ABSTRACT
Introduction
Nano-based systems have received a lot of attention owing to their particular properties and, hence, have been proposed for a wide variety of biomedical applications. These nanosystems could be potentially employed for diagnosis and therapy of different medical issues. Although these nanomaterials are designed for specific tasks, interactions, and transformations when administered to the human body affect their performance and behavior. In this regard, bacteria and other cells have been presented as alternative nanocarriers. These microorganisms can be genetically modified and customized for a more specific therapeutic action and, in combination with nanomaterials, can lead to bio-hybrids with a unique potential for biomedical purposes.
Areas covered
Literature regarding bacteria and cells employed in combination with nanomaterials for biomedical applications is revised and discussed in this review. The potential as well as the limitations of these novel bio-hybrid systems are evaluated. Several examples are presented to show the performance of these alternative nanocarriers.
Expert opinion
Bio-hybrid systems have shown their potential as alternative nanocarriers as they contribute to better performance than traditional nano-based systems. Nevertheless, their limitations must be studied, and advantages and drawbacks assessed before their application to medicine.
1. Introduction
Up to now, nanomaterials (NMs) have been proven to constitute an interesting and powerful strategy with great potential for biomedical applications [Citation1–6]. Due to their small size and the particular properties derived from it, NMs have been proposed as an alternative to strategies of diagnosis and therapy [Citation7–10] typically employed on the medical field. As it is already known, the advancement and development of technology and instrumentation have allowed the controlled synthesis, design, and modification of NMs as a function of their final application [Citation11–14]. As a result, nano-based systems have been engineered and evaluated for a wide variety of purposes, such as localized and targeted drug delivery, improved contrast imaging and enhanced sensing analysis, all of them applied for both diagnostic and therapeutic purposes.
Nevertheless, despite their extensive range of applications and their numerous advantages, NMs are still far from perfection. Although their small size may be an advantage for reaching specific places within the human body, it also leads to high reactivity and immediate interaction with biomolecules and other bio-entities as soon as in contact with a physiological fluid [Citation15,Citation16]. These interactions will further modify the specifically designed and engineered NM characteristics, leading to a different behavior and performance of the nanosystem. These could be translated into several consequences, including loss of active targeting, toxicity issues, changes in biodistribution, and different pharmacological profile, among many others [Citation17–19]. As the NM properties are largely determined by its size, morphology, and functionalization, all these parameters and their relationship with the bio-interactions that occur in the biological media must be studied and understood in order to explain the alterations on their expected performance [Citation20–23]. It is important to highlight that the use of nano-based systems has not been yet generalized, which is mainly caused by the inability to fully control and understand the interactions of NMs once they enter the human body and their behavior and performances [Citation24–26].
Taking this information into consideration, several scientific groups have proposed the use of microorganisms as alternative carriers for several nano-based systems [Citation27–30]. It is common knowledge that the human body is inhabited by a wide and diverse population of microorganisms that include bacteria, fungi, and derived viruses [Citation31,Citation32]. In addition, our body is made up of an extensive amount of many different types of cells. As both of these – bacteria and cells – are found in the human body, they constitute interesting alternative carriers of NMs for biomedical applications [Citation27–29].
In this regard, bacteria have a long history of application to medical therapy, and their potential for treatment and cure of diseases has been studied and demonstrated [Citation30]. Bacteria exhibit interesting properties such as carriers as taxis and appendices that allow them to navigate through the different tissues and elements within the body [Citation33,Citation34]. In addition, their motion can be modulated by using internal and external stimuli, and they can be genetically altered in order to display a determined set of characteristics [Citation35,Citation36]. In the case of eukaryotic cells, there are different cell types that have been proposed and evaluated as nano-carriers, such as macrophages, dendritic cells, stem cells, or red blood cells [Citation37]. Biocompatibility, prolonged circulation, and phagocytic ability are just some of the useful and particular properties that some of these cell types display. In particular, stem cells have received a lot of attention owing to their intimate relationship with cancer tissues and tumors [Citation38,Citation39].
In this review, we will specifically focus on bacteria and eukaryotic cells as alternative NM carriers for medical purposes. Appealing characteristics of both bacteria and eukaryotic cells will be reviewed and their connection to their potential as NM carriers will be described. Advantages and limitations of these novel systems will be revised and explained, as well as the proposed strategies to overcome their particular challenges. Moreover, specific examples from the literature of these NM-bacteria/cell systems and their biomedical applications will be shown and elucidated. Although NMs have already proven their great potential for their use for medical purposes, their limitations must be acknowledged. The combination of NMs with bacteria/cells leads to the formation of a bio-hybrid with unique properties and constitutes a new and complex system with added potential for biomedical purposes.
2. Bacteria as alternative carrier for nanomedicine
As mentioned before, bacteria display very interesting properties, including self-propulsion, transport, sensing, and ability to respond to different external signals, as well as acting as information providers of a treatment or disease stage [Citation29,Citation30]. During the following section, we will provide a brief summary about the historical use of bacteria for medical purposes, detailed information about bacteria interesting characteristics, and the reason they give an advantage and added value in combination with NMs. Moreover, several examples of bio-hybrid NM-Bacteria systems will be provided and explained, as well as the drawbacks and benefits they bring for biomedical applications.
2.1. Piece of history – bacteria and probiotics
Bacteria is just one of the numerous microorganisms that are known to inhabit the human body [Citation31]. Actually, it is by far the most present, outnumbering cells by approximately 10-fold [Citation32]. These prokaryotic microorganisms can be found in different places within our bodies, such as the skin and mucous membranes of mouth or nose, as well as within the reproductive and digestive systems. Due to their abundance, especially within the intestine, bacteria display the capacity to participate in and regulate the metabolism of a wide array of compounds [Citation40]. This is translated in a strong impact on the circulation and distribution of said compounds among different organs and parts of the body, which influences their physiological response [Citation41]. For these reasons, bacteria have a long history of therapeutic use because of their inferred ability to treat or cure certain diseases, usually in the form of probiotics [Citation42–44] present in food or through their application to fecal transplants as a treatment for an especially severe form of diarrhea caused by Clostridium difficile [Citation45–47].
The history of probiotics goes back centuries ago, basically ever since people started to drink fermented milk for their health. One of the first studies reporting the benefits of bacteria was carried out by Henry Tessler in 1899, where he reported the existence of bifidobacterium within the intestines of breast-fed infants [Citation42]. This study suggested that the presence of said bacteria in the intestine was beneficial for the children, as they were less likely to suffer from diarrhea episodes. Nevertheless, it was not until 1907 that the use of probiotics for health benefits was proposed. This idea was first presented by a Russian scientist called Elie Metchnikoff, as a result of his observations of the longer life-expectancy of Bulgarians in comparison with other inhabitants of Europe [Citation48]. Elie hypothesized that this outcome was the consequence of the consumption of fermented milk containing viable and beneficial bacteria. During the same year, Metchnikoff stated that the strain producing lactic acid, Lactobacillus bulgaricus (L. bulgaricus), was able to influence and even modify the pathologic microbiology of the intestine. Consequently, he suggested the possibility of improving and enhancing our health through the manipulation of the intestinal microbiome, more concretely, by ingestion of host-friendly bacteria present in products containing fermented milk. Since then, several documents and reports about the use of probiotics can be found, though majority of them lack a well-designed experimental protocol and, especially, good data. However, these ideas about changing the colonic florae were regarded as not very relevant and principally as a form of unorthodox medicine [Citation49,Citation50].
Despite the initial lack of attention, this idea was recovered during the mid-1990s, when an exponential growth in microbiome research was observed. In fact, by 2001 the term ‘microbiome’ was already coined and commonly employed for the description of the ‘collective genome of microorganisms inhabiting the human body’ [Citation51]. The same year, the word ‘probiotic’ was officially used for defining ‘living organisms which, administered in adequate amounts, provide health benefits to the host’ [Citation52]. Nowadays, probiotics have become a serious field of medical research with a high level of investments, and their extensive use and known benefits have led to the constitution of multibillion-dollar industry. Probiotics are mainly related and applied for the treatment of gastrointestinal illnesses, such as infectious diarrhea, traveler diarrhea, antibiotic-associated diarrhea, acute diarrhea in children, inflammatory bowel disease, or atopic dermatitis. Among the bacteria employed for the treatment of some of these medical problems, lactobacillus strains are the most common ones [Citation42]. For example, L. rhamnosus is by far the most studied strain regarding infectious diarrhea, and it has been proven that it is able to reduce diarrhea duration in one day [Citation53]. In the case of antibiotic-associated diarrhea, L. acidophilus, L. casei and L. rhamnosus are some of the most common options [Citation54]. In addition, they have been also utilized for a variety of dental health problems including periodontal infection, halitosis and cavities [Citation55].
As it can be seen, bacteria have a wide variety of applications from the probiotic point of view, and it has been used for the treatment of several diseases through the combination of probiotics and other drugs. In the next section, we will explore other applications of bacteria in medicine, as well as the utilization of bacteria as a drug delivery system for cancer, among others.
