ABSTRACT
Introduction
Intramuscular (IM) injections deliver a plethora of drugs. The majority of IM-related literature detail s dissolution and/or pharmacokinetic (PK) studies, using methods with limited assessments of post-injection events that can impact drug fate, and absorption parameters. Food and Drug Association guidelines no longer require preclinical in vivo modeling in the U.S.A. Preclinical animal models fail to correlate with clinical outcomes, highlighting the need to study, and understand, IM drug fate in vitro using bespoke models emulating human IM sites. Post-IM injection events, i.e. underlying processes that influence PK outcomes, remain unacknowledged, complicating application of in vitro methods in preclinical drug development. Understanding such events could guide approaches to predict and modulate IM drug fate in humans.
Areas covered
This article reviews challenges in biorelevant IM site modeling (i.e. modeling drug fate outcomes), the value of technologies available for developing IM injectables, methods for studying drug fate, and technologies for training in performing IM administrations. PubMed, Web-of-Science, and Lens databases provided papers published between 2014 and 2024.
Expert opinion
IM drug research is expanding what injectable therapeutics can achieve. However, post-injection events that influence PK outcomes remain poorly understood. Until addressed, advances in IM drug development will not realize their full potential.
KEYWORDS:
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1. Introduction
Intramuscular (IM) injections deposit a formulation into the belly of a skeletal muscle (i.e., muscle associated with the skeletal system). This parenteral route evades the digestive conditions of the gastrointestinal tract, which are generally unsuitable for delivering e.g., macromolecules [1]. Indeed, the IM route presents some advantages over the commonly employed subcutaneous (SC) route. Blood flow per mass of SC tissue ranges between 0.02 and 0.15 L/min/Kg [2]. Injection volumes are usually limited to 1.5 mL [3], while increased volumes are now possible [4]. This larger injectable volume can be beneficial for administration of long-acting injectable (LAI) formulations. Skeletal muscles, by comparison, are vascularized to a far greater degree than the hypodermis [5]. Blood flow per mass of skeletal muscle tissue can be as much as 1 L/min/Kg at rest, which is essential for efficient muscle function (via simultaneous delivery of oxygen, and removal of metabolic waste products) [6]. This increased blood flow, compared to the SC compartment, can permit rapid access of drug to the systemic vasculature [7,8,9]. This higher vascular flow, and larger injectable volumes (up to a theoretical 5 mL) are critical factors in selecting the IM route over the SC route for administering drugs types ranging from small molecules to macromolecules [10]. Drugs considered for IM administration can be formulated for a rapid onset of action, such as epinephrine in treating anaphylactic shock, or for controlled, extended periods of release per individual injection, such as the LAI formulations of the prodrug haloperidol decanoate for managing schizophrenia. A more detailed review of IM-approved pharmaceuticals has been published previously [10].
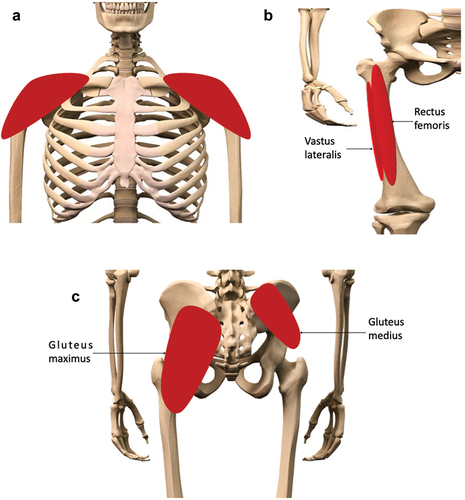
The typical sites of IM administration in humans are the deltoid, vastus lateralis, rectus femoris, and ventrogluteal plane of the gluteus medius muscles () [11]. The dorsogluteal muscle, whilst once an accepted injection site, is no longer recommended, owing to the risk of sciatic nerve damage from a poorly placed injection [12]. The ventrogluteal site, by comparison, is sufficiently distanced from the sciatic nerve to essentially eliminate this risk. Furthermore, generally, the ventrogluteal site presents a thinner layer of adipose tissue than the dorsogluteal site, increasing the likelihood of successful IM administration, as opposed to an erroneous SC injection [13]. The choice of injection site is, usually, dependent on the volume of material to be injected. Smaller volumes (< 1 mL) are typically administered in the deltoid muscle; this route is favored for e.g., vaccine administration, due to ease of access [14]. Larger volumes (up to an absolute theoretical maximum of 5 mL) can be administered to the other listed muscles [7]. If volumes > 5 mL are required at any one time per site, injections can be split between two sites, e.g., the left and right ventrogluteal sites.
Figure 1. Preferred sites for intramuscular (IM) injections in the human body. Depending on volume of injectate to be administered, IM injections are performed into the mass of the A) deltoid muscle, B) rectus femoris, vastus lateralis, or C) the dorsogluteal plane of the gluteus medius muscle. The gluteus maximus muscle, though no longer recommended for IM injections, due to risk of sciatic nerve damage, is shown here for context.
Since the 1940s, when IM administrations became common for administering antibiotics, the IM injection of therapeutics has continuously grown. The successful development of IM-administered pharmaceuticals has been well appraised [10,15,16]. Indeed, ongoing efforts in development of IM-administered LAI format drugs continues to push the boundaries of formulation science, encompassing such technologies as in situ forming implants, and nanosuspensions, allowing controlled drug release from a single IM depot site over extended time periods per individual administration [17,18,19,20,21]. In situ forming implants, forming within the IM site itself (as opposed to physical implants, generally administered in the SC site [22]), could be developed through a bioengineered approach, taking advantage of biomimetic materials, to further refine the design of injectable, controlled release formulations. Indeed, risperidone pharmacokinetic (PK) profiles from in situ “micro-implants” (ISM; a polymeric matrix that precipitates in vivo), allowed a rapid transition from introductory oral risperidone to the ISM injectable after 24 hours, compared to the present 7 days transition period from oral to injectable formulations [17]. These materials can mimic the natural properties of the extracellular matrix (ECM), promoting better integration with the surrounding tissues, which may help prevent deformation of the implant. Yet, conformational changes may result from the compressive force of muscle tissue via dynamic movement [18]. Excessive “bursts” of drug release, for example, could result from such compression of the in situ forming implant. The implant could also split into smaller pieces, increasing the surface area, and thereby affecting PK profiles further. Such potential events need suitable IM-specific in vitro tools to allow proper investigation, and understanding, of subsequent phenomena. Nanosuspensions (nanocrystals of pure drug nanoparticles with a typical diameter 200-500 nm), on the other hand, can enhance the solubility of hydrophobic drugs, improving systemic uptake from the highly vascularized IM compartment [19]. Indeed, the prodrug paliperidone palmitate was successfully marketed as a nanosuspension in 2015, requiring a single IM injection every 3 months. As an amorphous suspension, such technologies can distribute along paths of least resistance after injection, adapting to the physical conformation of the skeletal muscle at the site of injection. Particle size appears to be a determinant of PK outcomes, as observed with in vivo models administered with celecoxib, and prodrugs of testosterone-cholesterol [23,24]. Combinations of nansosuspensions-in-hydrogels, further controlling PK outcomes through “bioadhesion” parameters, improving integration with host tissue, have been discussed previously [25].
Such technologies can simultaneously enhance patient adherence to therapeutics for indications such as schizophrenia, while enhancing patient quality of life by minimizing frequency of clinic visits. Despite these advances noted in publications, the majority of articles related to IM-administrations describe PK outcomes of a drug and its formulation. Despite the long-term success of IM drug development, this focus on PK studies (often with in vitro tools of little biorelavance, and in vivo models) has resulted in a critical knowledge gap that requires urgent attention. There remains an ongoing, persistent gap in understanding how drugs and their formulations behave within the local skeletal muscle environment post-IM injection, i.e., “drug fate” at the injection site. There are several factors that could affect drug fate following IM injection. For example, solubility of the injectable could be affected by a rapid shift from “formulation” to “physiological” conditions, such as the transition to physiological temperature of 37 °C and/or pH of 7.4. It is also possible that the elements of the injectable could interact with physical ECM elements, such as collagens, fibronectin, and hyaluronic acid (HA). Such post-injection events could directly influence PK outcomes of a drug, and, therefore, therapeutic success.
Acellular in vitro modelling of intramuscular injection site events provides an avenue to efficient and effective development of intramuscular drugs, as this would provide critical insights into possible PK outcomes for a formulation that might be undesirable in the clinic. By simulating the complex environment of muscle tissue, researchers can scrutinize how drugs diffuse through the IM environment and access the systemic circulation under tractable, controlled conditions. Cellular models could then further investigate potential interactions between drug formulations and skeletal muscle tissue related to metabolism and cytotoxicity. This detailed understanding can aid in optimizing drug formulations, ensuring consistent efficacy, and minimizing potential adverse reactions in the clinic. Such approaches could replace many of the currently utilized in vivo preclinical models, which may or may not be predictive of clinical outcomes as highlighted by the recently changing regulatory landscape.
In preclinical development of novel pharmaceuticals, in vivo modelling has been a regulatory requirement in providing evidence of safety, and efficacy, of novel drugs prior to clinical trials. These requirements have been a fixture of the Food and Drug Association (FDA) of the USA for over eighty years [26]. Likewise, the European Medicines Agency (EMA) has required such evidence for regulatory processes [27]. However, these preclinical in vivo models have not accurately predicted outcomes observed in clinical trials. The FDA has reported that > 90 % of preclinical drugs tested with in vivo models “fail to demonstrate either safety or efficacy when tested in humans” [26]. This puts patients at unnecessary risk of overdosing in clinical trials, and risks premature termination of research into promising new drugs. Due to this regulatory requirement for in vivo preclinical testing, development of IM-biorelevant alternatives to in vivo models has been generally disregarded. However, as of December 2022, the official FDA guidelines were amended. In the “FDA Modernization Act 2.0”, the word “animal” was replaced by the term “nonclinical tests” [26]. Whilst the EMA has not yet amended their requirements thusly, the EMA is “reinforc[ing] and further embed[ding] application of the 3Rs principles” in research programs [28]. Indeed, official documentation detailing the approach to “regulatory science” between 2020 and 2025 stipulates the need to “strengthen cooperation between all stakeholders and international partners” in decreasing the application of in vivo models [28]. This is to be achieved through “ … support[ing] qualification of new alternative 3R-compliant method/models including in silico and novel in vitro assays” alongside “development of clear guidance to encourage and prioritize the use of new approach methodologies (NAMs) that can be used to fulfil testing requirements in lieu of traditional animal tests” [28]. Since this publication in March 2020, an official “mid-point achievements” report from March 2023 noted that an “innovation task force” had been successfully established to “address the impact of emerging […] technologies on current scientific, legal and regulatory requirements”, to act as a point of reference for industry and academia [29].
In a seismic change for the pharmaceutical industry, the historical FDA requirement of in vivo modelling in preclinical testing in the USA has ended. Furthermore, such changes are likely to materialize in Europe in the foreseeable future, based on aims and current progress detailed in official EMA documentation. As an alternative to in vivo modelling, in vitro methods can be explored in greater depths.
