1. Introduction
Prodrugs are inactive precursors of an active drug designed to be bioconverted (activated) post administration with the main aim of improving the pharmacokinetic properties of the parent drug. Prodrugs have been successful for a longtime. Sulfasalazine, one of the earliest prodrugs, reaches the colon and is metabolized by bacteria into two active metabolites: sulfapyridine and 5-aminosalicylic acid (5-ASA). Sulfasalazine was approved for use in the USA in 1950 and still is considered first-line treatment in autoimmune conditions such as Crohn’s disease and ulcerative colitis [Citation1]. It has been demonstrated that the prodrug approach has reached vast success in the past few years. It is estimated that around 10% of all marketed drugs are prodrugs, 20% of small molecular weight drugs approved between 2000 and 2008 were prodrugs, and between 2008 and 2017 the share of prodrugs in the drug market was 12% [Citation2].
Various strategies are employed in the prodrug approach. The most common of which is making a prodrug susceptible to abundant enzymes by functionalization with a group that can be cleaved to produce the active form of the drug. The prodrug approach to drug optimization offers chemical stability such as an inactive oral prodrug can be stable in the gastrointestinal tract and only be bioconverted by CYP450 in the liver, plasma, or GIT mucosal esterase, or other enzymes. Examples of this include phosphate groups which are susceptible to alkaline phosphatase, ester groups which are susceptible to esterases, and carbamates or amidine groups which are susceptible to amidases. Newer strategies include pegylation, which is used to increase cellular uptake, and dimer prodrugs, which are cleaved to two active moieties.
Also, prodrugs can be used as precursors in biological conversion pathways, as is the case with L-dopa, a prodrug of dopamine. L-dopa crosses the blood-brain barrier through L-type amino acid transporter-1 and is metabolized by aromatic amino acid decarboxylase to active dopamine in the CNS. Targeted prodrugs have also been explored in oncology in order to minimize side effects and improve the tolerability of chemotherapy [Citation3].
Prodrugs are also used to increase the duration of action of medicines, acting as chemical sustained release forms. Lisdexamfetamine dimesylate is an inactive prodrug of amphetamine used mainly in the treatment of attention deficit hyperactivity disorder (ADHD). The prodrug is hydrolyzed by red blood cells to L-lysine and active d-amphetamine. The duration of action of lisdexamfetamine is 12 h [Citation4] compared to that of instant release amphetamine, which is 3-6 h.
In cardiovascular medicine, prodrugs have been successful. Older prodrugs such as angiotensin-converting enzyme inhibitors (ACEi) are considered cornerstones in the management of hypertension. ACE inhibitors are dicarboxyl ester prodrugs converted to their active -rilat form by liver esterase (such as enalapril and enalaprilat). The exceptions for this are lisinopril and captopril which are not prodrugs, and fosinopril, which is a phosphonic acid prodrug hydrolyzed by liver and GIT mucosa esterases. Newer prodrugs, such as dabigatran etexilate and prasugrel (), are anticoagulants indicated for the treatment and prevention of blood clotting.
Table 1. Chemical structures of prodrugs to gain FDA approval during 2008–2018. Some of the listed prodrugs were cited in reference [Citation2].
Prodrugs of nucleoside analogs are used to improve pharmacokinetic properties such as intestinal permeability and oral absorption [Citation15]. For instance, valacyclovir and valganciclovir are valine ester prodrugs of acyclovir and ganciclovir, respectively, target intestinal oligopeptide transporter aiming to improve the oral absorption of the parent drug.
2. Continued need for prodrugs
During the years 2008–2017, a total of 249 new molecular entities were approved, 31 of which were prodrugs. With the exception of 2016, which had no novel drug approvals of prodrugs, and 2012, which had one, each year at least 2 approvals of prodrugs were reported. summarizes novel prodrugs granted FDA approval during 2008–2018.
While the majority of the prodrugs mentioned above are hydrolyzed by esterases; romidepsin, sofosbuvir, benznidazole, and secnidazole reach the intracellular environment as inactive entities. Romidepsin is a novel anticancer agent. It is activated by intracellular glutathione by cleavage of a disulfide bond and producing a thiol group. This group then binds to a zinc atom in Zn-dependent histone deacetylase.