2.2. Bacteria applications in medicine
Apart from the widely known use of probiotics, bacteria are known to have a key role in medicine through their use for the production of a wide array of compounds, such as enzymes, hormones, vaccines, or antibiotics [Citation28,Citation30]. All these substances are currently utilized in the medical field for the diagnosis, treatment, and prevention of an extensive variety of diseases and medical problems. Firstly, enzymes are molecules that act as very specific biocatalysts and are present in all living organisms. In medicine, enzymes can be employed as analytical tools for diagnosis purposes as well as therapeutic agents for treating enzyme deficiencies and other medical conditions. In humans, enzymes can support food digestion and detoxification or strengthen the immune system, among others [Citation56]. Secondly, hormones are also being applied for the treatment of different medical issues like infertility, menopause, or even for different types of cancer [Citation57,Citation58]. In addition, invasive bacteria, such as Salmonella or Listeria, have been tested as vaccine vectors, as they are capable of inducing a very potent humoral and cellular immune response [Citation59,Citation60]. Of course, these bacteria have been genetically modified in order to control their invasive behavior, their infectious ability, and their adverse effects [Citation61,Citation62]. Finally, bacteria have the ability of naturally produced antibiotics, which can be used for the prevention or treatment of bacterial infections [Citation63]. These bacteria usually produce these antibiotic compounds in order to fight with other bacteria. In addition, there are other substances that also exhibit antibiotic behavior and are not naturally produced by other bacteria.
In all cases mentioned before, bacteria have helped to fight different diseases and medical issues including diabetes, tuberculosis, AIDS, tetanus, or even cholera. Nevertheless, bacteria have demonstrated that they have even more to offer regarding their applications to the medical field and have been proposed as alternative therapeutic agents for medical conditions such as cancer, gastrointestinal infection, diabetes disorder, or viral infection, among many others [Citation42,Citation64,Citation65]. Due to their particular properties, such as self-propulsion, taxis, or stimuli-related responses, bacteria have been proven to be very interesting as alternative drug delivery systems by themselves or in combination with NMs. During the following section, we will explore some of the interesting and useful properties of bacteria for drug delivery on specific target sites and illustrate their properties with several examples.
2.3. Useful bacteria properties as nanocarriers
As mentioned previously, bacteria exhibit several interesting properties, such as self-propulsion and mobility, taxis, ability to respond to a wide variety of different stimuli, on-site production and delivery of proteins or other molecules, bacterial transfection, and capability of acting as filters of toxins or acting as cellular envelopes () [Citation29,Citation30]. These characteristics will be individually explained along the next paragraphs.
Figure 1. Representative scheme of interesting bacterial properties for drug delivery systems: self-propulsion thanks to the use of flagella and pili; taxis movement of bacteria toward nutrients; stimuli-responsive bacteria that can be activated with an adequate stimuli; batofection or the ability to transfect their plasmid material to cells; protein-production by the bacteria.
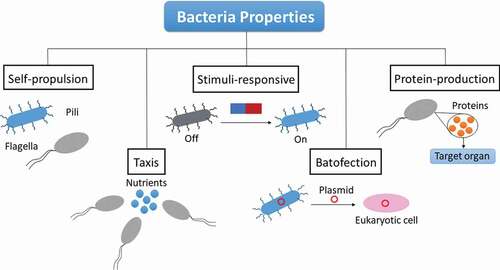
2.3.1. Self-propulsion and mobility
Bacteria possess appendices known as flagella and pili that allow them to navigate the media they inhabit [Citation66]. A pilus can be described as a hair-like appendage commonly found on the surface of bacteria. These bacterial elements can be used for self-propulsion, attachment, and bacterial conjugation. Pili are fundamental for bacteria attachment and biofilm formation, as well as for reproduction of the bacteria [Citation67]. In fact, pili are responsible for the virulence of many pathogenic bacteria, such as E. Coli, Vibrio cholarae, and several strains of Streptococcus. This is due to main role played by pili for the binding of bacteria to body tissues, which increases their replication rates and the interaction with the host [Citation67,Citation68].
Besides pili, flagella are the other physical bacterial elements that help motility and self-propulsion [Citation33,Citation34]. A flagellum is described as a whip-like appendage that protrudes from the cell body of certain cells called flagellates. Bacteria in general, as a type of prokaryotic cell, may display none, one or several flagella depending on the strain. Flagella primary function is locomotion, though they also act as sensory organelles with sensitivity toward the temperature of the media as well as to a range of chemical stimuli. The ability to respond to different stimuli is usually known as taxis [Citation35,Citation69,Citation70] and will be explained in detail in the next section. Helicobacter pylori is a great example of flagellated bacterium, where flagella plays an important role on the ability of the bacteria to cross the mucus lining until it reaches the stomach epithelium [Citation71].
In general, pili and, especially, flagella allow bacteria to propel in many different kinds of environments, liquid, or semi-solid, by generating a range of movement behaviors, including swimming, sliding, gliding and twitching among others [Citation66]. The movement of bacteria is generally stochastic, though its speed and direction may vary as a functional of external physical and chemical stimuli [Citation69].
2.3.2. Bacterial taxis
Under favorable environmental conditions, bacteria undergo active migration toward specific locations in a phenomenon known as bacterial taxis. In general, taxis is commonly defined as a movement of a particular organism in response to stimuli, such as the presence of food, different pH medias, light, or even oxygen [Citation70]. Different types of bacteria will lead to a variety of taxis behavior depending on the stimuli responsible for the bacteria reaction. It is important to distinguish between the terms taxis and tropism. While tropism is referred to the directional movement of plants in response to a range of environmental factors, taxis is utilized to describe the movement of animals, bacteria in this case, as a result of changes in the environment. Taxis phenomenon is based on the attractant of repellent stimuli faced by the bacteria, which will induce a direct movement up the attractant gradient or toward diminishing the repellent gradient, respectively [Citation35,Citation69].
2.3.3. Stimuli-responsive bacteria
The ability of self-propulsion of bacteria in response to certain stimuli and to perceive modifications of the environment can be employed to direct them toward specific locations inside the human body [Citation35,Citation70,Citation72]. These properties can be utilized for targeting and sensing purposes and, in general, for the use of bacteria as alternative drug delivery systems. As there are several different types of bacteria depending on the stimuli, relevant examples of these systems will be explained in more detail in the next sections.
2.3.3.1. Light-sensitive bacteria
As it has been mentioned before, bacteria can be genetically altered in order to modify their properties as a function of their therapeutic goal [Citation36]. One of the possibilities consists in the genetic engineering of bacterial cells to modify their light-related properties. In this case, bacteria are genetically modified to display light-sensitive ion channels or pores on their membranes. These channels and pores play a key role in the regulation of the flow of ions that travel across the membrane and, therefore, in the cell volume [Citation73]. Light-sensitive bacteria could be applied for the control of the transcription process, and their performance has been shown to be superior in comparison with the use of traditional promoter systems [Citation73–76].
The potential of the genetic modification of bacteria to induce light sensitivity or to create optogenetic drug delivery systems has been demonstrated by Motta-Mena et al. [Citation74]. In their publication, they present an optogenetic gene expression system based on a genetically engineered version of EL222, a bacterial light-activated protein (originated from Erytrhobacter Litoralis) that binds DNA when irradiated with blue light, hence promoting the transcription process in a controlled manner. The bio-engineered system was reported to display a large dynamic range of protein expression as well as rapid activation-deactivation kinetics. In addition, the performance of the system was tested for light-activated transcription in several mammalian cell lines and zebrafish embryos, where gene activation and no toxicity were observed. Another optogenetic system was designed and proposed by Polstein and Gersbach [Citation75]. They developed an engineered light-activated CRISPR-Cas9 effector that induces transcription of endogenous genes in the presence of blue light. This publication was particularly important, as it offered a versatile system that could be easily directed toward new DNA sequences and hence be used as a powerful tool for the design of bacterial switches.
2.3.3.2. Magnetically responsive bacteria
Magnetotactic bacteria are a specific bacteria type that is characterized by containing nanocrystals that allow them to synchronize to the magnetic field of the earth as well as respond to magnetic-stimuli [Citation77]. This means that the location of these bacteria, and therefore their action could be controlled by using a remote, long-range magnetic field. This idea was studied by Felfoul and Martel [Citation78,Citation79], whose group employed a magnetic resonance imaging machine as external magnetic field to induce a targeted navigation of magnetotactic bacteria. They demonstrated the effectiveness of their method by using a complex microchannel network, stating the potential of this strategy for drug delivery and tumor and infection targeting. This idea was further developed by the combination of magnetotactic bacteria with different therapeutic agents for the targeting of an angiogenic network in a tumor environment.
Unfortunately, though magnetotactic bacteria holds a great potential for biomedical applications, there are important concerns about their safety and their use for clinical purposes. Consequently, the focus of the study of magnetic directed systems has shifted toward non-magnetotactic bacteria loaded with magnetic nanoparticles as potential drug delivery systems [Citation80]. In a different research line, safer biohybrid systems constituted safer and commensal bacteria combined with magnetic nanomaterials are being studied. As an example of these types of systems, Carlsen et al. [Citation81]. presented a biohybrid system constituted by micro-swimmers propelled by multiple bacterial cells. These micro-swimmers are composed of Serratia Marcescens bacteria attached to superparamagnetic beads of micrometer size. Researchers demonstrated that this method of remote magnetic control allowed for higher control of bacterial navigation, reducing the stochastic motion. Nevertheless, it is important to highlight that the electromagnetic force from a source such as MRI decreases significantly with the particle size, which makes it challenging to propel smaller nanoparticles by using this method.
2.3.3.3. Thermo and pH-responsive bacteria
Changes in temperature and pH are known to be great indicators of the presence of medical issues. One of the most studied and important examples is the different temperature and pH that characterizes tumor environment derived from a great variety of cancer types [Citation82]. It is widely known that the fast reproduction of tumoral cells leads to the rapid and imperfect formations of tumors. Due to the high formation speed, the tumor does not possess a correctly vascularized structure, which leads to a hypoxic environment and deficient blood perfusion, and is known to exhibit a more acidic pH and higher temperature than the surrounding tissue. These distinctive differences between healthy and tumor tissues allow bacteria to differentiate and identify tumor cells. These bacterial cells could hence be potentially used for thermo- or pH-responsive drug delivery or targeting [Citation83].