Accurate in vitro modelling of the human IM injection site, taking cues from the physiology and physical structure of skeletal muscle, is a realistic alternative to previous in vivo animal models. Researchers can investigate fully tractable, biorelevant technologies to better understand post-injection events, i.e. “drug fate”, which have been previously evaluated and discussed in detail [10]. In the context of regulatory changes, his article identifies and assesses: a) the complexities of modelling the IM injection site, b) the value of current in vitro pre-clinical models, c) the associated issues regarding modelling post-injection drug fate outcomes, and d) teaching technologies for training medical professionals to confidently perform IM injections.
2. Challenges of modelling the IM injection site
2.1 “Critical parameters”
In modelling the IM injection site, it is reasonable to propose that characteristics of the skeletal muscle tissue should be represented. The physiology and physical structure of skeletal muscle has been previously described in detail [10]. Critically, trace amounts of an injected material enter myocytes, the viable muscle cells that make up the bulk of skeletal muscle. This occurs primarily in the needle tract due to physical tissue damage resulting from the injection event. Most of an IM injected medicine is dispersed between functional organizations of myocytes required for coordinated contraction. Thus, the IM injection site itself is in proximity of acellular sheaths (or fasciae) that stabilize these functional organizations.
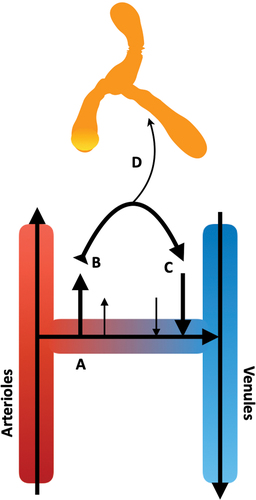
The fascial layers (endomysium, perimysium, and epimysium) are formed from extended sheaths of acellular, structural matrices: the ECM. The fascial layers provide a robust frame to physically support the structure of functional skeletal muscle elements: the endomysium supports single fibrils, the perimysium supports fascicles, and the epimysium envelopes the entire muscle organ () [30]. Whilst robust, these layers are simultaneously flexible to allow coordinated movement of the functional elements of skeletal muscle. Studies examining post-injection movement of injected material have described injectate distribution within planes of perimysial fasciae () [31,32,33,34,35]. Exact composition of the ECM is unique to each fascial layer. These compositions are well documented, with the perimysial layer being primarily composed of the structural type-I collagen (Col-I) and the non-structural HA [30,36,37,38,39]. Perfusing these regions is the interstitial fluid (ISF), an amalgamation of electrolytes, and soluble protein (e.g., serum albumin), formed from extravasated blood plasma [10]. As the ISF returns to the vasculature under “colloid and hydrostatic pressures”, the ISF transports injected material away from the injection site, into the circulatory or lymphatic systems () [10].
Figure 2. The structure, and fascial layers of, the skeletal muscle organ. The fascial layers provide structural support for the functional elements of the skeletal muscle, whilst retaining sufficient elasticity to adapt to conformational changes within the organ during dynamic movement. Reproduced from [10] with permission.
![Figure 2. The structure, and fascial layers of, the skeletal muscle organ. The fascial layers provide structural support for the functional elements of the skeletal muscle, whilst retaining sufficient elasticity to adapt to conformational changes within the organ during dynamic movement. Reproduced from [10] with permission.](/cms/asset/d2e6b773-7846-4c30-b0d9-9240cc532854/iedd_a_2388841_f0002_c.jpg)
Figure 3. Distribution of material post-IM injection. A, B) 1 mL of an oil vehicle (10% benzyl alcohol and 90% sesame oil was injected into A) the deltoid and B) vastus lateralis muscles of a human volunteer. Immediately following administration, the resulting depots (approx. length 5 cm), distributing along fascial planes, were visualised with 3-dimensional graphics. Adapted from [34] with permission. C) After injection of cabotegravir (40 mg/kg dose, 200 mg/mL stock) into rat “upper hindlimb” muscle, a depot formed of approx. 2 mm diameter. Distribution of drug from this central depot followed discreet routes following interfascial planes, highlighting a “path of least resistance” for the injected material. Adapted from [35] with permission.
![Figure 3. Distribution of material post-IM injection. A, B) 1 mL of an oil vehicle (10% benzyl alcohol and 90% sesame oil was injected into A) the deltoid and B) vastus lateralis muscles of a human volunteer. Immediately following administration, the resulting depots (approx. length 5 cm), distributing along fascial planes, were visualised with 3-dimensional graphics. Adapted from [34] with permission. C) After injection of cabotegravir (40 mg/kg dose, 200 mg/mL stock) into rat “upper hindlimb” muscle, a depot formed of approx. 2 mm diameter. Distribution of drug from this central depot followed discreet routes following interfascial planes, highlighting a “path of least resistance” for the injected material. Adapted from [35] with permission.](/cms/asset/b502a064-7ed1-4051-95c5-8cdac6e3d7b0/iedd_a_2388841_f0003_c.jpg)
Figure 4. Movement of the interstitial fluid (ISF) in vivo. A) As blood transfers from arterial to venous vessels through tissue beds, B) blood pressure forces plasma fluid out of the systemic circulation into the tissue, generating interstitial fluid. C) As these forces are exceeded by osmotic gradients, the extravasated fluid begins to return to the venous end of the local vasculature. D) Some of this fluid is taken up by the lymphatic system, carrying lipophilic elements, e.g., proteins and drugs. In skeletal muscle, the dynamic movement of the local tissues, and pulse pressure of local arterioles, provides the necessary forces to move the lymph through the one-way bicuspid valves of the lymphatic system.
It is the composition of fascial layers, the ISF, and their homeostatic conditions, that have been previously described as “critical parameters” for modelling IM injection sites [10]. As the field of IM research develops, and improvements in analytical technologies become available, these “critical parameters” should be drawn from the most up-to-date research available. The IM site is multifaceted; combining all parameters into a single model would not only be complicated, but likely prohibitively expensive. Thus, the design of the model must be informed by the type of administration to be tested, and the associated hypothesis. By this, we refer to the characteristics of the drug and the formulation itself. Injectable formulations can be broadly organized into two categories: aqueous and non-aqueous (i.e., oil) formats. Hypotheses regarding drug fate of aqueous formulations ultimately reflect solubility of the injectable at the injection site, to facilitate drug release into the systemic circulation. With oil-based formulations, such as for testosterone, we must also consider the fate of the oil vehicle [34]. As such, if both kinds of formulations were to be scrutinized in studies, the in vitro model would need to accommodate both formats in a biorelevant manner, to not skew the data in favor of a specific formulation format. Here, we present recent advances available in the literature, which can improve the study of skeletal muscle organs and, therefore, improve the ability to effectively model the IM injection site in vitro.
2.2 In vitro modelling IM “critical parameters”
2.2.1 The ISF
In modelling the IM environment, knowledge of the physical composition of the IM injection site, and the fascial layers, is well understood. Defining the composition of the ISF, however, is problematic. As the ISF is a form of extravasated blood plasma, many assumptions are made regarding electrolyte composition, based on blood plasma composition [40,41,42,43,44,45,46]. Further complications arise from the lack of studies concerning other elements of the ISF. For example, the bicarbonate buffering axis is essential for maintaining physiological pH in the ISF, and measured amounts in the ISF range between 5 – 54 mM bicarbonate ([HCO3−]ISF) [47]. A previous calculation of 17.3 mM [HCO3−]ISF, lies within this range [10].
To improve our knowledge of ISF composition, sophisticated techniques to sample ISF without altering the composition of ISF samples are needed; the “composition” of sampled ISF is dependent on the method used [40,45,48]. Damage to the local tissue environment can cause e.g., intracellular material to “contaminate” the ISF sample. However, advancements in targeted study of individual components of the ISF are being made. Wang and colleagues have described a microneedle patch, specifically designed as a point-of-care diagnostic tool for protein biomarkers in the ISF [49]. This technique is “minimally invasive”, whilst avoiding the common issue of extracting minute quantities of ISF (2 μL of fluid from 4 cm2 of tissue) using e.g., suction techniques [50]. Using this “targeted” sampling method, the proportion of e.g., serum albumin (a member of the albumin protein family) in the ISF could be studied in greater detail. Serum albumin can act as a “secondary depot” for an injected material, potentially enabling drug transport away from the injection site and uptake into the circulation, as a mobile drug reservoir [51,52]. As such, using physiologically appropriate concentrations of serum albumin can result in more accurate data output from in vitro studies using serum albumin, studying drug release from the injection site. Yet, serum albumin is not necessarily the sole agent having this role, as a range of soluble proteins have been identified from tissue fluids of human trapezius muscles [45]. Based on such reports, and bioinformatic analysis, it is possible to propose additional proteins that may act as secondary depots. Incorporating these into tractable in vitro experiments could be used to address such hypotheses.
Several ISF-modelling solutions have been proposed over the years [53,54,55,56,57,58,59]. These range from oversimplified options such as “simulated muscle fluid”, containing sodium chloride and imidazole only, to more sophisticated propositions such as “modified simulated body fluid”, containing a plethora of electrolytes and buffering elements [54,56]. The ISF is in a constant state of flux owing to its function in delivering nutrients and oxygen to respiring tissues and removing metabolic waste products. The pH, whilst generally strictly controlled, can deviate beyond homeostatic conditions during disease, muscle exertion, or as a result of tissue trauma [44,60,61]. Nevertheless, efforts have been made to model the ISF using a variety of solutions, including established tissue-fluid mimics [53,54,55,56,57,58,62]. These solutions have been described for explicit use with skeletal muscle; lactated Ringer’s, and Krebs Henseleit solutions have been used to study Xenopus skeletal muscle, and ex vivo isolated human skeletal muscle, respectively [53,58]. Comparing the composition of these solutions identifies similarities in components and concentrations () [63]. From the described solutions, the most common components are NaCl, MgCl2, KCl, and CaCl2. Indeed, these reflect the most common ions found in tissue fluids: Ca2+, Na+, K+, and Cl− [64]. The buffering elements, however, as discussed, requires dedicated investigations to better understand the proportion of endogenous ISF buffering elements. So far, NaHCO3 is the typical buffering element used for in vitro solutions. However, pH must be carefully maintained (e.g., by CO2 infusion) to prevent precipitation of CaCO3, and loss of buffering action through loss of CO2(g). Alternatively, chemicals such as HEPES can be used instead [59,65]. The pKa of HEPES is approximately 7.3, offering optimal buffering capacity in solutions at physiological pH 7.4 [66].
Table 1 Composition of interstitial fluid mimicking solutions.
