antibacterial activity indices of selected fresh and processed culinary herbs.
3.3. Antidiabetic assay
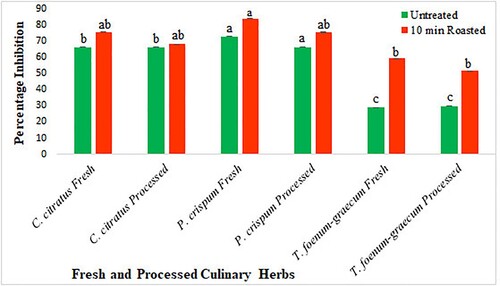
Fresh and processed culinary herbs with 10 min roasting treatment were selected to determine the antidiabetic potential as they displayed the highest antioxidant and antibacterial activities compared with untreated samples. Results revealed that the potency of selected herbs was increased on heat-treatment, and among all samples, FPR10 (83.8%), PPR10 (75.4%), and FCR10 (75.4%) displayed the highest α-amylase inhibition activity (Figure ).
3.4. Antihemolytic assay
Both fresh and processed P. crispum herbs roasted at 10 min were used to assess H2O2 induced haemolysis, and results were compared with untreated samples. Results revealed that the selected species exhibit less than 5% inhibition, indicating that these are least effective in providing protection against haemolysis (Table ).
Table 3. Antihemolytic activity of fresh and processed samples of P. crispum observed at different concentrations.
3.5. Phytochemical analysis
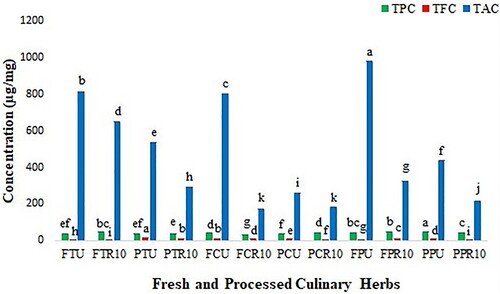
Untreated and 10 min roasted samples were further selected to observe total phenolic, flavonoid, and alkaloid contents. Among heat-treated samples, TPC were found in the decreasing order of FTR10 > PCR10 > PPR10 > FPR10 > PTR10 > FCR10 and TFC were revealed as PTR10 > FPR10 > FCR10 > PCR10 > PPR10 > FTR10. Similarly, TAC was observed in the descending order of FTR10 > FPR10 > PTR10 > PPR10 > PCR10 > FCR10, respectively. Overall, fresh samples of heat-treated P. crispum and T. foenum-graecum showed the highest phenolic (44.3 and 44 µg/mg) and alkaloid (326 and 648 µg/mg) contents. It was observed that phenolic contents were considerably increased after giving heat-treatments, while alkaloid contents were reduced on heating. Two-way ANOVA of fresh and processed samples confirmed that the obtained results were statistically significant (p < 0.05) (Figure ).
Figure 3. Total phenolic, flavonoid and alkaloid contents detected in the untreated and treated samples of fresh and processed selected culinary herbs.
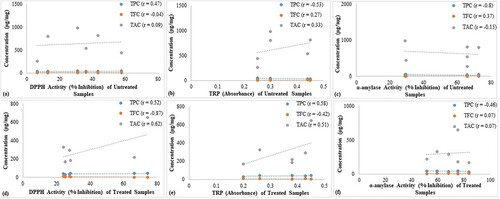
The Pearson correlation analysis revealed a moderate relationship (r = 0.47) between phenolic contents and the DPPH activity of untreated samples and a strong correlation (r = 0.52) in the case of roasted samples. The relationship between alkaloid contents and DPPH activity was highly significant (r = 0.62) in these herbs (Figure (a,d)). Similarly, the correlation between TRP and phytochemicals indicated an insignificant relationship (≤0.33) in untreated samples (Figure (b)) and a positive correlation among treated samples (r ≥ 0.51) (Figure (e)). However, a very weak correlation was observed between α-amylase assay and phytochemical contents (Figure (c,f)).