Bacterial infections are also accompanied by changes in pH, usually derived from anaerobic fermentation on the affected tissue, and in temperature, due to the immune response. These factors open the possibility to employ pH and/or temperature as the main stimuli for the guidance and targeting of bacteria to specific tissues and parts of the body, for example, toward tumors [Citation64]. The work of Zhuang and coworkers constitutes a good example of the potential of pH-driven bacteria [Citation83]. These researchers reported the design and fabrication of multi-bacteria propelled micro-robots as bio-hybrid systems guided by pH gradients. These micro-robots are constituted by the combination of pH-sensitive flagelled bacteria (Serratia marcenses) and microbeads attached to them. Under the presence of specific pH gradients, the system displays unidirectional and bidirectional pH-taxis. The strength of this method lies the knowledge of the bacterial pathway and allows for its customization depending on the specific disease in terms of temperature, pH or even other secreted biomolecules. In addition, this method could be coupled to other signaling pathways, leading to the possibility of temperature/pH induced gene expression.
2.3.3.4. Oxygen-driven bacteria
Bacterial cells display the ability to respond to different concentrations of oxygen in the environment. While anaerobic bacteria are characterized by their affinity and taxis toward low oxygen regions, aerobic bacteria seek oxygen. As mentioned before, one of the most important characteristics of solid tumors is their hypoxic nature and, hence, anaerobic bacteria strands have been studied for cancer treatment. In particular, species of Clostridium and Streptococcus are known to accumulate on the hypoxic regions of solid tumors, which originally lead to the discovery of the tumor-targeting bacteria [Citation84]. The most important advantage with respect to nanomaterials is the ability of bacteria to actually penetrate to the tumor core, whereas nanomaterials tend to stay on the peripheral regions [Citation85]. As an example of anaerobic bacteria for tumor location and treatment, researchers have successfully genetically modified Clostridium to achieve the transport of immune-stimulant proteins for directing the killing of tumor cells as well as for the enhancement of IL-2 antitumor activity [Citation86].
Although strict anaerobic bacteria are good candidates for tumor therapy, they are only able to survive at the core of the tumor and note on the more external regions. In this regard, facultative anaerobes, such as E. Coli or Salmonella are a good alternative, as they are able to replicate at the border between healthy and necrotic tissue. One of the problems that arises is the control of the replication of this bacteria to the desired tissue, which can be sorted by genetic modification of the bacteria [Citation87]. A relevant example is a genetically modified version E. Coli that contains Yersinia invasion gene and hence allows uptake into mammalian cells [Citation88]. In this case, bacterial invasion was restricted by the use of a hypoxia promoter with a lux quorum sensing circuit of Vibrio fischeri, making sure the invasion takes place exclusively in low-oxygen tumor environments. In addition, Listeria listeriolysin protein can be utilized to enable endosomal escape and can be used to deliver different types of biomolecules, such as nucleic acids, specific genes, and many others [Citation89].
2.3.4. Protein production on site
One of the main challenges for drug delivery systems for therapeutic purposes is the nonspecific biodistribution that affects healthy organs and leads to important side effects. In this regard, bacterial cells offer the possibility of in situ protein expression, which can be applied for in situ production of a variety of therapeutic proteins [Citation90,Citation91]. This would help to drastically reduce the cost of the treatment, as it does not require any purification step, the dosage can be easily decreased by several orders of magnitude and it avoids exposure of the protein to harsh environments. As an example, Salmonella is one of the most studied bacteria for cancer treatment applications due to its high tumor-targeting capacity [Citation92,Citation93]. This bacterium has been proposed for immunotherapy coupled with in situ delivery of immunomodulatory proteins, such as cytokines. Of course, protein production is not limited to immuno-stimulating agents, as proteins can also act as drugs or prodrugs. However, it should be noted that there are some issues related to the transcription and translation process of eukaryotic proteins that can affect the activity and yield in the case of some proteins, leading to difficulties for protein expression [Citation94].
2.3.5. Batofection
Batofection is described as bacterial transfection, the ability of bacterial cells to transfer their genetic material into mammal cells. This property of bacteria has been applied to the delivery of oligonucleotides on site [Citation95,Citation96]. For this purpose, the genes of interest are coded in a bacterial plasmid, which is finally transferred to mammalian cells via batofection to release the material in the nucleus. Engineered bifidobacterium has been reported to enable delivery of genes to cancer cells. A specific example would be the delivery of endostatin gene, which displays high productivity and specificity for liver tumors [Citation97]. In general, batofection is directed toward therapy of infections and tumors through DNA vaccines [Citation98,Citation99]. This type of vaccines is usually constituted by promoter, antigen and specific plasmid and have been reported to be delivered to macrophages by using different bacteria strands.
2.3.6. Bacteria ghost
One of the main issues in the utilization of bacteria for biomedical issues is the need to accurately control bacteria invasion and replication. Bacterial cellular envelopes are proposed as an alternative solution as, unlike their alive counterparts, they lack the ability to colonize vital organs. The inner and outer surfaces of the envelope can be altered by genetic modification of the originally live bacteria. Interestingly, these bacterial envelopes are able to retain their surface structures, their bio-adhesion characteristics and keep their immunomodulation capacity [Citation100].
Among bacterial cellular envelopes, the most common form is bacterial ghosts [Citation101]. This form of bacterial envelope is described as a hollow, vacant, and non-living envelope of gram-negative bacteria. Bacterial ghosts are formed by the controlled expression of cloned gene E, which leads to the production of the membrane protein E that enables oligomerization and formation of transmembrane tunnel structures. This leads to a difference in the osmotic pressure between the cytoplasm and its surrounding, which causes the lysis of the bacteria and an empty non-living cell shell is left. In the case of gram-positive bacteria, the controlled expression of cloned gene E leads directly to the death of bacteria cell without lysis and, hence, no empty bacteria cellular envelope is formed through other strategies have been explored [Citation102].
2.4. Limitations of bacteria for medicine
Bacterial ghosts have been studied and developed for their potential medical application as delivery systems of bioactive molecules at least during the last two decades [Citation103]. One of the first publications regarding this topic was reported in 1999, where bacterial ghosts derived from E. Coli NM522 were genetically altered to carry biotinylated agents inserted into their cytoplasmic membrane. Researchers demonstrated the potential of these platforms as drug carriers with active targeting [Citation104]. Paukner and coworkers were the first ones to develop leakage-proof bacterial ghosts [Citation105]. Once again, bacterial ghosts were obtained from E. Coli NM522 and later loaded with calcein. Leakage was prevented by sealing the bacterial ghosts with membrane vesicles in the presence of calcium ions. Moreover, they were able to minimize the side effects of doxorubicin by loading the drug into bacterial ghosts formed from Mannheimia haemolytica, demonstrating that these bacterial envelopes could be used as slow-release drug delivery vehicles. In addition, Lin et al. developed bacterial cellulose and bacterial cellulose-chitosan membranes for their application to wound healing [Citation106]. In their publication, Lin and coworkers showed that these systems allowed the formation of new blood vessels and facilitated integration in treated wounds. The repaired dermis was very similar to normal skin. Numerous additional examples of bacterial ghosts for biomedical purposes can be found in the literature [Citation103].
Although the powerful potential of bacteria in the field of nanomedicine has been demonstrated, there are some serious challenges that must be overcome in order to achieve clinical translation of these alternative carriers. Due to their invasive nature, the dosage amount of bacterial vehicles that can be administered must be limited, which may lead to a less efficient performance. This is mainly due to the high immune response to high bacterial concentrations, which may lead to autoimmune reaction or rapid clearance of bacteria [Citation95].
Genetic modification is the main alternative to this issue. In general, bacteria are genetically altered in order to display a lower invasive character, attenuated toxicity and reduced immune response [Citation87]. These modifications must be carried out carefully for maintaining an adequate invasive and replication bacterial rate. It is important to notice that, even if the engineering of bacteria properties allows for a safer alternative, the removal of the toxic genes there may be a residual toxicity and could have especially dangerous consequences for immune-depressed patients. In addition, the use of antibiotics may also interfere with bacteria cell treatment, killing the bacteria and rendering the therapy useless. Regarding the problem of bacteria virulence, the use of commensal bacteria has been proposed [Citation107]. Commensal bacteria are considered a good alternative due to their nontoxic nature and their beneficial properties. However, these characteristics are usually limited to a specific body part, which means that even these bacteria may threaten the delicate equilibrium of microbiota and hence become problematic at other sites [Citation41].
Another important concern is the loss of functionality and behavior of genetically modified bacteria, especially because they will be introduced in a highly complex physiological environment very different from the one where the bacteria were developed. An alternative option would be to directly alter the bacterial chromosome to modify bacterial function [Citation29,Citation30]. The possibility of mutations must be taken into account when turning to this strategy as they can also pose potential complications. Bacteria are ultimately living and evolving organisms and their potential as drug carriers will be limited by their design for keeping a reliable performance in a complex environment.
Lastly, bacterial use for drug delivery must meet very distinctive regulatory requirements and guidelines regarding safety, toxicity, and manufacture technology [Citation29]. Bacteria behavior and performance at complex biological environments needs to be controlled and the risk of problems needs to be handled through extra safety layers and containment strategies. These biohybrid systems must be thoroughly characterized and understood before translation to the clinical field.