Sofosbuvir is a novel and promising antiviral medication indicated for the treatment of hepatitis C and is used heavily in combination therapy. Sofosbuvir is a substrate for cathepsin A and carboxylesterase 1, which metabolize it by removing its terminal ester and phenyl. Finally, phosphorylation by uridine monophosphate-cytidine monophosphate kinase leads to the active GS-461203. While the structures of these drugs do not resemble those of drugs susceptible to tissue and plasma esterases their affinity to intracellular enzymes made them prime prodrugs exhibiting targeted pharmacological action.
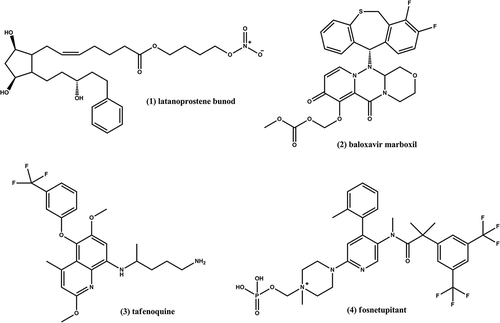
Interestingly, latanoprostene bunod (1 in ) delivers two active agents in a 1:1 ratio. Upon hydrolysis, the prodrug releases latanoprost acid, a prostaglandin F2-alpha analog, and butanediol mononitrate which undergoes further metabolism to NO leading to vascular relaxation. Hence, this prodrug acts via a dual mechanism which leads to lowering of the intraocular pressure.
Figure 1. Chemical structures of latanoprostene bunod (1), baloxavir marboxil (2), tafenoquine (3), and fosnetupitant (4).
Although the prodrug approach is advancing and reaching successes in providing effective medications to a variety of diseases it still needs the utilization of the sophisticated computational methods used for the design of drugs. Kinetics and thermodynamics for biological systems (active sites of receptors and enzymes, and etc.) that have biomedicinal interests have been intensively researched and have been proved to be fruitful. Today, quantum mechanics, such as ab initio, semi-empirical, and density functional theory (DFT), and molecular mechanics (MM) including docking are increasingly being utilized to characterize active sites of receptors and enzymes. These widely used methods have proven as successful tools for providing structure-energy calculations for an accurate prediction of potential drugs [Citation16].
3. Expert opinion
The strategies and aims of prodrugs to be approved in the past decade are reported in . Phosphorylation to improve aqueous solubility was successfully used in the design of fospropofol, fosaprepitant, and fosnetupitant allowing for injections. On the other hand, gabapentin enacarbil was designed to increase the bioavailability of gabapentin by targeting intestinal transporters monocarboxylate transporter type 1 and sodium-dependent multivitamin transporter.
Prodrugs can also be designed to overcome metabolic drawbacks. Tolterodine and fesoterodine are both indicated for overactive bladder disease and both metabolized to 5-hydroxymethyl tolterodine. The bioactivation of tolterodine is highly dependent on CYP2D6 activity which resulted in interpatient variability. Fesoterodine, on the other hand, is rapidly and completely hydrolyzed by non-specific esterases. Also, eslicarbazepine acetate solved the problem of its predecessors: carbamazepine and oxcarbazepine which produced toxic metabolites such as epoxides.
Taking a leaf from the book of sofosbuvir, the design of prodrugs with the aim of intracellular bioconversion rather than the classical approach to amidase, esterase, or CYP450 activation would be beneficial in the treatment of conditions such as viral infections, cancers, and in the revolution of future antibiotics.
As per November 2018, three prodrugs have been approved; tafenoquine, and fosnetupitant. Baloxavir marboxil (2 in ) is a novel agent with a novel mechanism of action. It is indicated for the treatment of influenza A and B and given as a single dose during the first 48 h hours of influenza symptoms. It inhibits viral shedding through the inhibition of viral CAP endonuclease. Tafenoquine (3 in ) is an 8-aminoquinoline analog of primaquine indicated for the treatment of malaria. Fosnetupitant (4 in ) is the prodrug form of netupitant and one of the active ingredients in Akynzeo, a drug approved for the treatment of acute and delayed nausea due to chemotherapy. It is dephosphorylated by plasma phosphatase to produce the active form.