3.6. GCMS analysis
The spectral analysis displayed various acyclic, monocyclic, and heterocyclic hydrocarbons along with some ethers, aldehydes, and aromatic dicarboxylic acids. Cyclohexanone was only detected at the lowest retention time (5.07 min) in the treated, processed P. crispum, while all other compounds were observed after a 20 min retention time. Some compounds were present in untreated herbs which disappeared upon roasting, like eicosane and tert-hexadecanethiol. On the contrary, some compounds appeared vividly after roasting the herb, such as 2-methyl dodecane, Cis-9-hexadecenal, 2-methyl tetracosane, and 2-methyl tetracosane. In contrast, the concentration of some compounds was increased considerably after roasting; the concentration of diisooctyl ester-1, 2 – benzenedicarboxylic acid was enhanced in fresh (81.66%) and processed (42.01%) herbs. An aromatic dicarboxylic acid, namely diisooctyl ester-1, 2-benzenedicarboxylic acid, was determined as a major compound (i.e. ranging from 38 to 82%) in all treated and untreated fresh and processed P. crispum. Total identified compounds revealed that compounds concentration was increased on roasting in fresh (99.52%) and processed (99.50%) samples compared with untreated herbs (Table ).
Table 4. Compounds identified at respective retention times in selected P. crispum samples using the GCMS method.
4. Discussion
Plant-derived products, such as spices, herbs, and tea, are the pillars of home-cooked meals and traditional medicinal systems due to their organoleptic and medicinal properties [Citation35]. The aqueous extracts of some plants exhibit low-level biological activities than those prepared in strongly polar solvents like methanol [Citation4,Citation5]. Hence, different heating methods can be used as an imperative strategy to increase the efficiency of aqueous plant extracts. The present study focused on the investigation of the effects of roasting, baking, and boiling of three culinary herbs consumed by the local people of Pakistan. Heat-treatments were applied at different time intervals to confirm that the biological activities of these herbs are retained, reduced, or destroyed during cooking. Results revealed that DPPH activity and TRP increased when herbs were roasted and baked for 10 min and then gradually declined on further heating. It indicates that an increase in heating duration adversely affects biological activities. It can be inferred that the degradation of some enzymes occurs when herbs are heat-treated for a longer duration [Citation36].
On the contrary, boiling for 10 min increased the antioxidant activity, and heating for 20 and 30 min resulted in a further increase of activity. The thermal process substantially influences bioactivities depending on the magnitude, duration and different heating methods [Citation37]. In the case of T. foenum-graecum, fresh samples presented more antioxidant activity than their processed alternates. P. crispum revealed an opposite trend to T. foenum-graecum in which processed samples displayed more activity than the fresh samples, whereas in C. citratus trend was indifferent to treated and untreated samples.
The antioxidant potential is regulated by the phenolic and flavonoid compounds that neutralize free radicals via different mechanisms [Citation38]. These compounds are considered one of the responsible agents of biological activities in plants [Citation6]. Drying treatments ameliorate the antioxidant activity and phenolic contents of C. citratus [Citation39]. Naidu et al. [Citation19] depicted the usefulness of dehydrated (i.e. 40°C) T. foenum-graecum as a valuable food additive to enhance nutrition through their phenolic contents and antioxidant activities. Likewise, total antioxidant activity and reducing the power of processed P. crispum were found to be increased on applying heat-treatment (86.6 ± 0.2 mmol Trolox equivalent/kg and 14.7 ± 1.2 mmol TE/kg) compared with the untreated control (81.8 ± 1.8 mmol TE/kg and 7.4 ± 0.0 mmol TE/kg), while TPC was decreased (75.8 ± 1.3 mmol GAE/kg) on heating (80.8 ± 0.3 mmol GAE/kg in untreated sample) [Citation40]. Thus, different heating methods demonstrated that the antioxidant potential does not directly depend on the chemical composition but also on the medium where the reaction occurs.
Innovation of antimicrobials has long paved the way for human health, but the discovery of antimicrobial agents is always in demand due to the increasing microbial resistance [Citation41]. In the present study, antibacterial activity revealed that heat-treated P. crispum exhibited the highest activity (≥ 15 mm inhibition zones) against most tested microorganisms. Among fresh herbs, P. crispum was very effective against K. pneumoniae when boiled at different time intervals, whereas the processed sample was highly active on 20 min roasting, 20 min baking, and 10 min boiling against B. subtilis.