2.5. Bacteria based nano-system for biomedical applications
During the last years, a variety of different nanomaterials have been combined with bacteria to form a biohybrid drug delivery system with enhanced properties [Citation29,Citation30]. One example of this research work was presented by Dong and coworkers in 2018 [Citation108]. The authors studied the methodology of fabrication of living bacteria expressing peptides on their surface with metallic nanoparticles (NPs). The peptides have been used in order to attach gold nanoparticles (AuNPs), and it was demonstrated that the bacteria were still viable even after interaction with this nanomaterial. Summarizing, this group designed and optimized a fabrication method of living bacteria and NPs to create biohybrid agents with enhanced properties. These hybrids were shown to retain their viability as well as their ability to grow and divide. The peptides expressed on the surface of bacteria can be engineered depending on the nanomaterial that needs to be coupled with them.
Another important publication regarding bacterial hybrid systems was presented by Suh et al. in 2019 [Citation109]. In this case, their group designed a biohybrid system based on Salmonella enterica and poly(lactic-co-glycolic acid) NPs for cancer treatment. The system was named as NanoBEADS. In order to study penetration of the system in a tumoral tissue, the authors utilized 3D tumor spheroids for in vitro experiments, while the biodistribution of the system was investigated in vivo using a mammary tumor model. Results indicated that nanoparticle conjugation did not affect the ability of transport of the bacteria nor its penetration capacity. In addition, the system showed and enhanced particle retention and distribution in solid the solid tumor until 100-fold without the need for external stimuli. This autonomous biohybrid system shows the potential of the use of bacteria in combination with NMs for enhanced performance in cancer treatment by a more targeted drug delivery and, consequently, minimized side effects.
This group has published a very recent paper regarding the use of living bacteria as nanocarriers for NPs and drug delivery. In this case, Moreno and coworkers presented an alternative approach for the delivery of NPs achieving a high penetration in the model tumoral matrices [Citation27,Citation110]. In their work, researchers combined bacteria E. coli with mesoporous silica nanoparticles (MSNs) loaded with an anticancer drug. In order to couple the NPs with the bacteria, the bacterial surface was modified with azide groups, while MSNs were functionalized with dibenzocyclooctyne. In addition, MSNs were loaded with doxorubicin as cytotoxic compound. The dibenzocyclooctyne groups of the NP reacted in a click-type reaction with the azide groups of the bacterial surface, allowing the formation of the biohybrid system as it can be seen in .
Figure 2. Attachment of mesoporous silica nanoparticles to the surface of bacteria through click chemistry means [Citation110].
![Figure 2. Attachment of mesoporous silica nanoparticles to the surface of bacteria through click chemistry means [Citation110].](/cms/asset/d17094ae-8dae-4702-b8ec-014c6bd5d670/iedd_a_2029844_f0002_oc.jpg)
Motility and penetration ability of the system alone was tested first in a collagen matrix containing nutrients (). Later, these parameters were again tested but, this time, by using a 3D tumoral model constituted by a collagen matrix with human fibrosarcome cells embedded (). Results indicated that this novel biohybrid system has the ability to transport the NPs through the collagen matrix and, in addition, it is also capable of destroying up to 80% of the tumoral cells. This work presents a powerful strategy for cancer therapy that can be applied to other types of bacteria and that allows the loading of a variety of anticancer agents thanks to the remarkable properties of MSNs.
Figure 3. Illustration of the mobility and penetration studies of the biohybrid system using a nutrient-rich collagen 3D gel [Citation110].
![Figure 3. Illustration of the mobility and penetration studies of the biohybrid system using a nutrient-rich collagen 3D gel [Citation110].](/cms/asset/a7834449-ad66-4c44-b108-6c54af5480c6/iedd_a_2029844_f0003_oc.jpg)
Although these examples have focussed on mesoporous, metallic and polymeric NPs, there is a great variety of different NMs that have been coupled or can be coupled to bacteria for enhanced performance in targeted drug delivery for an array of medical issues [Citation28,Citation29]. These examples illustrate the potential of this type of biohybrid systems and the possibilities of novel platforms for biomedical applications.
3. Cells as alternative nanocarriers for nanomedicine
The use of cells and cell-based platforms as drug delivery systems was proposed as an alternative to purely nanomaterial-based synthetic vehicles due to the issues of the latter for an adequate targeting and delivery of the material to a specific site. Years of research on cells and their derivatives have led to the discovery of delivery pathways already provided by the human body and that could be potentially employed for biomedical purposes. Cells display their own unique properties, such as long-term blood circulation, crossing of barriers, site-specific migration among many others. In particular, circulating cells could possibly serve as delivery vehicles of different compounds due to their intrinsic features, which include high fluidity, unparalleled systemic circulation, natural delivery mechanisms, and lack of immunogenicity [Citation37,Citation111]. The following sections will be dedicated to present a brief summary of the beginnings of the use of cells in medicine, different types of cells that can be applied to biomedical applications as well as cell-mimicking platforms. Advantages of cells-NM systems over NMs will be discussed, and several examples of these biohybrid vehicles will be given.
3.1. Piece of history – cells and medicine
The use of cells for medical applications goes way back to before cells were actually known and described. There is evidence of exsanguination and reinjection as well as ingestion of blood and other tissues as soon as the 16th century. In the late 1500s, the first known device designed for infusion of tissue from a donor to a recipient was reported [Citation112]. It was not until 1665 that scientist Robert Hook discovered cells and first proposed the term ‘cell’ [Citation113]. This discovery was possible thanks to the improvements that Hook applied to existing microscopes, which allowed him to visualize cells. From then on, cells have been extensively studied by researchers all over the world with the aim of understanding cell functions as well as their relationship with medical issues.
Up to the 20th century, medical procedures, such as blood transfusion, assisted fertility, or organ transplantation, have become common, as well as using functional tissue for treatment of several diseases. However, the use of donor tissue to treat medical problems was limited to like for like, for instance: blood for blood or skin for skin tissues until the beginning of the 21st century. This paradigm changed with the discovery and description of the ‘stem cells’ by Ernest McCulloch and James Till in the early 1960s [Citation114]. Pluripotent stem cells have the potential of differentiating into many or any tissue type, which can nowadays be achieved owing to the development of the necessary tools for this purpose. These particular cell type is known to be a constituent of embryonic tissue and bone marrow, tissues that are particularly expensive and difficult to obtain. Due to their origin, stem cell use raises some ethical and legal questions regarding their collection and their utilization for commercial purposes [Citation115]. Fortunately, these cells were acknowledged in another kind of tissue, which provided an alternative for the collection, manipulation, and potential use of stem cells – or other different types of undifferentiated cells – for alternative therapies avoiding the need of embryonic or bone marrow materials [Citation116]. This leads to the possibility of commercialization at large scale of cell-therapy products.
Over the past years, the potential for repair and tissue regeneration of mesenchymal stem cells has been widely recognized, leading to the proposal of the use of these cells for therapeutic purposes. This has derived in the development and research of numerous cell-based strategies for biomedical applications by using a combination of different types of cells, drugs, enzymes, bioactive molecules and nanomaterials [Citation38,Citation39]. During the next sections, the most studied and commonly used cells, as well as examples of hybrid nanomaterial-cell systems for biomedical purposes will be described, and the advantages and limitations of their utilization mentioned.
3.2. Types of cells used for biomedical applications
Different types of cells will be utilized depending on their properties and the final applications. The most investigated types are blood cells, immune cells, and stem cells. Among other advantages, they help extend the circulation time, contribute to a better targeting capacity and are able to cross several biological barriers. In addition to cells, particles mimicking cell function and morphology have been also developed for their use in drug delivery [Citation28,Citation37]. During the next sections, type cells and cell derivatives will be individually explained, and some examples of their applications will be provided ().
Figure 4. Representative scheme of different types of cells and cell-mimicking platforms for drug delivery.
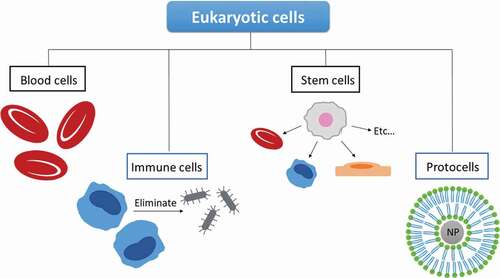
3.2.1. Blood cells
3.2.1.1. Red blood cells
Red blood cells (RBCs), also known as erythrocytes, are the most abundant type of cell present in the blood, consisting in 99% of the total amount of red blood cells in the human body. Being their major function, the transport of blood through the whole organism, RBCs present a large internal capacity volume, high surface area, and high flexibility that allows them to navigate and alter their shape and squeeze through the blood vessels when circulating in the cardiovascular system. In addition, RBCs are known to be biocompatible, biodegradable, and exhibit targeting ability. All these properties make them ideal alternative vehicles for the transport of a variety of therapeutic molecules [Citation117,Citation118].
Up to now, scientists have taken advantage of the prolonged circulation time and slow rate of drug release of RBCs for releasing bioactive molecules on the circulatory system [Citation119]. RBCs have been utilized for the delivery of drugs for the treatment of a variety of medical complications, such as parasitic infections, viral infections, or cardiovascular issues [Citation28]. Their ability to extend circulation times was reported by Chambers et al. [Citation120], where they were used to avoid rapid clearance of polymeric NPs. Their results indicated that attaching the NPs to the RBCs by non-covalent adhesion elongated their circulation from 10 min (NPs alone) to 10 h. Apart from NPs, it also prevents other active biomolecules, such as proteins or enzymes, from being rapidly eliminated through the liver. For instance, RBCs have been loaded with the enzyme L-Asparaginase and have been successfully employed for the treatment of acute lymphoblastic leukemia [Citation121].