To the best knowledge of the authors, there are no published studies comparing the use of multiple ISF models in studying e.g., PK outcomes of a single formulation. This would investigate the impact of solution composition in modelling dissolution under tractable conditions, identifying if specific components are “critical parameters” for modelling the ISF, and therefore post-injection events. Such studies would be invaluable in establishing in vitro-in vivo correlations (IVIVCs) but may also be unique for a specific drug and formulation. To generate IVIVCs, however, the corresponding data from human studies must be available for comparison against the in vitro data. As evidenced by these discussions, further development of ISF-mimicking solutions is a key concern for better understanding of post-IM injection events. Nevertheless, the physical injection site environment should accurately replicate in vivo conditions. Such methods can either be cellular, incorporating viable material, or acellular, using non-viable material, such as the ECM. The choice is dependent on the hypothesis being tested, as both avenues have unique advantages.
2.2.2 The physical IM injection site
In modelling the physical elements of the IM injection site, researchers have explored options in both acellular and cellular modelling options. Advances in acellular modelling the IM injection site has recently been reviewed [10]. A range of recent advances in cellular modelling described in the literature will be explored here.
2.2.2.1 Cellular modelling
The terms “intramuscular injection”, “in vitro”, and/or “cellular modelling” were applied in combination, and singularly, to PubMed (NCBI), Web of Science (Clarivate), and Lens (lens.org) databases, with articles requiring publication between 2014 and 2024. Articles specific to in vitro applications were selected for review and inclusion in this article.
There have been advances in cell culture methods to develop biorelevant tissue models, as reviewed by Shyam and colleagues [67]. Two-dimensional (2D) models, usually in multi-well culture plates, are relatively easy to generate and use for efficacy and toxicity testing. However, despite widespread application in preclinical drug development, their continued use has been questioned; “there is no good science in bad models” [68]. Results obtained from 2D cultures do not reflect three-dimensional (3D) model data [69,70]. This could be due to a paucity of unique cellular interactions present in 3D arrangements which are not represented in 2D formats. The local ECM of 3D arrangements itself provides signaling cues [71]. Furthermore, the partial (or absence of) cell polarization observed in 2D is not reflective in vivo polarization [72]. In combination, this highlights a lack of signaling diversity in 2D cultures, resulting in oversimplified cellular organizations. The models themselves can be considered oversimplified too, as unique attributes of local microenvironments, such as mechanical forces, are difficult to replicate in 2D settings [73].
Generating 3D cultures can provide better models in vivo conditions, including several cell interactions [74]. Indeed, 3D cultures have been considered the “missing link” in preclinical efforts where the complexities of cell interactions can be modelled [75,76]. Such 3D models are typically either “spheroids” or “organoids”. Spheroids are spherical masses of primary cells, suspended in cell culture medium [67]. Organoids, by comparison, are collections of various cell types that organize into architectures consistent with the organ of interest with associated behaviors and functions [67]. Organoids are increasingly favored over spheroids in preclinical research, as they better represent the nature of a target organ. This allows informed conclusions to be drawn from assays studying e.g., pharmacology. Compared to 2D models, the more elaborate ECM composition required to physically support organoid architecture and organization can also offer benefits for studying drug fate events, by better mimicking in vivo events [73,77].
The ECM forming the fascial layers is as essential to the dynamic movement of the skeletal muscle as the functional myocytes themselves. Small-scale assessment of potential interactions between a formulation and ECM elements at the IM injection site (e.g., Col-I) can provide valuable insights into events that might occur in a patients [59]. Such models, by replicating the organization of skeletal muscle at the IM injection site, can establish conditions for such interactions. Thus, the impact of both cellular and ECM components, which may have equal or unequal importance, can be determined. By accurately replicating these conditions, hypotheses can be tested in a biorelevant manner, maximizing the value of the data. Ideally, incorporating vascular networks into organoids would allow examination of additional biorelevant events. However, at present, such networks are limited to open channels within the model, lacking cell types that might affect vascular function such as smooth muscle cells [78,79]. Thus, this “vasculature” may be more akin to skeletal muscle arterioles in size and distribution [78,79]. At present, the system is limited to “passive tissue perfusion”, but there is scope to develop “active and controlled tissue perfusion under fluidic pressure” [79]. Incorporation of vasculature could enhance organoid viability by allowing nutrient and waste-product exchange in cells within the organoid. This would avoid necrotic events due to organoid size that would otherwise prevent effective penetration of nutrients into the organoid. As such, the scale of such models could be increased as desired, to enhance ease of use in assays and handling, for example. Incorporation of cells specific to the vasculature and the challenge of maintaining their viability, however, presents a significant challenge [79]. The high degree of vascularization is a key physiological characteristic of skeletal muscle. As such, the potential for an IM-injection site model where drug movement into the vasculature can be modelled, as well as the physical injection site itself, should be considered. Such models would permit more informative study of drug diffusion and toxicity. For a more comprehensive review of engineered, cellular skeletal muscle models, the reader is referred to a recent, in-depth article by Ostrovidov and colleagues [80].
Despite these advantages of 3D over 2D modelling options, the primary caveat of 3D models is their sheer complexity and variability. Generating and maintaining viable 3D cultures is technically challenging, time consuming, and costly [81]. The reproducibility of models themselves can limit standardization efforts [82,83]. Further, technical challenges arise from the need for specialized, costly techniques to establish the models, such as bioprinting [84,85]. These complexities can inhibit widespread adoption and scalability of 3D modelling in research settings. Compared to 2D models, challenges of scaling a 3D model can also limit use in screening assays [85]. Despite low throughput, the amount of data that can be generated from these models can be overwhelming, requiring robust, sophisticated tools to identify, extract, and analyze relevant data [86].
Efforts to model the IM injection site have included using isolated skeletal muscle tissue [87]. The functional elements, and composition of these tissues are true representations of in vivo conditions for that species. However, these kinds of models require ethical approval, are costly, and have a reduced period of viability, limiting the range of assays that can be performed [10]. Yet, skeletal muscle can also be cultured directly from cells obtained from skeletal muscle tissue. Such models accurately reflecting in vivo conditions can be used to better understand post-injection outcomes and PK parameters [88]. Studies have described how “explant culture enables efficient and high yield derivation of myogenic cells from porcine skeletal muscle”, implying that skeletal muscle physiology is well reflected in the in vitro model. Additionally, the cultured cells “retained in vitro myogenic differentiation potential up to the 8th passage”, with each passage requiring 10 – 14 days (i.e., a potential total viability of 112 days). Such a long viability timeframe presents an opportunity to broaden the range of assays that can be performed on such models if the physiology does not drift over these passages. Thus, studying drug fate, and PK outcomes, over the course of multiple days/weeks per assay for different cell passages may be required. If this model were to be further developed for pharmaceutical applications, human skeletal muscle models could be cultured directly from viable muscle and to a scale where assays could be readily conducted (i.e., akin to an organoid, as opposed to 2D cultures). But, at present, this method requires euthanasia of the host (i.e., a piglet) to allow muscle tissue collection. Sophisticated sampling methods could be developed and employed to avoid euthanasia, improving the ethical implications of the work.
2.2.2.2 Biomaterial scaffolds for cellular models
The terms “intramuscular injection”, “in vitro”, and “biomaterials” were applied in combination, and singularly, to PubMed (NCBI), Web of Science (Clarivate), and Lens (lens.org) databases, with articles requiring publication between 2014 and 2024. Articles specific to in vitro applications were selected for review and inclusion in this article.
The use of biomaterials as a “scaffold” for 3D cultures to be utilized if e.g., a specific conformation is desired, has been proposed. In the context of skeletal muscle, ECM components could be used. These include (but are not limited to) the structural collagens (Col-I, type-III collagen), HA, and fibrin [89,90,91,92]. Such materials, which are observed in the ECM in vivo, provide necessary interactions and biological cues for cells in 3D cultures. Alternative materials have been previously employed, such as sodium alginate, and poly(lactic-co-glycolic acid) (PLGA) [93]. However, whilst these materials bring advantages in pliability and usability, these are not found in vivo, and data obtained using such materials should be scrutinized accordingly. Following on from using such non-biorelevant material in generating skeletal muscle scaffolds, is the development of “biohybrid” skeletal muscle models: cellular models that incorporate both biorelevant and non-biorelevant materials to mimic skeletal muscle elements. By using a “bioink” of gelatin methacrylate, and sodium alginate (derived from seaweed), to grant sufficient viscoelasticity of the scaffold, Filippi and colleagues designed an ingenious bio-printed skeletal muscle model with “vasculature” throughout the construct [79]. These designs can even undergo “passive mechanical tension”, somewhat replicating forces from muscle movement.
While the scaffold makes use of sodium alginate, the model does exploit gelatin, the partially hydrolyzed form of collagen. Gelatin is traditionally derived from bovine bones, and used as a thickening agent in foodstuffs [94]. Whilst this “processed” collagen material does not retain the functionality and physical form of mature collagen fibrils, the amino acid composition remains conserved, such as the hallmark of the collagen family, the “collagenous motif”. Glycine (Gly) is followed by proline (Pro) and 4-hydroxyproline (4-Hyp), in the Xxx and Yyy positions, respectively, in the sequence Gly-Xxx-Yyy. [89,95]. This sequence is critical in the structural function of collagens and is considered a fundamental feature of collagens. Gly alone forms one third of collagen structures, while the combination of Gly, Pro, and 4-Hyp account for 57 % of the total amino acids in collagens [96]. Comparisons of the amino acid content of bovine Col-I with that of bovine gelatin (extracted with acidic methods, i.e., Type A gelatin) have been made [97]. The amino acids of the collagenous motif are well conserved in bovine gelatin, when compared to structurally intact Col-I fibrils (). Gly accounts for approximately one third of all the amino acids in both Col-I and type A gelatin, whilst the combination of Gly, Pro, and 4-Hyp account for approximately 55 %, agreeing with previous reports [96,97].
Table 2 Collagenous motif amino acid content, per 1000 amino acids, of bovine type-I collagen (Col1) and acid-extracted (type A) bovine gelatin. Adapted from [97].
When generating gelatin, the extraction method employed can impart distinct properties in the product. Type A methods use acids, and type B methods use bases. For biorelevant skeletal muscle modelling, type A gelatin has an isoelectric point (pI) between pH 7 and 9, whereas Col-I has an pI of 8.26 [98]. Thus, it is likely that a type A gelatin matrix would be positively charged at physiological pH 7.4, reflecting the positive charge of collagen matrices in vivo [94,99,100]. Bovine Col-I materials, including gelatin, could be used as a viable alternative to human collagens in modelling human skeletal muscle in vitro. Bioinformatic analysis of human versus bovine Col-I reported an amino acid sequence homology of 97.4 % for α chains, and 92.39 % for β chains [59]. From ease of use of material, relatively low costs, and amino acid homology, gelatin may become common in 3D modelling of skeletal muscle fascial layers.
The interwoven lattice of the ECM is also formed of non-structural elements like hyaluronic acid (HA). Such elements contribute their own properties to the overall behavior of the ECM. An anionic polysaccharide of indeterminate length, but exhibiting very large molecular weights, HA readily takes up water, essentially acting as a biological hydration reservoir, whilst stabilizing the ECM by extensive distribution throughout connective tissues [36,37,101]. While structural collagens are the physical framework of the ECM, elements like HA provide viscoelasticity to the ECM. Such elasticity helps minimize friction within the ECM during dynamic movement, maximizing efficient function of skeletal muscle organs. Indeed, rheological study found that adding HA to bovine-derived Col-I hydrogels enhanced the viscoelastic properties of the hydrogel [59]. HA can be readily harvested from bacterial Streptococcus species cultures [102]. This allows subsequent HA products to be used for many applications, including as a beauty product in the cosmetics industry, and in controlled-release drug delivery [103,104].