Antibacterial activity is greatly influenced by the evaporation, solubility, and diffusion rate of bioactive compounds [Citation42]. In a previous study, untreated T. foenum-graecum showed <15 mm inhibition zones against various bacterial strains [Citation43]. Similarly, Al-Haadi et al. [Citation44] and Fatima et al. [Citation45] also revealed significant inhibitory activities of untreated P. crispum and C. citratus against some bacterial species. On the contrary, Wong and Kitts [Citation46] observed significant antimicrobial activity of aqueous P. crispum leaves and stem extracts after freeze-drying and providing irradiations to the leaves with an electron beam. Herbs remain moderately active in an untreated form; however, they become highly active after heating.
Moreover, diabetes mellitus is caused by naturally occurring cytotoxic chemicals, such as streptozotocin that affect the pancreas and insulin-producing beta cells [Citation47]. Results revealed that P. crispum and C. citratus exhibited the highest activity (68% to 84% inhibition) in all samples. Previous studies showed significant antidiabetic activity in C. citratus and T. foenum-graecum extracts [Citation47,Citation48]. However, in another study C. citratus and P. crispum grown in Thailand displayed an insignificant antidiabetic activity [Citation49], indicating that the climatic factor also influences the biological potential of medicinal herbs.
In general, α-amylase inhibitory potential considerably increases in all heat-treated fresh and processed herbs, exactly like the antioxidant activity, which increased in heat-treated samples. Secondary metabolites in plants directly influence the epigenetic pathways, such as histone modifications to respond against physiological functions [Citation50]. Exposure of medicinal herbs to different temperatures further induces reprogramming of gene expression in a coordinated manner by changing the primary and secondary metabolites along with DNA profiles [Citation51]. In the present study, phenolic contents were increased on roasting the selected herbs, while alkaloid contents were significantly reduced on heat-treatment.
The Pearson correlation analysis showed a strong relationship (r > 0.50) between phytochemicals and antioxidant activities in roasted samples, while an inverse correlation (r < 0.50) was shown in the case of untreated samples. Phytochemical contents increase on heating the culinary herbs contributing to biological activities. Our study is also coherent with the previous findings of Oboh et al. [Citation52], indicating that heat-treatments greatly influence the antioxidant properties due to the release of phytochemicals, thus contributing to the health-promoting abilities of C. citratus. However, antidiabetic activity was independent of TPC, TFC, and TAC in medicinal herbs. Other phytochemicals, such as terpenoids, are chiefly responsible for the antidiabetic potential of herbs [Citation53].
Antihemolytic activity is useful for studies on plant extracts and compounds that show antioxidant potential [Citation54]. In the present study, 10 min roasted P. crispum showed significant antioxidant, antibacterial and antidiabetic activity; thus was selected further to analyze antihemolytic potential compared to the untreated herb. However, results revealed insignificant antihemolytic activity, and thus, the Pearson correlation analysis of antihemolytic activity and phytochemicals was also not performed. The selected herb is not suitable for use in drugs to treat haemolytic diseases.
In the end, organic compounds present in untreated and heat-treated P. crispum were determined using the GCMS method. It is used for the quantitative estimation of fatty acids and esters and has the potential to withstand high temperature during gas-chromatographic separations [Citation55,Citation56]. Our results indicated the presence of 23 compounds in different concentrations in the selected P. crispum samples. The obtained data of 10 min roasted fresh and processed herb were compared with the untreated samples. Comparatively, the untreated processed herb (99.13%) was more effective than the untreated fresh herb (89.99%) as it comprises a higher concentration of compounds. However, fresh (99.52%) and processed (99.50%) herbs displayed almost equal concentrations of compounds on 10 min roasting. Untreated processed herb exhibited a variety of compounds and among these, some compounds disappeared or were damaged on heating, while some compounds showed an increase in concentration after roasting.