RBCs also display targeting the ability of the spleen and the liver, as the majority of these cells are captured by the reticuloendothelial system (RES) of these particular organs after undergoing some structural changes. One example of this application is the loading of the glucocerebrosidase enzyme in RBCs for the treatment of Gaucher disease [Citation122]. In addition, RBCs containing deferoxamine can be applied to the treatment of iron accumulation in patients suffering from thalassemia [Citation111].
3.2.1.2. Platelets
Platelets are blood cells characterized by their small size and lack of nucleus. Although their life span (7–10 days) is much smaller than the one of RBCs (100–120 days), they display some features that make them interesting as drug vehicles. Long life-spans, high abundancy, high drug loading efficiency, and targeting ability are some of their desirable properties as drug delivery systems. One of the most important advantages of these cell types is its ability to migrate to parts of the body where the proliferation rate is higher, which makes them ideal for cancer therapy [Citation123]. Researchers have reported that doxorubicin-loaded platelets have a better performance, both in vitro and in vivo, than doxorubicin alone for the elimination of tumor cells [Citation124]. Moreover, Xu and coworkers also demonstrated that platelets release their cargo at a faster rate in more acidic environments [Citation124,Citation125]. Cancer tissues are known to be more acidic than healthy tissues and, therefore, the drug release is controlled by the presence of cancer cells. Metastatic cells are able to activate the aggregation of platelets around tumor cells, which helps them spread to other tissues through blood circulation. Remarkably, some scientists have discovered the tendency of metastatic cells to adhere to platelets in order to travel to different parts of the body. This fact offers the possibility of the use of platelets for the elimination of metastatic cells and tumors by the release of a variety of anticancer compounds [Citation126]. It was revealed that doxorubicin-loaded platelets functionalized with antibodies for facilitated endocytosis are able to release the drug not only near the tumor cells but also inside the tumor [Citation125].
3.2.2. Immune cells
3.2.2.1. Macrophages
Macrophages are a particular type of phagocytic blood cells that play a fundamental role as first-level defenders of the human immune system. These cells are derivatives of monocytes, which are able to migrate to locations undergoing infection and inflammation, such as pathologic and tumor sites [Citation127]. In addition, their ability to cross the blood-brain barrier make them attractive for therapy of neurological disorders [Citation128]. Their ability to deliver active drugs at a specific location was demonstrated by Dou et al. [Citation127]. In this publication, bone-marrow derived macrophages were coupled with lipid NPs containing indinavir for the treatment of HIV. They reported the accumulation of their biohybrid platform at the targeted location as well as a 2-week release of the drug that did not cause significant toxicity to the rest of the body.
Unfortunately, macrophages present some shortcomings such as drug delivery vehicles [Citation128,Citation129]. As live cells, a high amount of drug or NP cargo may interfere with cell migration, function, or survival, which limits the drug load. Moreover, due to their phagocytic nature, macrophages activity is not limited to uptake but rather followed by the release of acids and enzymes in order to destroy pathogens. This means that macrophages may digest their cargo by degrading the active biomolecules, reducing the effectiveness of the system.
3.2.2.2. Leucocytes
Leukocytes are a type of blood cells that are involved in the innate and adaptive immune systems. They display interesting features such as the ability to cross biological barriers as well as travel to specific sites affected by a disease. In addition, leukocytes are able to interact with cancer cells in solid tumors and also in the blood stream, providing an interesting pathway for cancer therapeutics [Citation130,Citation131]. Furthermore, leukocytes share some similarities with cancer cells regarding their physical and adhesive properties.
Neutrophils are a type of granular leukocytes, also known as polymorphonuclear granulocytes. Generated at the bone marrow, they are the most abundant immune cell type within the human peripheral blood. Interestingly [Citation132]. Neutrophils display natural chemotaxis toward inflammatory signals, and they can move freely through the circulatory system and its walls in order to rapidly attack antigens [Citation133]. Further, neutrophils are the first cells to arrive at an infection/inflammation site, where they produce cytokines for recruiting other cells [Citation134]. Although they have a short circulation life span, neutrophils have been successfully applied as alternative carrier platforms. For instance, Xue and coworker [Citation135] developed an alternative therapy for glioblastoma where they used neutrophils as carriers of chemotherapeutic agents. This strategy was also applied to the gastric cancer therapy [Citation136], where neutrophils contained abraxane and the chemotherapeutic drug was released at the tumor site. In both cases, neutrophils proved to be adequate drug vehicles that overcome the performance of the therapeutic agents alone.
Another type of leukocytes that has been proposed as a potential drug delivery system is lymphocytes [Citation137]. There are three main subtypes of lymphocytes: T cells, B cells, and natural killer cells. Among them, T cells are the ones that have been more extensively investigated as alternative drug carriers. These cells are specialized in destroying foreign invaders, increasing the B cell response and activating near-localized cells to stimulate the immune response. In addition to cell specificity and induced apoptosis, T cells are also able to cross the blood-brain barriers, which gives them access to restricted parts of the body. Stephan and coworkers [Citation138] took advantage of the ability of these cells to target and accumulate in tumors to design a drug carrier attaching drug-loaded NPs on the surface of T cells for potential cancer treatment. Another study reported the use of T-cells combined with lipid nanocapsules for the therapy of lymphomas (hematological cancer), which was revealed to increase survival and reduce tumor growth [Citation139].
3.2.2.3. Stem cells
Stem cells are described as self-renewable cells that have the potential to differentiate into several kinds of cell types, which is the main reason they have been applied for tissue regeneration and repair [Citation140]. Although research has been mainly focused on stem cells for tissue and cell replacement, the ability of stem cells to migrate toward tumors has made them interesting options for drug delivery in cancer therapy [Citation141]. In addition, stem cells can be genetically modified in order to express therapeutic genes encoding anti-tumor biomolecules [Citation142,Citation143].
Mesenchymal stem cells, a specific type of stem cells, have been genetically altered to produce INTB and have been successfully employed to target tumor cells in cases of breast carcinoma, prostate cancer, or lung metastasis [Citation129]. For these studies, mesenchymal stem cells were administered by local/intravenous injection, which led to a higher survival time and reduced toxicity in animal models. A variety of signal agents have been inserted into stem cells for cancer treatment. Another example of the use of these cells as delivery vehicles was published by Roger at al. [Citation144], where they designed a biohybrid system combining stem cells and polymeric NPs and lipid nanocapsules loaded with coumarin-6, a known anticancer drug. Their results revealed that drug loading did not affect the tumor tropic nature of stem cells.
3.2.3. Cell-mimicking platforms
In a similar way to bacteria ghosts, cell membranes can also be utilized for drug delivery purposes as they are natural drug carriers. Nevertheless, due to their lipid-based structure and how it is generated, it is complicated to load hydrophilic compounds and, therefore, deliver several drugs at the same time and control the process of release [Citation28]. In order to solve these issues, cell membranes have been combined with nanomaterials, which creates an improved delivery platform. It is important to note that the development of lipid-enveloped nanomaterials, which mimic membrane composition and structure, are also considered as cell-mimicking platforms. These lipid-coated nanoparticles are also known as ‘protocells’ [Citation145]. In this case, nanoparticles are covered by a supported lipid bilayer or hybrid lipid bilayer, which helps avoid the premature escape of the encapsulated drug, increases stability in solution and gives opportunity for an easy functionalization. In addition, lipid-coating helps tumor penetration and has a great potential for alternative therapies. Supported lipid bilayers are usually utilized for coating silica nanoparticles, as these nanomaterial type exhibits a hydrophilic surface [Citation146]. On the other hand, hybrid lipid bilayers are commonly applied on metallic nanoparticles [Citation147].
An interesting example of protocells prepared with supported lipid bilayers was reported by Villegas et al. [Citation85]. In their publication, researchers propose a combination of silica nanoparticles and supported lipid bilayers to form a protocell with the objective of improving tumor penetration and controlling drug release. The use of the supported lipid bilayers allows for the functionalization with additional polymeric nanocapsules that carry proteolytic enzymes. These enzymes are introduced in order to digest the extracellular matrix and facilitated the diffusion of the carrier toward the inside of the tumor tissue. Results indicated that this hybrid system successfully improves penetration ability and drug release control. In addition, Durfee and coworkers [Citation148] developed protocells for active targeting and delivery of drugs to leukemia cells. In their publication, the authors reported a robust synthetic route for silica nanoparticles coated with supported lipid bilayers, which was applied to silica nanoparticles of different size, shape, and pore morphology. They reported that, for silica nanoparticles of larger pores, the adequate coating was not possible by the proposed methodology. Researchers demonstrated that the designed protocells exhibited high specific targeting and were able to deliver the cytotoxic drug to leukemia cells without damaging healthy cells. Finally, super paramagnetic iron nanoparticles with hybrid lipid coating have been developed for their application as contrast agents for magnetic resonance imaging [Citation149]. In this case, protocells were loaded with an amphiphilic drug and were tested under in vitro and in vivo conditions. Other groups have further modified these nanoparticles and functionalization to improve their properties.