Biohybrid skeletal muscle models developed to date have been relatively small in size: 15 mm long, 3.7 mm deep, and 2.4 mm in height [79]. This may not necessarily allow the development of an extended ECM network akin to the perimysial fascial layer observed in vivo. Histological staining, however, can identify any organized collagen matrices present. In studying interactions with the IM injection site ECM, data from previous in vivo drug distribution studies could be used to assess and compare the outcomes observed with biohybrid skeletal muscle models [31,32,33]. If these “surrogate” fascial layers are not observed in the biohybrid muscle model, an ECM environment could be generated separately and “transplanted” onto the model. Such a system could utilize a hydrogel environment of Col-I and HA, for example, to grant viscoelasticity whilst using biorelevant ECM components [59]. There is also potential to use ECM matrices derived from decellularized animal tissues [105]. However, reports indicate that the complex cellular constructs of 3D models can be difficult to physically handle [106]. Reproducibility of these models is also questionable, complicating assays and outcomes, thus complicating acquisition of definitive research outcomes [106]. Additionally, such techniques require complicated preparation procedures, and careful maintenance to ensure proper growth conditions to maintain viability after reaching “maturity”. However, when comparing the financial outlay to conventional in vivo models, such as rats, cell culture costs are typically lower. Besides this, using organoids would aid reduction and replacement of in vivo models, enhancing application of the “3Rs” principals in research.
To the best knowledge of the authors, there are no published studies comparing models of the physical IM environment and the PK outcomes of injectables. However, a previous study has addressed the impact of individual ECM components on post-injection outcomes [59]. This model is discussed further in 3.2.1. Regardless of the model design, the model should replicate in vivo conditions sufficiently to generate informative data of how injectables may behave after injection into the IM environment (). For example, the non-physiological pH of a formulation will transition to homeostatic pH. This pH shift may cross the pI of an injected drug, altering its overall charge, risking drug precipitation [107]. Similarly, a pH shift, and indeed temperature transitions, can catalyze microparticle degradation, which can further catalyze accelerated degradation processes [108,109,110]. Temperature transitions from e.g., refrigerated to physiological temperature, can affect stability of a drug and result in detrimental precipitation, particularly in the case of macromolecules [10]. Furthermore, the dramatic range of physical pressures that can be applied at the injection site, as a result of dynamic muscle movement, may impact depot formation and the distribution of the injected material [111]. Alongside these pressures, the transient nature of hydrostatic and osmotic pressures in vivo can limit or accelerate drug release, depending on formulation characteristics [112,113].
Table 3 Acellular post-intramuscular injection events.
3. Current IM injection site modelling technologies
3.1 PK and dissolution methods
A large proportion of the literature describing in vitro IM “models” refer to long-established technologies as outlined in e.g., the United States Pharmacopeia (USP). Such classical models have become established through their ease of use, standardization, and value in studying e.g., drug dissolution.
3.1.1 USP apparatus
Despite these models being widely employed in pharmaceutical research, it has been well documented that inappropriate assumptions can be made. In attempts to model IM conditions, the typical USP model is the “paddle” setup, i.e., USP II apparatus [114]. USP apparatus, however, are typically better suited to solid dosage drugs, and not injectables [115]. A major variable of this method involves the dissolution conditions used. Phosphate-based buffers that include NaCl (such as phosphate-buffered saline, (PBS)) are often used as simple solutions designed to maintain a consistent pH throughout experiments e.g., pH 7.4, at 37 °C. These are sometimes used in conjunction with “sink” conditions, i.e., “the maximum drug concentration in the bulk fluid does not exceed about 20 % of the drug’s solubility” [116]. PBS, however, does not represent the complexities of the ISF [117]. Additionally, solution additives are sometimes added to “accelerate” drug release. The addition of surfactants, to reduce the surface tension of the solution to increase the rate of drug dissolution into the receiver solution, can reduce the time required for assays, but may compromise prediction of in vivo PK profiles [118]. In some instances, drug release is “enhanced” with highly acidic or alkaline receiver solutions that are heated to high temperatures. For example, in a “reactive dissolution” assay (studying prodrug dissolution, and chemical conversion to the active pharmaceutical ingredient (API)), a medium has been described of 250 mL distilled water, containing 1 % sodium dodecyl sulfate, and 2 % isopropyl alcohol, that is combined with 4 mL of a 2.5 % lithium hydroxide solution [118]. This medium was maintained at 70 °C for the duration of the assay, to ensure complete conversion of paliperidone palmitate (suspensions of which were added directly to the reaction vessel) to the API, paliperidone. Here, the use of non-physiological conditions may have limited the value of the data generated. Such temperatures would be incompatible for using e.g., serum albumin in the dissolution medium, as protein denaturation would prevent study of serum albumin-mediated drug uptake (i.e., as a secondary depot). Furthermore, experiments using USP apparatus also exclude physical elements, such as collagens, for modelling physical interactions at e.g., an “injection site”. Dialysis bags are preferentially selected for specifying a molecular weight cut-off, to study release characteristics of an API, for example [114]. However, dialysis bags generally use cellulose, or synthetic polymers, to generate the membranes [119]. Excluding biorelevant materials to mimic the aqueous and physical ECM environments may limit the value of data generated by not accounting for potential in vivo events post-injection. Furthermore, the USP method employed, and the conditions utilized, can dictate outcomes of an experiment, skewing the data to support a specific outcome [114,120].
It is possible to attempt to modify USP equipment, such as Apparatus II, to better represent in vivo IM conditions. Publications have described modifications to better model gastrointestinal conditions, for example, by introducing “peristaltic movement” [121]. For IM administration modelling, using a hydrogel “injection site” containing a drug formulation in Apparatus II could potentially offer a more physiologically relevant model for assessing intramuscular drug release compared to solely using traditional dissolution media. However, the viability of this approach depends on several factors. The hydrogel used to simulate the injection site should closely mimic the physical properties of muscle tissue (using such parameters as proposed in 2.2.2), including elasticity (readily defined via rheological characterization), and indeed porosity of the matrix (collagen fibril diameter, and thus matrix porosity) can be controlled by incubation temperature [59,63,122]. Hydrogel composition may need optimization to ensure it adequately represents conditions necessary for hypothesis-driven studies. Once the hydrogel is suitably prepared, the drug should either be uniformly dispersed or encapsulated within the hydrogel matrix, or deposited as a bolus directly into the center of the hydrogel itself by injection. Whichever method is employed, drug incorporation into the hydrogel should be thoroughly characterized to ensure consistency and reproducibility using methods such as scanning electron microscopy (to determine drug distribution) and rheology (to determine whether viscoelasticity is affected by drug incorporation, or bolus deposition). The hydrogel could either be left to move with fluid currents created by paddle movement, or the Apparatus II could be modified to hold the hydrogel in a constant position within the reservoir, using cuvettes with semi-permeable, non-specific dialysis membranes, for example. If injecting the hydrogel with formulations, a further question is when to inject material: prior to hydrogel submersion, or after the hydrogel has been allowed to acclimatize to e.g., temperature conditions of the fluid in the reservoir. This ultimately rests with the hypothesis being addressed.
3.2 Drug fate study methods
While IM-related literature is dominated by PK and dissolution studies, little has been accomplished in understanding post-injection events that determine PK outcomes in vivo, i.e., drug fate. If IM drug fate can be understood, then the PK of individual drugs can be better predicted, allowing informed choices regarding formulation design.
The terms “intramuscular injection”, “in vitro” or “in silico”, and “modelling tools” were applied in combination, and singularly, to PubMed (NCBI), Web of Science (Clarivate), and Lens (lens.org) databases, with articles requiring publication between 2014 and 2024. Articles specific to in vitro, and in silico applications were selected for review and inclusion in this article.
3.2.1 The Simulator of IntraMuscular Injections (SIMI)
An inexpensive tool for modelling the “critical parameters” of the IM injection site was described in 2022, termed the Simulator of IntraMuscular Injections, abbreviated to SIMI () [59]. This tool utilizes a physical “injection site”, into which materials are injected into the system. This physical environment was formed from a tractable hydrogel of Col-I and HA, with sufficient viscoelasticity to allow formation of a bolus. Col-I hydrogels are by no means a novel concept, having previously been exploited in tissue regeneration and wound healing (e.g., post-trauma studies) [123,124]. Col-I is the dominant structural element in fascial layers, and the most abundant protein of human tissues, with this protein representing 90 % of the total protein found in humans [30,125,126]. To represent non-structural elements of fasciae, HA was then selected for its relative abundance in fascial layers and its vital role in fascial “lubrication”, maximizing function with minimal friction. Col-I accounts for 10 - 13 mg/g of “wet tissue”, whilst HA accounts for between 0.09 - 0.13 mg/g [36,127,128]. Thus, from sheer abundance within skeletal muscle fasciae, Col-I was used as the dominant component of the SIMI hydrogels. This hydrogel matrix was contained in a semi permeable cuvette with non-specific, semi porous polycarbonate membranes, and submerged in an ISF-mimicking aqueous phase. As injected materials move away from the central depot site in the hydrogel, they must interact with the physical hydrogel matrix of Col-I and HA before releasing into the ISF-mimic solution. This solution can then be sampled, and analyzed, to determine the rate of drug released from the injection site hydrogel matrix over time.
Figure 5. The Simulator of IntraMuscular Injections (SIMI) research tool. (A) A polystyrene float (1) suspends a type-I collagen and hyaluronic acid hydrogel, contained within a semi-porous cuvette (2). The hydrogel-containing cuvette is suspended in interstitial fluid (ISF)-mimicking solution, maintained at 37 ◦C, pH 7.4 (3). This ISF mimic is constantly stirred via magnetic stirring (4) to mimic ISF movement in vivo using a heated stirring plate (5). (B) Materials are injected into the centre of the hydrogel at a 90o angle relative to the surface, forming a bolus deposit. (C) Over time, injected material traverses (and interacts with) the collagen/hyaluronic acid matrix. The injected material then exits the matrix and moves across the non-specific membranes, releasing into the buffer system. Reproduced from [59] with permission.