While current trends point toward an age of biological treatments such as antibodies, prodrugs still prove viable products. I believe that the prodrug approach has a strong potential to grow rapidly in such a way that it will provide more than 25% of the marketed therapeutics during the coming decade if the researchers in the field utilize the computational methods used in the drug design and discovery. Computational methods based on quantum mechanics and MM could be used for the design of prodrugs. Prodrugs targeting transporters could be designed with the assistance of computational methods such as docking and etc. In a similar way, prodrugs targeting esterases, amidases and, etc., can be more effective if their design is relied on DFT and ab-inito methods that they have a great ability to predict kinetics of chemical systems. In our group we have used DFT methods for unraveling mechanisms of intramolecular processes that were previously studied in the labs of others to comprehend enzyme catalysis. Our goal was to establish a correlation between experimental and calculated kinetic values and to use the resulting correlation’s equation for the design of a number of novel prodrugs [Citation17].
For example, DFT and MM methods we have researched the mechanisms for the intramolecular proton transfer in Kirby’s acetals and Bruice’s cyclization of dicarboxylic semiesters which led to the design and synthesis of the following novel prodrugs: aza-nucleosides prodrugs for the treatment for myelodysplastic syndromes, tranexamic acid prodrugs for the treatment of hemorrhage conditions, dopamine prodrugs for Parrkinson’s disease, atovaquone prodrugs as antimalarial agents, bitterless paracetamol prodrugs as antipyretic and pain killer for pediatrics and geriatrics, and prodrugs of the decongestant phenylephrine. In the above-mentioned examples, the prodrug linker was bound to the amine or hydroxyl group in the parent drug in such a way that the drug-linker entity (prodrug) intraconverts upon an exposure to the physiological environment such as stomach, intestine, and/or blood circulation, with intramolecular reaction rates that are only determined on the chemical structural features of the pharmacologically inactive promoiety (linker) [Citation18].
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Peppercorn MA. Sulfasalazine: pharmacology, clinical use, toxicity, and related new drug development. Ann Intern Med. 1984;101(3):377–386.
- Rautio J, Meanwell NA, Di L, et al. The expanding role of prodrugs in contemporary drug design and development [Review Article]. Nat Rev Drug Discov. 2018 Aug;17(8):559–587. PubMed PMID: 29700501.
- Najjar A, Rajabi N, Karaman R. Recent approaches to platinum(iv) prodrugs: a variety of strategies for enhanced delivery and efficacy. Curr Pharm Des. 2017;23(16):2366–2376. PubMed PMID: 28155621; eng.
- Brams M, Mao AR, Doyle RL. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad Med. 2008 Sep;120(3):69–88. PubMed PMID: 18824827; eng.
- Hughes B. 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93–96. PubMed PMID: 19180096; eng.
- Hughes B. 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89–92. PubMed PMID: 20118952; eng.
- Mullard A. 2010 FDA drug approvals. Nat Rev Drug Discov. 2011 Feb;10(2):82–85. PubMed PMID: 21283092; eng.
- Mullard A. 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91–94. PubMed PMID: 22293555; eng.
- Mullard A. 2012 FDA drug approvals. Nat Rev Drug Discov. 2013 Feb;12(2):87–90. PubMed PMID: 23370234; eng.
- Mullard A. 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85–89. PubMed PMID: 24481294; eng.
- Mullard A. 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77–81. PubMed PMID: 25633781; eng.
- Mullard A. 2015 FDA drug approvals. Nat Rev Drug Discov. 2016 Feb;15(2):73–76. PubMed PMID: 26837582; eng.
- Mullard A. 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73–76. PubMed PMID: 28148938; eng.
- Mullard A. 2017 FDA drug approvals. Nat Rev Drug Discov. 2018 Feb 1;17(2):150. PubMed PMID: 29386602; eng.
- Sinokrot H, Smerat T, Najjar A, et al. Advanced prodrug strategies in nucleoside and non-nucleoside antiviral agents: a review of the recent five years. 2017 Oct 16;22(10). PubMed PMID: 29035325. DOI:10.3390/molecules22101736
- Karaman R. Prodrugs design: a new era. New York, USA: Nova Science Publishers, Incorporated; 2014.
- Haddad F, Sawalha M, Khawaja Y, et al. Dopamine and levodopa prodrugs for the treatment of parkinson’s disease. Molecules . 2018;23(1):40.
- Karaman R. Prodrugs design based on inter- and intramolecular chemical processes. Chem Biol Drug Des. 2013;82(6):643–668.