Regarding cell membrane-coated nanoparticles, Zhang and coworkers were responsible for the development of a coating methodology using red blood cell membranes, which could be applied to a variety of systems with different purposes. In their work, the authors fabricated membrane-coated nanoparticles by a three-step method: (1) separation of red blood cells from blood and hemoglobin; (2) obtain nanovesicles of around 100 nm by extrusion; (3) co-extrusion of membranes and nanoparticles to fuse them together [Citation150]. The potential of these platforms was evaluated by loading them with doxorubicin and using them for the therapy of acute myeloid leukemia [Citation151]. Although the majority of studies have employed red blood cell membranes, there are several examples of other types of cells. For example, cancer cell-membrane was employed to coat the surface of polymeric nanoparticles to fabricate a hybrid drug delivery carrier [Citation152]. The main objective of these hybrid systems is to deliver tumor-associated antigens into dendritic cells to induce a specific immune response. In addition, leukocyte membrane-coated mesoporous nanoparticles for cancer therapy [Citation153]. Their surface was further modified by glycans and other molecules in order to avoid uptake by macrophages. These vectors are then able to extend the circulation time and, therefore, enhance their efficiency again in cancer treatment after loading with doxorubicin.
3.3. Limitations of eukaryotic cells for medicine
Although the use of cells and cell-mimicking platforms holds a great potential for biomedical applications, it also poses some limitations, which may depend on the type of cell [Citation28,Citation37]. Two of the most general problems for the application of cells to medical purposes are their reproduction to larger amounts and their administration. In general, cells are very delicate organisms that must be handled carefully in order to maintain them alive and in good conditions. The cells need to be grown, stored, and transported, and they need to survive every step. In addition, their administration is difficult and may lead to infections or complications if not carefully performed. Also, cells are live organisms that will keep proliferating, and it is important to take into account that all these phenomena must be controlled. Finally, there are several factors that will depend on the cell type, such as the circulation time in the blood, the capacity for drug loading or the immune response that some may cause upon application.
3.4. Cell-based nano-systems for biomedical applications
Similar to bacteria, cells have been considered as interesting alternatives for targeted delivery of therapeutic compounds. Moreover, it has been demonstrated that the combination of cells and nanomaterials leads to improved characteristics and performance for biomedical applications [Citation28,Citation37,Citation111]. Among the wide variety of cells that can be used for this purpose, Xuan et al. reported the creation of a platform constituted by macrophages and silica-based nanoparticles containing doxorubicin as therapeutic agent for cancer treatment [Citation153]. It was proved that the silica-based nanocapsules minimally affect the migration ability of macrophages during the first 6–12 h and, therefore, give time for the delivery of the cytotoxic drug. This platform was tested using a xenograft model, where the box-loaded macrophages were intravenously injected. Results show a halt in the growth of the tumor and a minimal systematic toxicity produced by the system. A new cell-platform is presented, which can be modified for the loading of other bioactive compounds for the treatment of other medical issues.
This group has also published an interesting study regarding cells as carriers for nanoparticles and drugs, in this case, decidua-derived mesenchymal stem cells [Citation27,Citation154]. In their work, Paris et al. use these type of mesenchymal cells as a platform to carry mesoporous silica nanoparticles for cancer treatment. For this purpose, the first step consists in the internalization of the nanoparticles by the cells and the consequent co-localization study (). Once the nanoparticles were shown to be nontoxic for the cells, authors continued to test the migration capability of the cells under in vitro and in vivo conditions. Results indicated that the migration remained unaffected by the presence of the nanoparticles and the doxorubicin-loaded system was shown to induce cancer cell death under in vitro conditions.
Figure 5. Illustration scheme and confocal images of the fabrication of the biohybrid platform by combining mesenchymal stem cells with mesoporous silica nanoparticles [Citation154].
![Figure 5. Illustration scheme and confocal images of the fabrication of the biohybrid platform by combining mesenchymal stem cells with mesoporous silica nanoparticles [Citation154].](/cms/asset/b91af3d8-a61b-4e8d-9073-8ce60c1d225e/iedd_a_2029844_f0005_oc.jpg)
Immune cells such as T cells have also been studied as alternative nanocarriers for drug delivery. Tang and coworkers designed a platform combining T cells with protein-containing nanogels for tumor treatment [Citation155]. The authors focused on the design of an alternative platform for applying adoptive cell therapy with antigen-specific T cells. They propose the loading of T cells with protein drugs contained in nanogels that can be selectively released in response to receptor activation. After several assays under in vitro and in vivo conditions, results indicated the selective control over the cargo release from the nanogels as well as a higher tumor clearance.
These are merely a few examples of the multiple combinations that have been and are currently under study, employing a variety of different cell types and cell-membranes, as well as numerous distinct nanomaterials [Citation28,Citation37,Citation111]. These alternative biohybrid systems have been demonstrated to present improved and enhanced properties and performance of nanoparticles or cells on their own. Research regarding these novel carriers will continue to evolve and show the possibilities given by this interesting approach.
4. Conclusion
Bacteria and cells have been utilized for medical purposes for a very long time. The interesting properties and behavior they exhibit as a function of cell or bacteria type are remarkable characteristics that make them a relevant alternative for biomedical applications, such as cancer therapy or as drug delivery platforms. On the one hand, bacteria possess intrinsic characteristics such as self-propulsion, bacterial taxis, or stimuli-responsive capacities. In addition, they can be used for internalization of genetic material through batofection, and they can be emptied and employed in the form of bacteria ghosts as drug delivery platforms. On the other hand, eukaryotic cells also have a long history of medical applications, with examples as common nowadays, such as blood transfusion or skin transplants. Among the numerous types of cells existing in our organisms, a variety of them display remarkable properties like long-term circulation or tumor-targeting nature, which makes them useful for a potential use in biomedical applications. Moreover, in a similar way to bacterial ghosts, cell membranes, exosomes, and lipid mixtures are used as envelopes for bioactive molecules or other therapeutic agents.
Owing to their interesting properties, bacteria and cells have been combined with nanomaterials in order to improve the performance of the latter. One of the most studied issues in scientific research is cancer and its corresponding therapeutic strategy. Scientists have proposed the design of a nanomaterial with the ability to target tumors or tumor cells while also being able to release a drug of choice. Although several publications have demonstrated the potential of nanomaterials for biomedical purposes, there are several issues, such as the short-circulation time, lack of tumor penetration and insufficient targeting ability. The combination of nanomaterials with bacteria or cells is a different approach that has the potential to overcome these problems. Several research publications have actually reported an improvement in nanomaterial performance by creating these hybrid drug delivery platforms. Nowadays, there is no doubt that alternative approaches based on the use of microorganisms must be taken into consideration for biomedical applications and that the fabrication and design of these novel systems has just started.
5. Expert opinion
The use of bacteria and cells for the treatment of medical issues is not something new. However, the development of nanomaterials and manipulation techniques for bacteria and cells have opened a new world of possibilities for alternative therapies. While all of them – nanomaterials, bacteria, and cells – have been proposed individually for the diagnosis and treatment of a wide variety of medical problems, researchers have realized that their combination can lead to a novel platform with additional properties and enhanced performance.
Nevertheless, though this interesting combination holds a lot of potential, there are some limitations that need to be considered. To begin with, bacteria are living organisms that tend to proliferate under the right circumstances. Its properties and colonization abilities need to be carefully controlled in order to avoid additional complications. The main approach against this problem is based on the genetic modification of the bacteria properties or by the use of the so-called bacterial ghosts, non-living bacteria empty walls that lack the ability to proliferate. In the case of eukaryotic cells, the main problem lies in the production of higher quantities as well as the transportation and distribution means for their use. Cells are very delicate and need to be treated very carefully in order to keep them alive, which can be challenging. Analogous to bacteria, cells are living organisms that will keep proliferation under adequate conditions, which needs to be controlled. An alternative proposed, instead of using cells, is based on the development and use of cell membranes for the coating of nanomaterials to create protocells.
This novel field of research is extremely interesting though very new and, thus, numerous efforts are needed in order to define the limits of these alternative drug delivery platforms. During the coming years, it is quite probable that scientists will be testing a variety of different nanomaterials, such as liposomes, metallic nanoparticles, silica nanoparticles, and many others, in combination with either bacteria or cells of different types, and testing their properties before and after coupling with microorganisms. In addition, the effects and limits regarding the loading or coupling to bacteria and cells must be defined. A particular topic regarding this novel field would be possible negative consequences toward the organisms of these new carriers, which will be challenging taking into account the complexity of the system. This new field is certainly exciting as well as complex, and it is definitely going to bring a whole lot of new ideas for biomedical applications.
Article highlights
1. Bacteria and cells exhibit a variety of properties that make them attractive for their use in nanomedicine.
2. In addition, these interesting characteristics can be combined with an extensive array of nanomaterials in order to create biohybrid systems with improved properties.
3. Among their interesting characteristics, bacteria present self-propulsion and mobility, bacterial taxis, stimuli-response behavior, ability to batofection, and to produce proteins in-situ, while their membrane – bacteria ghosts – can also be used for biomedical purposes.
4. There are many different types of cells with their own set of properties that can be used for medicine such as red blood cells, immune cells, or stem cells, among many others.
5. Bio-inspired and biohybrid systems potential for biomedical applications has been demonstrated, though a lot of research remains to be done in order to improve these systems, warrant their safety, and, finally, be applied to the clinic.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Hua S, Wu SY . Advances and Challenges in Nanomedicine. Vol. 9. Belgium: Frontiers in Pharmacology. 2019.
- Kim BYS, Rutka JT, Chan WCW. Nanomedicine. N Engl J Med. 2010;363:10.
- Vallet-Regí M, Rámila A, Del Real RP, et al. A New Property of MCM-41: drug Delivery System. Chem Mater. 2001;13:4.
- Vallet-Regí M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem. 2007;46(4010):7548.
- Sau TK, Goia DV. Biomedical Applications of Gold Nanoparticles. United States: Springer; 2012.
- Vallet-Regí M. Our Contributions to Applications of Mesoporous Silica Nanoparticles. United States: Acta Biomaterialia, 2021; In Press.