![Figure 5. The Simulator of IntraMuscular Injections (SIMI) research tool. (A) A polystyrene float (1) suspends a type-I collagen and hyaluronic acid hydrogel, contained within a semi-porous cuvette (2). The hydrogel-containing cuvette is suspended in interstitial fluid (ISF)-mimicking solution, maintained at 37 ◦C, pH 7.4 (3). This ISF mimic is constantly stirred via magnetic stirring (4) to mimic ISF movement in vivo using a heated stirring plate (5). (B) Materials are injected into the centre of the hydrogel at a 90o angle relative to the surface, forming a bolus deposit. (C) Over time, injected material traverses (and interacts with) the collagen/hyaluronic acid matrix. The injected material then exits the matrix and moves across the non-specific membranes, releasing into the buffer system. Reproduced from [59] with permission.](/cms/asset/ea0e00bd-57a5-4d98-88da-29858ff71969/iedd_a_2388841_f0005_c.jpg)
Figure 6. The ex vivo skeletal muscle model for studying nanoparticle fate post-intramuscular injection. Fluorescently-labelled nanoparticles were injected into a whole, intact mouse soleus muscle, close to the tendon. The distribution of the nanoparticles was studied via cryosections of the proximal region over increasing timeframes. Reproduced from [138] under Commons Licence CC BY-NC 4.0.
![Figure 6. The ex vivo skeletal muscle model for studying nanoparticle fate post-intramuscular injection. Fluorescently-labelled nanoparticles were injected into a whole, intact mouse soleus muscle, close to the tendon. The distribution of the nanoparticles was studied via cryosections of the proximal region over increasing timeframes. Reproduced from [138] under Commons Licence CC BY-NC 4.0.](/cms/asset/ec4bf0b3-4a13-4ff0-a672-910d40128786/iedd_a_2388841_f0006_c.jpg)
The effects of Col-I, or Col-I and HA combined (Col-I/HA; utilized as a hydrogel matrix) on post-injection fate of a model macromolecule was studied using the SIMI. Hydrogels were injected with a “drug”, i.e., green fluorescent protein, of distinct electrostatic charge states (excessively positively, near neutral “wild-type”, or excessively negatively charged), and different “formulation” compositions (exploiting L-histidine, sodium acetate, or polysorbate 20). Over 24 hours, the total quantity of GFP released was greater after injection into Col-I/HA hydrogels, compared to Col-I-only hydrogels. Additionally, regardless of hydrogel composition, the total quantity of positively-charged GFP released was lower than “wild-type” and negatively-charged GFP [59]. However, the total “drug” release was lower from Col-I hydrogels, compared to the Col-I/HA hydrogels. The data implies that the physical composition of the IM injection site, and the design of the injected material (i.e., excipients, and drug charge) both influence post-injection outcomes. This study highlights how individual parameters can combine to influence post-injection outcomes, indicating the multifaceted nature of modelling post-injection outcomes, regardless of the injection route.
Indeed, when considering the charge of a therapeutic, Botulinum type A toxin (BoNT/A), palivizumab, and interferon β-1α are all positively charged at physiological pH, with respective pI’s of 9, 9.3, and 8.93 respectively [129,130,131]. BoNT/A is, however, for local treatment of facial muscle spasms; BoNT/A is not designed for systemic release [132]. Thus, the pI of BoNT/A may be a factor for BoNT/A residence at the injection site. These medicines are not modified, for example by “PEGylation”, to extend the duration of action [133]. BoNT/A doses should be made no less than 3 months apart, palivizumab monthly, and interferon β-1α weekly [134,135,136]. This correlation between positively charged drugs and extended release/duration of action is not absolute. Pegaspargase (derived from Escherichia coli) is a PEGylated form of L-asparaginase. As the pI lies between 4.6 and 5.5, L-asparaginase is likely negatively charged at physiological pH [137,138]. Due to PEGylation, this is dosed once every two weeks, whereas the non-PEGylated version is dosed every third day. Another example, triptorelin, with a pI of 7, is also likely negatively charged at physiological pH. Triptorelin is modified via esterification with pamoic acid (i.e., a pamoate) and packaged as nanoparticles for controlled, extended drug release after administration [139,140].
Whilst GFP was used as an investigational tool, the molecular weight of approximately 27 kDa is not dissimilar from the approximate 22.5 kDa weight of the IM-administered interferon β-1α [141,142]. However, the outcomes of this study may not necessarily reflect outcomes of small molecule therapeutics, which requires investigation.
The fully tractable SIMI enables study of individual elements found at the IM injection site. By including/excluding elements in the hydrogel injection site, or the ISF-mimic, assays can examine whether discrete components (e.g., serum albumin) have impacts on the post-injection outcomes of an injectable (e.g., increasing the rate of diffusion of a drug away from the injection depot). Whilst the SIMI is a relatively simple model, it has scope for technological expansion to both enhance user-friendliness and expand the kinds of assays that can be performed. Such technologies include auto-sampling systems (eliminating manual sampling) and integrating Raman spectroscopic probes to deepen the detail of investigations (e.g., drug degradation within the hydrogel).
3.2.2 Ex vivo muscle modelling
Whilst tools such as the SIMI can address hypotheses regarding post-injection interactions of an injectable with the IM injection site, other groups have sought to expand study on post-injection drug distribution, building on the knowledge of previous reports [31,32,143]. Calderan and colleagues described an ex vivo model of mouse soleus muscles, preserved in a bioreactor. Fluorescently-labelled liposomes and PLGA nanoparticles were injected into the intact muscles immediately after explantation, and the distribution of these nanoparticles was analyzed over time using fluorescence microscopy [144]. It was observed that both liposomes and PLGA nanoparticles entered the functional elements of skeletal muscle, being mainly found inside myofibres, while PLGA nanoparticles were mainly confined to the connective tissue, which is composed primarily of non-structural type-IV collagen [145]. The presence of immune cell populations allowed more in-depth study of drug distribution, and fluorescent signals were detected inside macrophages, indicating the physiological clearance of nanoparticles.
The bioreactor approach enables “fluid dynamic conditions”, enhancing the relevance of modelling skeletal muscle using whole explanted soleus muscles. This tool was described by the authors as “an intermediate step between early in vitro and final in vivo analyses”. Indeed, this model provides an ideal means of confirming in vitro data using actual muscle tissue, but without the procedural complications associated with in vivo experiments. The incorporation of technologies to induce muscle contractions, mimicking dynamic muscle movement, would further improve this model, allowing even more data to be generated per experiment. Indeed, the dynamic nature of skeletal muscle may influence PK outcomes by manipulating the physical dispersion of bolus deposits. Such studies could better inform the formation of bolus deposit(s) post-injection, and how the bolus formation impacts drug release, including the impact of injection pressure and needle selection on these outcomes.
3.2.2.1 Injection equipment selection impacts post-injection outcomes
Data from in vitro and ex vivo studies should be compared against corresponding human trial data, to begin correlating in vitro predictive data with “real-world” data (i.e., representative of clinical outcomes). Without comparison against such human-derived data, the value of such studies producing mathematical models for formulation development are, ultimately, limited. Access to such datasets can be difficult, limiting the appropriate comparisons required. However, there are reports describing using “real-world” conditions to better inform the post-injection outcomes resulting from the drug delivery technology employed, such as autoinjectors [108]. This was acknowledged as attempts to better understand post-injection events using in vivo modelling to improve an “understanding of the effects of route of administration [on PK outcomes]” [114].
Hill and colleagues have described how PK outcomes of IM-administered ketamine, diazepam, and epinephrine were influenced by the brand of autoinjector used, the force applied to the plunger of a manual injection syringe, the length of the needle, and the gauge of the needle used [146]. “Study 1” from this group compared the dispersion volumes of an injectate from a diazepam auto-injector with a manual 3 mL syringe. The data showed that autoinjectors produced larger peak dispersion volumes compared to manual syringes. Additionally, autoinjectors with 20-gauge, 0.8-inch needles had larger dispersion volumes than those with 22-gauge, 0.6-inch needles. Autoinjectors also resulted in more rapid and complete uptake of injectate compared to syringes. “Study 2” then evaluated the dispersion volumes, and uptake of epinephrine from several autoinjectors. Each autoinjector “brand” uses different mechanical properties for administering the pharmaceutical. The data indicated differences in dispersion volumes and uptake among the autoinjectors tested. Overall, the findings suggested that the functional characteristics of injection devices influence injectate dispersion, and therefore tissue uptake. Indeed, such considerations should apply to assays using technology like the SIMI, and should be explored, to determine the impact on assay data.
Recent comparisons of epinephrine autoinjectors have demonstrated PK outcome differences. In a review by Turner and colleagues, PK outcomes of epinephrine indeed appear to be entirely dependent on the brand of autoinjector [147]. In the context of the results described, the authors noted how the “the force and speed of injection (which varies from one device to another) [was] likely to be of greater importance” to PK outcomes, while the needle length (but no mention of gauge) is less impactful [147]. It is also noted how inter-patient variance of “skin-to-muscle depth” can lead to variance in PK outcomes within a study. Further support of such findings comes from a review of epinephrine autoinjectors by Dreborg and colleagues [148]. The use of ultrasound in PK studies would be suitable to ensure an IM injection into the target organism has been achieved when required [148].
From such publications, it seems that the impact of the injector technology, and needle selection/design on drug fate outcomes is as important as understanding post-injection events [114]. This is of particular relevance when administering viscous injectable material, such as haloperidol decanoate formulations [149]. Such outcomes highlight how predicted fate of the “same” drug is reliant on the injector technology. In critical situations, such as administering epinephrine, this could prove an unnecessary risk to the patient, by potentially delivering an inappropriate dose. Further consideration has been placed into the development of such medical devices (i.e., autoinjectors), and integrated drug development/delivery device strategies have been proposed [150].
3.3 In silico IM modelling
Mathematical models provide an alternative to a tangible IM injection site, being primarily applied to modelling PK outcomes as opposed to drug fate events. However, such considerations have been made by Bettonte and colleagues [151]. This article described development and verification of a mechanistic, physiologically based PK model for IM LAIs, namely the antiretrovirals cabotegravir and rilpivirine, and the antipsychotic paliperidone. This model aimed to simulate drug release from the depot and subsequent PK. Parameters such as media viscosity, drug solubility, and drug density were found to impact drug kinetics [151]. The authors recognized how, for example, the depot shape was simplified, and the absence of e.g., drug metabolizing enzymes in the IM environment. However, the model was suitable for simulating drug release scenarios and guiding clinical decisions, particularly focusing on drug-drug interactions and PK evaluation in populations. This model provides a useful tool for simulating drug release from a LAI depot after IM administration. By considering both drug properties and injection site physiology, the model may aid prediction of drug fate and PK outcomes.
In a similar line of thought, Di and colleagues investigated the local movement and absorption of lipid nanoparticles (LNPs) after IM administration using multiphysics simulations. The study evaluated the impact of critical formulation and tissue parameters on LNP transport [152]. The authors noted how tissue deformation occurs during injection, with a rapid rise and stabilization of tissue pressure, fluid velocity, and porosity. These factors all impacted the post-injection outcomes of the LNPs [152]. Indeed, the tissue porosity significantly affected LNP absorption kinetics; smaller porosity of the local tissue resulted in faster absorption. Furthermore, LNP absorption into the lymphatics was influenced by surface area per tissue volume. The formulation design itself impacted these processes, as particle size, structure, and PEGylation influenced LNP properties. Smaller LNPs exhibited enhanced lymphatic permeability. It is acknowledged that LNPs are primarily designed for cellular penetration and uptake in the locality of the injection site, and not for systemic circulation. These kinds of studies take the local injection environment into account and provide more informative predictions of post-IM injection outcomes as a result. This complex interplay between formulation attributes, tissue properties, and local absorption emphasizes the need for simulation and experimental validation in drug delivery research.