Previously, Li et al. [Citation57] determined the effect of cooking on the volatile compounds of Nelumbo nucifera and revealed that boiling (100°C) increased the concentration of compounds, while steaming decreased the total volatile compounds in the plant. Similarly, Cremer and Eichner [Citation24] found an increase in hexanal concentration and the concentration of other volatile compounds in Capsicum annuum after applying heat-treatment. However, Filho et al. [Citation13] demonstrated that cleaning and drying of parsley leaves at different temperatures (40, 50, and 60°C) did not affect the yield of the main compounds of essential oil obtained from parsley. Therefore, it can be suggested from the present study that cooking increased the concentration of some volatile compounds with the production of new compounds like 1,2-benzenedicarboxylic acid and decreased the concentration of other compounds.
Different compounds in the plants exhibit different medicinal properties, such as hexadecanoic acid, which is present in our samples is considered for anticancer and antimicrobial activities [Citation58]. Similarly, eicosane extracted from Cestrum nocturnum extract displayed inhibitory effects on food-borne pathogens [Citation59]. On the other hand, the biological activities of some compounds have not been investigated so far. In general, it can be suggested that P. crispum comprises a variety of compounds that exhibit considerable biological activities and the concentration of existing organic compounds increases further on heating for short duration. Hence, fresh and processed herbs could be preferably used in our food and medicinal system after roasting.
5. Conclusion
The present study implies that different heating methods produce variable impacts on the biological properties of herbs. Roasting and baking decrease the antioxidant activity with an increase in time-interval, while antioxidant activity increase on boiling even for a longer duration. Antidiabetic activity and phenolic contents were increased on roasting, while TFC and TAC were notably reduced in most samples. Overall, biological activities vividly increased on 10 min roasting, and a correlation analysis confirmed that antioxidant potential is highly dependent on TPC and TAC. P. crispum showed variations in volatile compounds’ concentration after roasting. However, further in vivo studies are recommended to design appropriate dosage forms for the treatment of antimicrobial infections and oxidative stress-related diseases and isolate bioactive compounds using HPLC and NMR techniques to be used in industries.
Acknowledgements
This study uses the human blood from a person. Study approval for the use of the blood was taken from the Ethical Committee (EC) of Fatima Jinnah Women University, Rawalpindi (Pakistan).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Chempakam B, Parthasarathy V, Zachariah TJ. Chemistry of spices. London: CABI Publication; 2008.
- Olorunnisola SK, Hammed AM, Simsek S. Biological properties of lemongrass: an overview. Int Food Res J. 2014;21(2):455–462.
- Chan EWC, Chan HJ, Lim JE, et al. Effects of different cooking methods on the bioactivities of some spices. Emir J Food Agric. 2015;27(8):610–616.
- Bursal E, Aras A, Kılıç Ö. Evaluation of antioxidant capacity of endemic plant Marrubium astracanicum subsp. macrodon: identification of its phenolic contents by using HPLC-MS/MS. Nat Prod Res. 2019;33(13):1975–1979.
- Bingol MN, Bursal E. LC-MS/MS analysis of phenolic compounds and in vitro antioxidant potential of stachys lavandulifolia vahl. var. brachydon boiss. Int Lett Nat Sci. 2018;72:28–36.
- Aras A, Bursal E, Alan Y, et al. Polyphenolic content, antioxidant potential and antimicrobial activity of Satureja boissieri. Iran J Chem Chem Eng. 2018;37(6):209–219.
- VanDer-Schaft N, Schoufur JD, Nano J, et al. Dietary antioxidant capacity and risk of type 2 T2DM mellitus, preT2DM and insulin resistance: The Rotterdam study. Eur J Epidemiol. 2019;34(9):853–861.
- Majeed M, Bhatti KH, Pieroni A, et al. Gathered wild food plants among diverse religious groups in Jhelum District, Punjab, Pakistan. Foods. 2021;10(3):594.
- Akhtar S, Riaz M, Naeem I, et al. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control. 2020;112:107–132.
- Aphrodite CT, Jean S, Kuoh YD, et al. Culinary temperature variably affects the antioxidant content of some local spices and green herbs. Turk J Agric Food Sci Technol. 2021;9(4):781–786.