- Bellah M, Christensen SM, Iqbal SM. Nanostructures for Medical Diagnosis. J Nanomater. 2012;1:21.
- Castillo RR, Colilla M, Vallet-Regí M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin Drug Deliv. 2017;14(2):14.
- Vallet-Regí M, González B, Izquierdo-Barba I. Nanomaterials as Promising Alternative in the Infection Treatment. Int J Mol Sci. 2019;20(15):18.
- Baeza A, Vallet-Regí M. Mesoporous Silica Nanoparticles as Theranostic Antitumoral Nanomedicines. Pharmaceutics. 2020;12(10):16.
- Zhao P, Li N, Astruc D. State of the art in gold nanoparticle synthesis. Coord Chem Rev. 2013;257:28.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
- Vallet-Regí M, Colilla M, Izquierdo-Barba I, et al. Mesoporous Silica Nanoparticles for Drug Delivery: current Insights. Molecules. 2017;23(1):47.
- Álvarez E, Estévez M, Jiménez-Jiménez C, et al. A versatile multicomponent mesoporous silica nanosystem with dual antimicrobial and osteogenic effects. Acta Biomater. 2021;136:570–581.
- Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle-protein Corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences (PNAS). 2007. 104: p. 6.
- Ke PC, Lin S, Parak WJ, et al. A Decade of the Protein Corona. ACS Nano. 2017;11(12):4.
- Monopoli M, Aberg C, Salvati A, et al. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:8.
- Ahsan SA, Rao CM, Ahmad MF. Nanoparticle-Protein Interaction: the Significance and Role of Protein Corona. Cellular and Molecular Toxicology of Nanoparticles - Advances. 2018;1:24.
- Nguyen VH, Lee B-J. Protein Corona: a new approach for nanomedicine design. Int J Nanomedicine. 2017;12:15.
- Zanganeh S, Spitler R, Erfanzadeh M, et al. Protein Corona. Opportunities and Challlenges. Int J Biochem Cell Biol. 2016;75:5.
- Lundqvist M, Stigler J, Elia G, et al. Nanoparticle size and surface properties determine the protein Corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences (PNAS). 2008. 105( 38): p. 6.
- Liu W, Rose J, Plantevin S, et al. Protein Corona formation for nanomaterials and proteins of a similar size: hard or soft Corona? Nanoscale. 2013;5:11.
- Caracciolo G, Pozzi D, Capriotti AL, et al. Lipid composition: a “key” factor for the rational manipulation of the liposome-protein Corona by liposome design. The Royal Society of Chemistry Advances. 2015;5. 9.
- Caracciolo G. Clinically approved liposomal nanomedicines: lessons learned from the biomolecular Corona. Nanoscale. 2018;10:6.
- Charbgoo F, Nejabat M, Abnous K, et al. Gold nanoparticle should understand protein Corona for being a clinical nanomaterial. J Control Release. 2018;272:15.
- Hadjdemetriou M, McAdam, S, Garner, G, et al. The Human In Vivo Biomolecule Corona onto PEGylated Liposomes: a Proof-of-Concept Clinical Study. Adv Mater. 2019;31(4):e1803335.
- Pérez JMM; María Vallet-Regí. GIBI. Bacteria and cells as ”cabs” for drug-loaded nanoparticles. 2021. https://www.youtube.com/watch?v=Wsbcpt-cE3E
- Yoo J-W, Irvine DJ, Discher DE, et al. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535.
- Shende P, Basarkar V. Recent trends and advances in microbe-based drug delivery systems. Daru. 2019;27(2):799–809.
- Hosseinidoust Z, Mostaghaci B, Yasa O, et al. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44. Pt A.
- Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18:2–4.
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214.
- Sowa Y, Rowe AD, Leake MC, et al. Direct observation of steps in rotation of the bacterial flagellar motor. Nature. 2005;437(7060):916–919.
- Martel S. Bacterial microsystems and microrobots. Biomed Microdevices. 2012;14(6):1033–1045.
- Storz G, Hengge R. Bacterial Stress Responses. 2nd ed. A.S.f.M. United States: ASM Press; 2010.
- Lim D, Song M. Development of bacteria as diagnostics and therapeutics by genetic engineering. J Microbiol. 2019;57(8):637–643.
- Lutz H, Hu S, Dinh P-U, et al. Cells and cell derivatives as drug carriers for targeted delivery. Medicine in Drug Discovery. 2019;3. 100014.
- Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev. 2012;64(8):739–748.
- Hu YL, Fu Y-H, Tabata Y, et al. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147(2):154–162.
- Wu GD, Lewis JD. Analysis of the Human Gut Microbiome and Association With Disease. Clin Gastroenterol Hepatol. 2013;11(7):774–777.
- Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157(1):121–141.
- Islam SU. Clinical Uses of Probiotics. Medicine (Baltimore). 2016;95(5):e2658.
- Wieërs G, Belkhir L, Enaud R, et al. How Probiotics Affect the Microbiota. Front Cell Infect Microbiol. 2019;9:454.
- Sharifi-Rad J, Rodrigues CF. Probiotics: versatile Bioactive Components in Promoting Human Health. Medicina (Kaunas). 2020; 56(9):43.
- Dimidi E, Christodoulides S, Scott SM, et al. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 2017;8(3):484–494.
- Valdés-Varela L, Gueimonde M, Ruas-Madiedo P. Probiotics for Prevention and Treatment of Clostridium difficile Infection. Adv Exp Med Biol. 2018;1050:161–176.
- Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;11(11): Cd003048.
- Metchnikoff E. The prolongation of life: optimistic studies. United Kingdom: Heinemann. 1908.
- Podolsky SH. Metchnikoff and the microbiome. Lancet. 2012;380(9856):1810–1811.
- Mackowiak P. Recycling Metchnikoff: probiotics, the Intestinal Microbiome and the Quest for Long Life. Front Public Health. 2013;1: 1–3.
- Prescott SL. History of medicine: origin of the term microbiome and why it matters. Human Microbiome Journal. 2017;4:24–25.
- Ozen M, Dinleyici EC. The history of probiotics: the untold story. Benef Microbes. 2015;6(2):159–165.
- Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30(1):54–60.
- Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;1(45 Suppl):S149–53.
- Bizzini B, Pizzo G, Scapagnini G, et al. Probiotics and oral health. Curr Pharm Des. 2012;18(34):5522–5531.
- Yari M, Ghoshoon MB, Vakili B, et al. Therapeutic Enzymes: applications and Approaches to Pharmacological Improvement. Curr Pharm Biotechnol. 2017;18(7):531–540.
- Drăgănescu M, Carmocan C. Hormone Therapy in Breast Cancer. Chirurgia (Bucur). 2017;112(4):413–417.
- Casarini L, Crépieux P, Reiter E, et al. FSH for the Treatment of Male Infertility. Int J Mol Sci. 2020;21(7):2270.
- Roland KL, Brenneman KE. Salmonella as a vaccine delivery vehicle. Expert Rev Vaccines. 2013;12(9):1033–1045.
- Bruhn KW, Craft N, Miller JF. Listeria as a vaccine vector. Microbes Infect. 2007;9(10):1226–1235.
- Somerville JE Jr., Cassiano L, Bainbridge B, et al. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Invest. 1996;97(2):359–365.
- Low KB, Ittensohn M, Le T, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17(1):37–41.
- Mohr KI. History of Antibiotics Research. Curr Top Microbiol Immunol. 2016;398:237–272.
- Duong MT, Qin Y, You S-H, et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51(12):1–15.
- Duan FF, Liu JH, March JC. Engineered Commensal Bacteria Reprogram Intestinal Cells Into Glucose-Responsive Insulin-Secreting Cells for the Treatment of Diabetes. Diabetes. 2015;64(5):1794–1803.
- Kearns DB. A field guide to bacterial swarming motility. Nature Rev Microbiol. 2010;8(9):634–644.
- Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66(4):613–635.
- Danne C, Dramsi S. Pili of gram-positive bacteria: roles in host colonization. Res Microbiol. 2012;163(9–10):645–658.
- Goldstein RA, Soyer OS. Evolution of Taxis Responses in Virtual Bacteria: non-Adaptive Dynamics. PLoS Comput Biol. 2008;4(5):e1000084.
- Krell T, Lacal J, Muñoz-Martinez F, et al. Diversity at its best: bacterial taxis. Environ Microbiol. 2011;13(5):1115–1124.
- Gu H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74(7):863–869.
- Taniguchi SI, Shimatani Y, Fujimori M. Tumor-Targeting Therapy Using Gene-Engineered Anaerobic-NonpathogenicBifidobacterium longum. In: Hoffman RM, editor. Bacterial Therapy of Cancer: methods and Protocols. New York: Springer New York; 2016. p. 49–60.
- Pastrana E. Optogenetics: controlling cell function with light. Nat Methods. 2011;8(1):24–25.
- Motta-Mena LB, Reade A, Mallory MJ, et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat Chem Biol. 2014;10(3):196–202.
- Polstein LR, Gersbach CA. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol. 2015;11(3):198–200.
- Olson EJ, Tabor JJ. Optogenetic characterization methods overcome key challenges in synthetic and systems biology. Nat Chem Biol. 2014;10(7):502–511.
- Faivre D, Schüler D. Magnetotactic Bacteria and Magnetosomes. Chem Rev. 2008;108(11):4875–4898.
- Felfoul O, Martel S. Assessment of navigation control strategy for magnetotactic bacteria in microchannel: toward targeting solid tumors. Biomed Microdevices. 2013;15(6):1015–1024.
- Martel S. Towards MRI-controlled ferromagnetic and MC-1 magnetotactic bacterial carriers for targeted therapies in arteriolocapillar networks stimulated by tumoral angiogenesis. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:3399–3402.