Such investigations could be either run in parallel with, or followed up by, in vitro assays to physically investigate injection site events influencing the PK predictions, such as those proposed in . Combining these kinds of assays would further strengthen the informative nature of the data. As such, the formulation design can be optimized, the PK predictions further improved, and therefore better inform decisions for in vivo assays and clinical trial design. In the context of better informing decision making for trials, Atoyebi and colleagues have developed an in silico PK model for antiretrovirals in pregnant patients, using both oral and IM administration routes [153]. While finding pregnant volunteers to participate in clinical trials is challenging in drug development, it is these populations who would benefit most from such human immunodeficiency virus and acquired immunodeficiency syndrome therapies, preventing transmission to their newborns [153]. Thus, better informing appropriate doses and dosage regimens in designing trials could minimize unnecessary risk due to inappropriate dosing.
Indeed, at present, in silico and in vitro research technologies for screening IM injectables are generally limited [154]. While a recent review described how in silico methods could be employed for improved development of vaccines, which are typically administered by IM injection [155], such technologies appear to be well established for SC administrations [156].
4. Technologies for teaching healthcare professionals IM injection techniques
With the continuous expansion of technologies for facilitating successful development of novel IM pharmaceuticals, the technologies used for teaching IM administrations has simultaneously developed. Whilst IM therapeutics show signs of gradually becoming self-administered medicines, healthcare professionals are still presently required, especially during national healthcare initiatives involving e.g., vaccination of a population en masse. This is to ensure an IM Injection, and not a SC injection, is performed. The needle gauge should be suitable for the injectable (e.g., a wider bore for more viscous materials), while the needle length should be selected according to the depth of SC adipose tissue. Thicker adipose layers require a longer needle to reach skeletal muscle tissue, and thinner layers require shorter needles, to minimize risk of striking bone. Technologies for teaching the IM injection procedure to healthcare professionals are infrequently described in the literature, as animals are more commonly employed in teaching [157].
The terms “intramuscular injection”, “simulator”, and “teaching” were applied in combination to PubMed (NCBI), Web of Science (Clarivate), and Lens (lens.org) databases, with articles requiring publication between 2014 and 2024. Articles specific to in vitro applications were selected for review and inclusion in this article.
4.1 Injectable silicone model
In simple models for IM injection practice, silicone 3D-printed models can simulate the skin, SC tissue, and a deltoid “muscle” below the SC space [157]. By using varying ratios of silicone material with or without a silicone “softener”, these distinct environments were generated to provide varying degrees of physical resistance to needle insertion into these “tissues” by a user. Injected material can drain from the bottom of the model through a hole introduced into the “muscle” layer during the curing process. These models took up to eight hours to fully prepare without the use of specialist heating equipment, to cure the silicone mixtures in separate stages, to form the “dermis”, the “SC space”, and the “muscle organ”. With heat sources capable of 80 °C temperatures, this timeframe was reduced to approximately two hours [84]. After being exposed to the model in hands-on usage, Canadian healthcare professionals were interviewed about the 3D printed model, giving an overall positive opinion of the model and its use in teaching IM injection procedures [157]. This model was then studied in the context of “cultural differences”. After initial study with Canadian healthcare professionals, the model was evaluated by healthcare staff in Singapore [158]. The Singaporean colleagues collectively noted the “fat” layer was “too thick”, possibly reflecting the nature of patients that these professionals regularly attend to, whereas Canadian professionals may regularly encounter such depth of SC adipose tissue. This type of observation highlights the need to be aware of the patient, and tailor the choice of needle gauge, and length, for IM injections. To accommodate for such physiological inter-patient variability, this model was proposed to be amended to accommodate differing degrees of “fat” thickness, to “better represent the [typically observed] patients in Singapore” [158]. Furthermore, there is ample scope that this model could readily be adapted to model other IM injection site muscles, e.g., the rectus femoris, and vastus lateralis muscles, by specifically studying administering IM drugs into a “leg”, for example.
4.2 Electronically modified model for pediatrics
Another silicone-based injection model, complete with electronic sensor capabilities, has also been described for educating nurses [159]. A physical manikin, representing an infant of up to 1 year of age, modelled the vastus lateralis with an alternating system of silicone and aluminum layers. The aluminum layers connected to a circuit that detects needle insertion, and “injection” into skeletal muscle. Likewise, it is capable of detecting injections into the SC space, or “striking bone”. The tool provides the user with an audible message, to clearly communicate whether an IM administration was correctly performed. In “incorrect” scenarios, the system does not discriminate between a SC injection or striking bone, limiting the feedback to the user. The model was specifically designed for practicing the IM procedure on infants, where the vastus lateralis is selected for IM injections due to the sheer mass of this muscle compared to e.g., the deltoid. Furthermore, newborns are less likely to remain still, and therefore may compromise the IM injection being performed. Thus, the confidence gained from having practiced the procedure amply beforehand will aid e.g., nurses in swiftly completing the procedure. This model could indeed be further developed for practicing injections on “adults”, possibly using modified prosthetic limbs, thereby widening the application of such a tool.
5. Conclusion
This review has considered challenges in modelling the IM injection site, advances in studying drug fate, and technologies available for teaching medical professionals to correctly perform IM injections. Following recent changes in FDA guidelines, the requirement for preclinical in vivo PK studies in the USA has ceased, primarily due to a lack of correlation with clinical outcomes. Thus, the value of in vitro modelling technologies that might help in predicting clinical PK outcomes has become more important in IM drug research. For modelling the IM environment, increasingly powerful techniques are being developed to monitor specific components of the ISF, which will allow ever more detailed compositions of ISF-mimics to be created. By taking cues from skeletal muscle physiology and composition, it is possible to model the physical and biological properties of the IM injection site in biorelevant, tractable experiments to test post-injection hypotheses, and conduct formulation screens. Ongoing research, by incorporating additional parameters such as dynamic muscle contractions and vasculature, will further expand the hypotheses that can be tested with in vitro technologies. In parallel to these advances, technologies to train healthcare professionals on how to correctly administer IM injections are becoming increasingly sophisticated. As such, confidence in medical professionals performing IM administrations increases, and minimizes the risk of accidental SC injections being performed. Together, these described advances highlight the ongoing, and increasing, value of the IM injection route for delivering pharmaceuticals.
6. Expert Opinion
The field of IM administration has witnessed substantial advancements over the years, transitioning from simple drug delivery systems to sophisticated LAIs. These developments have revolutionized patient care by offering long-term treatment options that reduce the frequency of clinical visits, thereby freeing up valuable time for healthcare professionals. Further, such medicines can ensure patients never miss a dose, which can be detrimental in cases such as anti-psychotic and anti-viral therapies. Now, as regulatory requirements from agencies such as the FDA begin to change the preclinical drug development landscape, the development of bespoke IM-modelling tools has never been more topical. Uniquely, this review has brought together the state of affairs of IM injection site modelling in the context of these changes to preclinical testing requirements. Thus, new perspectives can be taken on such technologies. As we delved deeper into these perspectives, several key areas emerge that warrant discussion and exploration.
Advancements in IM administration have significant implications for real-world outcomes, treatment guidelines, drug efficacy, and economics. The introduction of LAIs, for example, has transformed the management of chronic conditions such as schizophrenia. By ensuring sustained drug release over weeks or months, these formulations improve patient adherence to treatment regimens, thereby enhancing therapeutic outcomes and reducing the risk of relapse or disease progression. This, in turn, can lead to decreased healthcare costs by minimizing the need for frequent medical interventions, and hospitalizations. Furthermore, the development of bespoke in vitro tools to investigate post-IM injection events represents a major leap forward in preclinical drug development. These tools enable researchers to study the interactions between IM injectables and the skeletal muscle environment in a controlled setting, providing valuable insights into mechanisms underlying drug release, and absorption, from the injection site. Such knowledge can inform the design of more effective formulations tailored to specific PK outcomes, ultimately leading to better patient outcomes.
Despite promising advances, several challenges and limitations hinder the full realization of the potential of IM injectables. One of the primary obstacles is our limited understanding of interactions between injectable materials and the skeletal muscle environment. While in vitro tools offer a promising solution, they are still in the early stages of development; replicating the complexity of in vivo conditions which are relevant to post-injection outcomes is presently challenging. This present gap in knowledge can lead to suboptimal formulation designs, and inconsistent therapeutic outcomes. Further study of post-IM injection outcomes will, eventually, address this critical knowledge gap. Additionally, regulatory hurdles pose significant challenges to the adoption of new technologies in research endeavors. The stringent requirements for safety and efficacy testing, while necessary, can slow down translation of innovative research into approved therapies. The recent amendments to FDA guidelines are a step in the right direction, by removing the requirement for preclinical in vivo modelling which, ultimately, do not provide clinically relevant predictions. But further harmonization of such amended regulations across global jurisdictions is needed to facilitate widespread development, and adoption of, new preclinical technologies.
The future potential of IM injectables is immense. As the field continually develops, so too does the sophistication of the medicines envisaged, further expanding the potential of IM-injectables. By developing, and applying advanced in vitro tools, researchers can conduct high-throughput screening of formulation candidates under tractable conditions, accelerating the drug development process. This approach not only reduces reliance on animal models, but also provides more ethical, cost-effective alternatives for preclinical testing. Moreover, understanding the role of individual components at the injection site, such as Col-I, can pave the way for the development of formulations that consider specific tissue elements. This targeted approach can enhance drug efficacy, while minimizing off-target effects, thereby improving patient safety and treatment outcomes.
Looking ahead, the future of IM injectables may lie in the integration of cutting-edge technologies such as in situ forming implants, and the next generation of nano suspensions, as discussed in the introduction. Yet, it has been noted how further study of how in vivo drug fate, and PK outcomes, are influenced by factors such as, e.g., particle size in nanosuspensions, is urgently required [19,160]. Hence, it is imperative that the required modelling tools be considered now, so that necessary steps can be taken to begin development. The tools and technologies described in this article provide an ideal launchpad for future developments. This, coupled with the ongoing growth in the personalized medicine research, brings the promise of optimizing formulations for specific treatment outcomes based on e.g., tailored, condition-specific PK profiles.
In the next five to ten years, we can expect the development of in vitro IM-modelling tools to become more widespread, reducing the reliance on animal models, and streamlining the IM-administered drug development process. Advances in material science, and bioengineering, will lead to the creation of more sophisticated and effective formulations, while regulatory frameworks, ideally, should evolve to accommodate these innovations. Overall, the field of IM injectables is poised for transformative growth. By addressing the current challenges and leveraging emerging technologies, researchers can unlock new possibilities for drug delivery, ultimately benefiting patients, and advancing the frontiers of medical treatments. The journey towards this future is paved with exciting opportunities and the potential to make a profound impact on healthcare outcomes.