- Ng ZX, Than MJY, Yong PH. Peperomia pellucida (L.) Kunth herbal tea: effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti-inflammatory activities. Food Chem. 2021;344:128738.
- Mahmood S, Hussain S, Malik F. Critique of medicinal conspicuousness of Parsley (Petroselinum crispum): a culinary herb of Mediterranean region. Pak J Pharm Sci. 2014;27:193–202.
- Filho LC, Martinazzo AP, De Souza Teodoro CE, et al. Microbiological quality and essential oil of parsley (Petroselinum crispum) submitted to the hygienizing and drying process. Ind Crops Prod. 2018;114:180–184.
- Aissani N, Albouchi F, Sebai H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr Cancer. 2021;73(11–12):2605–2613.
- Dhull SB, Sandhu KS. Wheat-fenugreek composite flour noodles: effect on functional, pasting, cooking and sensory properties. Curr Res Nutr Food Sci. 2018;6:174–182.
- Dhull SB, Punia S, Sandhu KS, et al. Effect of debittered fenugreek (Trigonella foenum graecum L. flour addition on physical, nutritional, antioxidant and sensory properties of wheat flour rusk. Legume Sci. 2019;2:21.
- Farzaei MH, Abbasabadi Z, Ardekani MRS, et al. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J Tradit Chin Med. 2013;33:815–826.
- Wani SA, Kumar P. Fenugreek: a review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. 2018;17:97–106.
- Niknam R, Mousavi M, Kiani H. New studies on the galactomannan extracted from Trigonella foenum-graecum (Fenugreek) seed: effect of subsequent use of ultrasound and microwave on the physicochemical and rheological properties. Food Bioproc Tech. 2020;13(5):882–900.
- Naidu MM, Khanum H, Sulochanamma G, et al. Effect of drying methods on the quality characteristics of fenugreek (Trigonella foenum-graecum) greens. Dry Technol. 2012;30(8):808–816.
- Oladeji OS, Adelowo FE, Ayodele DT, et al. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci Afr. 2019;6:e00137.
- Shah G, Shri R, Panchal V, et al. Scientific basis for the therapeutic use of Cymbopogon citratus Stapf. Lemon grass). J Adv Pharm Technol Res. 2011;2(1):3.
- Zou Z, Xi W, Hu Y, et al. Antioxidant activity of citrus fruits. Food Chem. 2016;196:885–896.
- Cremer DR, Eichner K. Formation of volatile compounds during heating of spice paprika (Capsicum annuum) powder. J Agric Food Chem. 2000;48(6):2454–2460.
- Khalil RR, Mustafa YF. Phytochemical, antioxidant, and antitumor studies of coumarins extracted from Granny Smith apple seeds by different methods. Syst Rev Pharm. 2020;11(2):57–63.
- Uddin MS, Hossain MS, Al-Mamun A, et al. Phytochemical analysis and antioxidant profile of methanolic extract of seed, pulp and peel of Baccaurea ramiflora Lour. Asian Pac J Trop Med. 2018;11(7):443.
- Karuppiah P, Mustaffa M. Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pac J Trop Biomed. 2013;3(9):737–742.
- Kwon YI, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J Food Biochem. 2008;32(1):15–31.
- Yang ZG, Sun HX, Fang WH. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (Ams) on the immune responses to ovalb Sermakkani umin in mice. Vaccine. 2005;23:5196–5203.
- Sumczynski D, Bubelova Z, Sneyd J, et al. Total phenolics, flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem. 2015;174:319–325.
- Stankovic MS, Niciforovic N, Topuzovic M, et al. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, f. supinum (L.) Reichenb. Biotechnol Biotec Eq. 2011;25(1):2222–2227.
- Shamsa F, Monsef H, Ghamooshi R, et al. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci. 2008;32:17–20.
- NIST. NIST standard reference database number 69, 2011. https://webbooknistgov/chemistry/.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Vol. 4. Carol Stream (IL): Allured Publ Corp, 2007.
- Reinholds I, Pugajeva I, Bavrins K, et al. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit Contam: Part B. 2017;10(1):5–14.