- Tang YS, Wang D, Zhou C, et al. Bacterial magnetic particles as a novel and efficient gene vaccine delivery system. Gene Ther. 2012;19(12):1187–1195.
- Carlsen RW, Edwards MR, Zhuang J, et al. Magnetic steering control of multi-cellular bio-hybrid microswimmers. Lab Chip. 2014;14(19):3850–3859.
- Zhang X, Lin Y, Gillies RJ. Tumor pH and Its Measurement. J Nucl Med. 2010;51(8):1167–1170.
- Zhuang J, Wright Carlsen R, Sitti M. pH-Taxis of Biohybrid Microsystems. Sci Rep. 2015;5(1):11403.
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447.
- Villegas MR, Baeza A, Noureddine A, et al. Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices. Chem Mater. 2018;30(1):112–120.
- Chen CY, Chen C-F, Yi Y, et al. Construction of a microrobot system using magnetotactic bacteria for the separation of Staphylococcus aureus. Biomed Microdevices. 2014;16(5):761–770.
- Anderson JC, Clarke EJ, Arkin AP, et al. Environmentally Controlled Invasion of Cancer Cells by Engineered Bacteria. J Mol Biol. 2006;355(4):619–627.
- Stritzker J, Weibel S, Hill PJ, et al. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297(3):151–162.
- Xiang S, Fruehauf J, Li CJ. Short hairpin RNA–expressing bacteria elicit RNA interference in mammals. Nat Biotechnol. 2006;24(6):697–702.
- Seavey MM, Pan Z-K, Maciag PC, et al. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;15(3):924–932.
- Du ZQ, Wang JY. A novel lumazine synthase molecule from Brucella significantly promotes the immune-stimulation effects of antigenic protein. Genet Mol Res. 2015;14(4):13084–13095.
- Barbé S, Mellaert LV, Theys J, et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol Lett. 2005;246(1):67–73.
- Feltis BA, Miller JS, Sahar DE, et al. Liver and Circulating NK1.1+CD3− Cells Are Increased in Infection with Attenuated Salmonella typhimurium and Are Associated with Reduced Tumor in Murine Liver Cancer. J Surg Res. 2002;107(1):101–107.
- Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 2014;5(172):1–17.
- Pálffy R, Gardlik R, Hodosy J, et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther. 2006;13(2):101–105.
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells Proceedings of the National Academy of Sciences. 77( 4): p. 2163–2167.
- Yazawa K, Fujimori M, Nakamura T, et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat. 2001;66(2):165–170.
- Okuda K, Wada Y, Shimada M. Recent Developments in Preclinical DNA Vaccination. Vaccines (Basel). 2014;2(1):89–106.
- Sciaranghella G, Lakhashe S, Ayash-Rashkovsky S, et al. A live attenuated Listeria monocytogenes vaccine vector expressing SIV Gag is safe and immunogenic in macaques and can be administered repeatedly. Vaccine. 2011;29(3):476–486.
- Montanaro J, Inic-Kanada A, Ladurner A, et al. Escherichia coli Nissle 1917 bacterial ghosts retain crucial surface properties and express chlamydial antigen: an imaging study of a delivery system for the ocular surface. Drug Des Devel Ther. 2015;9:3741–3754.
- Kudela P, Koller VJ, Lubitz W. Bacterial ghosts (BGs)—Advanced antigen and drug delivery system. Vaccine. 2010;28(36):5760–5767.
- Langemann T, Koller VJ, Muhammad A, et al. The bacterial ghost platform system. Bioengineered Bugs. 2010;1(5):326–336.
- Tabrizi CA, Walcher P, Mayr UB, et al. Bacterial ghosts – biological particles as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15(6):530–537.
- Huter V, Szostak MP, Gampfer J, et al. Bacterial ghosts as drug carrier and targeting vehicles. J Control Release. 1999;61(1):51–63.
- Paukner S, Kohl G, Jalava K, et al. Sealed bacterial ghosts–novel targeting vehicles for advanced drug delivery of water-soluble substances. J Drug Target. 2003;11(3):151–161.
- Lin W-C, Lien C-C, Yeh H-J, et al. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr Polym. 2013;94(1):603–611.
- Claesen J, Fischbach MA. Synthetic Microbes As Drug Delivery Systems. ACS Synth Biol. 2015;4(4):358–364.
- Dong Z-M, Jin X, Zhao G-C. Amplified QCM biosensor for type IV collagenase based on collagenase-cleavage of gold nanoparticles functionalized peptide. Biosens Bioelectron. 2018;106:111–116.
- Suh S, Jo A, Traore MA, et al. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Advance Science (Weinh). 2019;6(3):1801309.
- Moreno VM, Álvarez E, Izquierdo-Barba I, et al. Bacteria as Nanoparticles Carrier for Enhancing Penetration in a Tumoral Matrix Model. Adv Mater Interfaces. 2020;7(11):1901942.
- Zargar SM, Hafshejani DK, Eskandarinia A, et al. A Review of Controlled Drug Delivery Systems Based on Cells and Cell Membranes. J Med Signals Sens. 2019;9(3):181–189.
- Hart GD. Descriptions of blood and blood disorders before the advent of laboratory studies. Br J Haematol. 2001;115(4):719–728.
- Hook R. Micrographia. United Kingdom: Echo Library. 1665.
- Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454.
- McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11(10):1026–1028.
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663–676.
- Hamidi M, Zarrin A, Foroozesh M, et al. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118(2):145–160.
- Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7(4):403–427.
- Gopal V, Kumar AR, Usha AN, et al. Effective drug targeting by Erythrocytes as Carrier Systems. Current Trends in Biotechnology and Pharmacy. 2007;1. 18–33.
- Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100(1):111–119.
- Domenech C, Thomas X, Chabaud S, et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153(1):58–65.
- Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: mechanism and extended circulation. Exp Biol Med (Maywood). 2007;232(7):958–966.
- Sun Y, Su J, Liu G, et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–128.
- Xu P, Zuo H, Chen B, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep. 2017;7:42632.
- Xu P, Zuo H, Zhou R, et al. Doxorubicin-Loaded Platelets Conjugated with Anti-CD22 Mabs: a Novel Targeted Delivery System for B-Cell Lymphoma Treatment with Cardiac Avoidance. Blood. 2017;130:1535.
- Modery-Pawlowski CL, Master AM, Pan V, et al. A platelet-mimetic paradigm for metastasis-targeted nanomedicine platforms. Biomacromolecules. 2013;14(3):910–919.
- Dou H, Destache CJ, Morehad JR, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835.
- Klyachko NL, Polak R, Haney MJ, et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials. 2017;140:79–87.
- Zhang W, Wang M, Tang W, et al. Nanoparticle-Laden Macrophages for Tumor-Tropic Drug Delivery. Advanced Materials. 2018;35:e1805557.
- Dong X, Chu D, Wang Z. Leukocyte-mediated Delivery of Nanotherapeutics in Inflammatory and Tumor Sites. Theranostics. 2017;7(3):751–763.
- Mitchell MJ, King MR. Leukocytes as carriers for targeted cancer drug delivery. Expert Opin Drug Deliv. 2015;12(3):375–392.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446.
- Chu D, Dhong X, Shi X, et al. Neutrophil-Based Drug Delivery Systems. Advanced Materials. 2018;322:e1706245
- Amulic B, Cazalet C, Hayes GL, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489.
- Xue J, Zhao Z, Zhang L, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692–700.
- Ju C, Wen Y, Zhang L, et al. Neoadjuvant Chemotherapy Based on Abraxane/Human Neutrophils Cytopharmaceuticals with Radiotherapy for Gastric Cancer. Small. 2019;15(5):e1804191.
- Yu H, Yang Z, Li F, et al. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020;27(1):1425–1437.
- Stephan MT, Moon JJ, Um SH, et al. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–1041.
- Huang B, Abraham WD, Zheng Y, et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7(291):291ra94.
- Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283.
- Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer. 2014;14(10):683–691.
- Dembinski JL, Wilson SM, Spaeth EL, et al. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013;15(1):20–32.
- Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106(12):4822–4827.
- Roger M, Clavreul A, Venier-Julienne M-C, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31(32):8393–8401.
- Miller DM, Gulbis JM. Engineering protocells: prospects for self-assembly and nanoscale production-lines. Life (Basel). 2015;5(2):1019–1053.
- Butler KS, Durfee PN, Theron C, et al. Protocells: modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery. Small. 2016;12(16):2173–2185.
- Luchini A, Vitiello G. Understanding the Nano-bio Interfaces: lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications. Front Chem. 2019;7(343):1–16.
- Durfee PN, Lin Y-S, Dunphy DR, et al. Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells. ACS Nano. 2016;10(9):8325–8345.
- Mahmoudi M, Sant S, Wang B, et al. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1):24–46.
- Zhang H. Erythrocytes in nanomedicine: an optimal blend of natural and synthetic materials. Biomater Sci. 2016;4(7):1024–1031.
- Marczak A, Kowalczyk A, Wrzesien-Kus A, et al. Interaction of doxorubicin and idarubicin with red blood cells from acute myeloid leukaemia patients. Cell Biol Int. 2006;30(2):127–132.
- Fang RH, Hu C-M, Luk BT, et al. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014;14(4):2181–2188.
- Xuan M, Shao J, Dai L, et al. Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv Healthc Mater. 2015;4(11):1645–1652.
- Paris JL, De la Torre P, Manzano M, et al. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016;33:275–282.
- Tang L, Zheng Y, Bandeira Melo M, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36(8):707–716.