Declarations of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Article highlights
Changes to FDA regulatory requirements no longer mandate preclinical in vivo modelling in drug development efforts.
Physiological elements of the intramuscular (IM) injection site, such as collagens, and interstitial fluid, should be considered when developing effective in vitro IM models.
The current state of the art for modelling human post-IM injection events with cellular elements is centred on 2D and 3D cell cultures, biomaterial hydrogels, and “biohybrid” scaffolds (e.g. gelatin and sodium alginate).
The current technologies available for studying post-IM injection drug fate in vitro and in silico consider parameters of both the skeletal muscle tissue, and injected formulations.
Useful tools are available for teaching medical professionals how to correctly administer IM injections (as opposed to e.g. an erroneous subcutaneous injection).
Additional information
Funding
References
- Vinarov Z, Abdallah M, Agundez JAG, et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur J Pharm Sci. 2021 Jul 1;162:105812. 10.1016/j.ejps.2021.105812
- Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obesity. 2014;38(8):1019–1026. doi: 10.1038/ijo.2013.200
- Schneider A, Mueller P, Jordi C, et al. Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors. Expert Opin Drug Delivery. 2020;17(2):225–236.
- Schneider A, Jost R, Jordi C, et al. Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions. Expert Opin Drug Delivery. 2023:1–16.
- Frontera WR, Ochala J Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015 Mar;96(3):183–195.10.1007/s00223-014-9915-y
- Evans EF, Proctor JD, Fratkin MJ, et al. Blood flow in muscle groups and drug absorption. Clin Pharmacol Ther. 1975 Jan;17(1):44–47. 10.1002/cpt197517144
- Rodger MA, King L Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs. 2000 Mar;31(3):574–582. 10.1046/j.1365-2648.2000.01312.x
- Diggle L. The WHO technique for intramuscular thigh vaccination in infants and toddlers had fewer adverse reactions than 2 other techniques. Evid Based Nurs. 2006 Jan;9(1):9.
- Ogston-Tuck S Intramuscular injection technique: an evidence-based approach. Nurs Stand. 2014 Sep;29(4):52–59. 10.7748/ns.29.4.52.e9183
- McCartan AJS, Curran DW, Mrsny RJ Evaluating parameters affecting drug fate at the intramuscular injection site. J Control Release. 2021 Aug 10;336:322–335.10.1016/j.jconrel.2021.06.023
- Kearns C, Houghton C, Dickinson E, et al. What variables should inform needle length choice for deltoid intramuscular injection? A systematic review. BMJ Open. 2023;13(1):e063530. doi: 10.1136/bmjopen-2022-063530
- Mishra P, Stringer M. Sciatic nerve injury from intramuscular injection: a persistent and global problem. Int J Clin Pract. 2010;64(11):1573–1579. doi: 10.1111/j.1742-1241.2009.02177.x
- Larkin TA, Ashcroft E, Hickey BA, et al. Influence of gender, BMI and body shape on theoretical injection outcome at the ventrogluteal and dorsogluteal sites. J Clin Nursing. 2018;27(1–2):e242–e250.
- Behrens R, Patel V. Avoiding shoulder injury from intramuscular vaccines. The Lancet. 2021;397(10273):471. doi: 10.1016/S0140-6736(21)00192-6
- Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. doi: 10.1007/s40263-020-00779-5
- Li W, Tang J, Lee D, et al.. Clinical translation of long-acting drug delivery formulations. Nat Rev Mater. 2022;7(5):406–420. doi: 10.1038/s41578-021-00405-w
- Walling DP, Hassman HA, Anta L, et al. The Steady-State Comparative Bioavailability of Intramuscular Risperidone ISM and Oral Risperidone: An Open-Label, One-Sequence Study. Drug Des Devel Ther. 2021;15:4371–4382. 10.2147/DDDT.S332026
- Joiner JB, King JL, Shrivastava R, et al. Effects of Injection Volume and Route of Administration on Dolutegravir In Situ Forming Implant Pharmacokinetics. Pharmaceutics. 2022 Mar 11;14(3). 615 10.3390/pharmaceutics14030615
- Ma Y, Cong Z, Gao P, et al. Nanosuspensions technology as a master key for nature products drug delivery and in vivo fate. Eur J Pharm Sci. 2023:106425. 185 10.1016/j.ejps.2023.106425
- Pandya A, Vora L, Umeyor C, et al. Polymeric in situ forming depots for long-acting drug delivery systems. Adv Drug Del Rev. 2023:115003.
- Zlotnikov ID, Malashkeevich SM, Belogurova NG, et al. Thermoreversible gels based on chitosan copolymers as “intelligent” drug delivery system with prolonged action for intramuscular injection. Pharmaceutics. 2023;15(5):1478. doi: 10.3390/pharmaceutics15051478
- Shi Y, Lu A, Wang X, et al. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B. 2021 Aug;11(8):2396–2415.10.1016/j.apsb.2021.05.002
- Qin M, Ye G, Xin J, et al. Comparison of in vivo behaviors of intramuscularly long-acting celecoxib nanosuspensions with different particle sizes for the postoperative pain treatment. Int J Pharm. 2023 Apr 5;636:122793. 10.1016/j.ijpharm.2023.122793
- Li S, Wang Z, Yu J, et al. Intramuscularly injected long-acting testosterone-cholesterol prodrug suspension with three different particle sizes: extended in vitro release and enhanced in vivo safety. Drug Deliv Transl Res. 2024 Apr;14(4):1093–1105. 10.1007/s13346-023-01460-2
- Rossier B, Jordan O, Allemann E, et al. Nanocrystals and nanosuspensions: an exploration from classic formulations to advanced drug delivery systems. Drug Deliv Transl Res. 2024 Mar 7. 10.1007/s13346-024-01559-0
- Moutinho S Researchers and regulators plan for a future without lab animals. Nat Med. 2023 Sep;29(9):2151–2154.10.1038/s41591-023-02362-z.
- ICH: S6(R1): Preclinical safety evaluation of biotechnology - derived pharmaceuticals - Step 5. European Medicines Agency; 2011.
- EMA regulatory science to 2025: strategic reflection. European Medicines Agency; 2020.
- • Official European documentation detailing the changing European guidelines, and how these were to be implemented in over the five year period of 2020 - 2025
- EMA regulatory science to 2025: Mid-point achievements to end 2022. European Medicines Agency; 2023.
- Purslow PP The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol. 2002 Dec;133(4):947–966. 10.1016/S1095-6433(02)00141-1
- Daugherty AL, Mrsny RJ Local tissue distribution and cellular fate of vascular endothelial growth factor (VEGF) following intramuscular injection. J Drug Target. 2010 Jan;18(1):27–35. 10.3109/10611860903134317
- Korthuis RJ. Anatomy of Skeletal Muscle and Its Vascular Supply. 2011. In: Skeletal Muscle Circulation [Internet]. San Rafael (CA): Morgan & Claypool Life Sciences.
- Probst M, Kühn JP, Scheuch E, et al. Simultaneous magnetic resonance imaging and pharmacokinetic analysis of intramuscular depots. J Control Release. 2016 Apr;227:1–12.10.1016/j.jconrel.2016.02.029
- Kalicharan RW, Oussoren C, Schot P, et al. The contribution of the in-vivo fate of an oil depot to drug absorption. Int J Pharm. 2017 Aug 7;528(1–2):595–601. 10.1016/j.ijpharm.2017.06.055
- Groseclose MR, Castellino S. Intramuscular and subcutaneous drug depot characterization of a long-acting cabotegravir nanoformulation by MALDI IMS. Int J Mass Spectrom. 2019 Mar;437:92–98..
- Piehl-Aulin K, Laurent C, Engström-Laurent A, et al. Hyaluronan in human skeletal muscle of lower extremity: concentration, distribution, and effect of exercise. J Appl Physiol 1985. 1991;71(6):2493.
- Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x
- Loukas M, Shoja MM, Thurston T, et al. Anatomy and biomechanics of the vertebral aponeurosis part of the posterior layer of the thoracolumbar fascia. Surg Radiol Anat. 2008 Mar;30(2):125–129.10.1007/s00276-007-0291-4
- Gordon MK, Hahn RA Collagens. Cell Tissue Res. 2010 Jan;339(1):247–257.10.1007/s00441-009-0844-4
- Sloop CH, Dory L, Roheim PS Interstitial fluid lipoproteins. J Lipid Res. 1987 Mar;28(3):225–237. 10.1016/S0022-2275(20)38701-0
- Fogh-Andersen N, Altura BM, Altura BT, et al. Composition of interstitial fluid. Clin Chem. 1995 Oct;41(10):1522–1525. 10.1093/clinchem/41.10.1522
- Reed RK, Rubin K Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res. 2010 Jul;87(2):211–217. 10.1093/cvr/cvq143
- Waterhouse BR, Farmery AD. The organization and composition of body fluids. Anaesth Intensive Care Med. 2012 Dec;13(12):603–608.
- Wiig H, Swartz MA Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012 Jul;92(3):1005–1060. 10.1152/physrev.00037.2011
- Turkina MV, Ghafouri N, Gerdle B, et al. Evaluation of dynamic changes in interstitial fluid proteome following microdialysis probe insertion trauma in trapezius muscle of healthy women. Sci Rep. 2017 03;7:43512. 1 10.1038/srep43512
- Roumelioti ME, Glew RH, Khitan ZJ, et al. Fluid balance concepts in medicine: Principles and practice. World J Nephrol. 2018 Jan;7(1):1–28. 10.5527/wjn.v7.i1.1
- Kumar V, Karon BS. Comparison of measured and calculated bicarbonate values. Clin Chem. 2008;54(9):1586–1587. doi: 10.1373/clinchem.2008.107441
- Markhus CE, Wiig H Isolation of interstitial fluid from skeletal muscle and subcutis in mice using a wick method. Am J Physiol Heart Circ Physiol. 2004 Nov;287(5):H2085–90. 10.1152/ajpheart.00379.2004
- Wang Z, Luan J, Seth A, et al.. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat Biomed Eng. 2021;5(1):64–76. doi: 10.1038/s41551-020-00672-y
- Samant PP, Prausnitz MR. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc Natl Acad Sci USA. 2018;115(18):4583–4588.
- Zhivkova ZD. Studies on drug-human serum albumin binding: the current state of the matter. Curr Pharm Des. 2015;21(14):1817–30.
- Kragh-Hansen U. Human Serum Albumin: A Multifunctional Protein. In: Otagiri M, Chuang VTG, editors. Albumin in Medicine: Pathological and Clinical Applications. Singapore: Springer Singapore; 2016. p. 1–24.
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J Physiol. 2000 Aug 01;526 Pt 3:597–611.
- Oyane A, Kim HM, Furuya T, et al. Preparation and assessment of revised simulated body fluids. J Biomed Mater Res A. 2003 May 01;65(2):188–95.
- Marques M, Loebenberg R, Almukainzi M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut Technol. 2011 AUG 2011;18(3):15–28.