- Ploenkutham R, Sripromma P, Amornraksa S, et al. Effect of roasting and kneading on antioxidant activity and consumer acceptance towards Asiatic Pennywort Tea. In MATEC Web of Conferences. 2018;187:01004.
- Hostetler GL, Riedl KM, Schwartz SJ. Endogenous enzymes, heat, and pH affect flavone profiles in parsley (Petroselinum crispum var. neapolitanum) and celery (Apium graveolens) during juice processing. J Agric Food Chem. 2012;60(1):202–208.
- Razali N, Razab R, Junit SM, et al. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008;111(1):38–44.
- Ng ZX, Yong PH, Lim SY. Customized drying treatments increased the extraction of phytochemicals and antioxidant activity from economically viable medicinal plants. Ind Crops Prod. 2020;155:112815.
- Kaiser A, Brinkmann M, Carle R, et al. Influence of thermal treatment on color, enzyme activities, and antioxidant capacity of innovative pastelike parsley products. J Agric Food Chem. 2012;60(12):3291–3301.
- Özçelik B, Kartal M, Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 2011;49(4):396–402.
- Manilal A, Sabu KR, Woldemariam M, et al. Antibacterial activity of Rosmarinus officinalis against multidrug-resistant clinical isolates and meat-borne pathogens. Evid Based Compl Altern Med. 2021;2021:6677420.
- Haque A, Khatun R, Yaakob Z. Gas chromatography mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum. Asian Pac J Trop Biomed. 2015;5(12):1033–1036.
- Al-Haadi AMH, Al-Rahbi SS, Akhtar MS, et al. Phytochemical screening, antibacterial and cytotoxic activities of Petroselinum crispum leaves grown in Oman. Iran J Pharm Sci. 2013;9(1):61–65.
- Fatima I, Kanwal S, Mahmood T. Microbiostatic, antioxidative and cytotoxic potentiation of some grasses of Bahawalpur, Pakistan. J Tradit Chin Med. 2019;39(4):482–491.
- Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97(3):505–515.
- Adeneye AA, Agbaje EO. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J Ethnopharmacol. 2007;112:440–444.
- Mathern JR, Raatz SK, Thomas W, et al. Effect of fenugreek fiber on satiety, blood glucose and insulin response and energy intake in obese subjects. Phytother Res. 2009;23(11):1543–1548.
- Wongsa P, Chaiwarit J, Zamaludien A. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 2012;131(3):964–971.
- Avramova Z. Transcriptional memory of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015;83:149–159.
- Hassanein RA, El-Khawas SA, Mohamed AMK. Effect of heat shock on some biochemical and molecular criteria of fenugreek (Trigonella foenum-graceum L). J Med Plants Res. 2012;6(9):1782–1794.
- Oboh G, Adefegha SA, Ademosun AO, et al. Effects of hot water treatment on the phenolic phytochemicals and antioxidant activities of lemon grass (Cymbopogon citratus). Elec J Env Agricult Food Chem. 2010;9:3.
- Faraone I, Rai DK, Russo D, et al. Antioxidant, antidiabetic, and anticholinesterase activities and phytochemical profile of Azorella glabra wedd. Plants. 2019;8(8):265.
- Djeridane A, Yousfi M, Nadjemi B, et al. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur Food Res Technol. 2006;224:801–809.
- Aboud SA, Altemimi AB, Al-HiIphy ARS, et al. A comprehensive review on infrared heating applications in food processing. Molecules. 2019;24(22):4125.
- Altemimi A, Lakhssassi N, Baharlouei A, et al. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42.
- Li S, Li X, Lamikanra O, et al. Effect of cooking on physicochemical properties and volatile compounds in lotus root (Nelumbo nucifera Gaertn). Food Chem. 2017;216:316–323.
- Wang S, Karthickeyan V, Sivakumar E, et al. Experimental investigation on pumpkin seed oil methyl ester blend in diesel engine with various injection pressure, injection timing and compression ratio. Fuel. 2020;264:116868.
- Tomita S, Nakamura T, Okada S. NMR-and GC/MS-based metabolomic characterization of sunki, an unsalted fermented pickle of turnip leaves. Food Chem. 2018;258:25–34.