- Simon A, de Almeida Borges VR, Cabral LM, et al. Development and validation of a discriminative dissolution test for betamethasone sodium phosphate and betamethasone dipropionate intramuscular injectable suspension. AAPS PharmSciTech. 2013 Mar;14(1):425–434.10.1208/s12249-012-9920-2
- Kinnunen HM, Sharma V, Contreras-Rojas LR, et al. A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. J Control Release. 2015 Sep;214:94–102.10.1016/j.jconrel.2015.07.016
- Fountain WA, Naruse M, Claiborne A, et al. Low-dose aspirin and COX inhibition in human skeletal muscle. J Appl Physiol (1985). 202012 01;129(6):1477–1482.
- McCartan A, Mackay J, Curran D, et al. Modelling intramuscular drug fate in vitro with tissue-relevant biomimetic hydrogels. Int J Pharm X. 2022 Dec;4:100125.
- Li S, Wang R, Zhang M, et al. Proteomic analysis of non-small cell lung cancer tissue interstitial fluids. World J Surg Oncol. 2013 Aug 5;11:173. 1 10.1186/1477-7819-11-173
- Marunaka Y Roles of interstitial fluid pH in diabetes mellitus: Glycolysis and mitochondrial function. World J Diabetes. 2015 Feb;6(1):125–135. 10.4239/wjd.v6.i1.125
- Reid MB, Shoji T, Moody MR, et al. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol (1985). 1992Nov;73(5):1805–9.
- McCartan A. Modelling critical parameters of the intramuscular injection site. The University of Bath.2022.
- Henderson MA, Gillon S, Al-Haddad M. Organization and composition of body fluids. Anaesth Intensive Care Med. 2018 Oct;19(10):568–574.
- Torres-Terán I, Venczel M, Klein S Prediction of subcutaneous drug absorption-do we have reliable data to design a simulated interstitial fluid? Int J Pharm. 2021;610:121257. 10.1016/j.ijpharm.2021.121257
- Korte I, Brenner KP, Pollmann KP. A comparison of two buffering systems for lens organ culture. Ophthalmic Res. 1982;14(4):265–268. doi: 10.1159/000265201
- Shyam R, Reddy LVK, Palaniappan A. Fabrication and characterization techniques of in vitro 3D tissue models. Int J Mol Sci. 2023;24(3):1912. doi: 10.3390/ijms24031912
- Pamies D, Hartung T. 21st Century Cell Culture for 21st Century Toxicology. Chem Res Toxicol. 2017 Jan 17;30(1):43–52.
- Choi JW, Bae S-H, Kim IY, et al. Testing in vitro toxicity of nanoparticles in 3D cell culture with various extracellular matrix scaffold. bioRxiv. 2021:2021.03. 18.436024.
- Juarez-Moreno K, Chávez-García D, Hirata G, et al. Monolayer (2D) or spheroids (3D) cell cultures for nanotoxicological studies? Comparison of cytotoxicity and cell internalization of nanoparticles. Toxicol In Vitro. 2022;85:105461.10.1016/j.tiv.2022.105461
- Rubashkin MG, Ou G, Weaver VM. Deconstructing signaling in three dimensions. Biochemistry. 2014;53(13):2078–2090. doi: 10.1021/bi401710d
- Antoni D, Burckel H, Josset E, et al. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16(3):5517–5527. doi: 10.3390/ijms16035517
- Duval K, Grover H, Han L-H, et al.. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016
- Lelièvre SA, Kwok T, Chittiboyina S Architecture in 3D cell culture: An essential feature for in vitro toxicology. Toxicol In Vitro. 2017;45:287–295.10.1016/j.tiv.2017.03.012
- Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22(5):456–472. doi: 10.1177/1087057117696795
- Borah A, Kumar DS. Overcoming the barriers of two-dimensional cell culture systems with three-dimensional cell culture systems: techniques, drug discovery, and biomedical applications. Biomedical Product and Materials Evaluation: Elsevier; 2022. p. 179–229.
- Dhaliwal A. 3D cell culture: A review. Mater Methods. 2012;2:162.
- Mastrullo V, Cathery W, Velliou E, et al. Angiogenesis in tissue engineering: as nature intended? Front Bioeng Biotechnol. 2020;8:188. 10.3389/fbioe.2020.00188
- Filippi M, Yasa O, Giachino J, et al. Perfusable Biohybrid Designs for Bioprinted Skeletal Muscle Tissue. Adv Healthc Mater. 2023 Jul;12(18):e2300151. 10.1002/adhm.202300151
- Ostrovidov S, Ramalingam M, Bae H, et al. Latest developments in engineered skeletal muscle tissues for drug discovery and development. Expert Opin Drug Discov. 20232023/01 /02;18(1):47–63..
- Hosseinkhani H. 3‐D culture systems for cell culture technology. J Cell Res. 2018;1:1–4.
- Huch M, Knoblich JA, Lutolf MP, et al. The hope and the hype of organoid research. Development. 2017;144(6):938–941. doi: 10.1242/dev.150201
- Velasco V, Shariati SA, Esfandyarpour R. Microtechnology-based methods for organoid models. Microsyst Nanoeng. 2020;6(1):76. doi: 10.1038/s41378-020-00185-3
- Sun M, Liu A, Yang X, et al. 3D cell culture—can it be as popular as 2d cell culture? Adv Nanobiomed Res. 2021;1(5):2000066.
- Tebon PJ, Wang B, Markowitz AL, et al. Drug screening at single-organoid resolution via bioprinting and interferometry. Nat Commun. 20232023/06 /06;14(1):3168.
- Tang J, Shi J, Liu J. Editorial: Advances in 3D cell culture for drug screening and toxicology evaluation. Front Bioeng Biotechnol. 2023;11:1266506.
- Napaporn J, Thomas M, Svetic KA, et al. Assessment of the myotoxicity of pharmaceutical buffers using an in vitro muscle model: effect of pH, capacity, tonicity, and buffer type [article]. Pharm Dev Technol. 2000;5(1):123–130. doi: 10.1081/PDT-100100527
- Dan-Jumbo SO. Development and validation of an in vitro porcine skeletal muscle model. 2023.
- Ricard-Blum S The collagen family. Cold Spring Harb Perspect Biol. 2011 Jan;3(1):a004978. 10.1101/cshperspect.a004978
- Ricklefs M, Korossis S, Haverich A, et al. Polymeric scaffolds for bioartificial cardiovascular prostheses. Scaffolds in Tissue Engineering-Materials, Technologies and Clinical Applications; IntechOpen: London, UK. 2017:267–291.
- Gilbert-Honick J, Iyer SR, Somers SM, et al. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials. 2018 May;164:70–79.10.1016/j.biomaterials.2018.02.006
- Shi M, Dong R, Hu J, et al. Conductive self-healing biodegradable hydrogel based on hyaluronic acid-grafted-polyaniline as cell recruitment niches and cell delivery carrier for myogenic differentiation and skeletal muscle regeneration. Chem Eng J. 2023;457:141110.
- Narayanan N, Lengemann P, Kim KH, et al. Harnessing nerve-muscle cell interactions for biomaterials-based skeletal muscle regeneration. J Biomed Mater Res A. 2021 Mar;109(3):289–299.
- Liu D, Nikoo M, Boran G, et al. Collagen and gelatin. Annu Rev Food Sci Technol. 2015;6(1):527–557. doi: 10.1146/annurev-food-031414-111800
- Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78(1):929–958. doi: 10.1146/annurev.biochem.77.032207.120833
- Li P, Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. 2018;50(1):29–38. doi: 10.1007/s00726-017-2490-6
- Handbook of Food Proteins. Phillips G, Williams P, editors. 2011. English. (Woodhead Publishing in Food Science Technology and Nutrition; 222).
- Zhang Z, Li G, Shi B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J Soc Leath Tech Ch. 2006;90(1):23.
- Djagny VB, Wang Z, Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001 Sep;41(6):481–92.
- Andrade ÂL, Ferreira JMF, Domingues RZ. Zeta potential measurement in bioactive collagen. Mater Res. 2004;7(4):631–634.
- Armstrong SE, Bell DR Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Am J Physiol Heart Circ Physiol. 2002 Dec;283(6):H2485–94. 10.1152/ajpheart.00385.2002
- Chong BF, Blank LM, McLaughlin R, et al. Microbial hyaluronic acid production. Appl Microbiol Biotechnol. 2005 Jan;66(4):341–351.10.1007/s00253-004-1774-4
- Bayer IS Hyaluronic Acid and Controlled Release: A Review. Molecules. 2020 Jun 6;25(11). 2649 10.3390/molecules25112649
- Juncan AM, Moisă DG, Santini A, et al. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules. 2021 Jul 22;26(15). 4429 10.3390/molecules26154429
- Wassenaar JW, Boss GR, Christman KL Decellularized skeletal muscle as an in vitro model for studying drug-extracellular matrix interactions. Biomaterials. 2015 Sep;64:108–114.10.1016/j.biomaterials.2015.06.033.
- Kapałczyńska M, Kolenda T, Przybyła W, et al. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–919.
- Thati S, McCallum M, Xu Y, et al.. Novel Applications of an In Vitro Injection Model System to Study Bioperformance: Case Studies with Different Drug Modalities. J Pharm Innov. 2020;15(2):268–280. doi: 10.1007/s12247-020-09437-1
- Rawat A, Stipper E, Shah VP, et al. Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal (R) Consta (R). Int J Pharm. 2011 Nov;420(2):198–205.
- Park K, Otte A, Sharifi F, et al. Potential roles of the glass transition temperature of PLGA microparticles in drug release kinetics. Mol Pharm. 2020;18(1):18–32.
- Yoo J, Won Y-Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater Sci Eng. 2020;6(11):6053–6062.
- Ward SR, Davis J, Kaufman KR, et al.. Relationship between muscle stress and intramuscular pressure during dynamic muscle contractions. Muscle and Nerve. 2007;36(3):313–319. doi: 10.1002/mus.20828
- Kurbel S, Kurbel B, Belovari T, et al. Model of interstitial pressure as a result of cyclical changes in the capillary wall fluid transport. Med Hypotheses. 2001 Aug;57(2):161–166.10.1054/mehy.2001.1288
- Michel CC, Woodcock TE, Curry FRE. Understanding and extending the Starling principle. Acta Anaesthesiol Scand. 2020;64(8):1032–1037. doi: 10.1111/aas.13603
- Wilkinson J, Ajulo D, Tamburrini V, et al. Lipid based intramuscular long-acting injectables: Current state of the art. Eur J Pharm Sci. 2022 Nov 1;178:106253. 10.1016/j.ejps.2022.106253
- Skwierczynski RD, Gray V, De Mutha J. Overview of the Activities of the USP Expert Panel on New Advancements in Product Performance Testing. Dissolut Technol. 2022;29(3):122–127.
- Siepmann J, Siepmann F Sink conditions do not guarantee the absence of saturation effects. Int J Pharm. 2020;577:119009. 10.1016/j.ijpharm.2019.119009
- Pavani P, Kumar K, Rani A, et al. The influence of sodium phosphate buffer on the stability of various proteins: Insights into protein-buffer interactions. J Mol Liq. 2021;331:115753.
- Sonntag E, Kolář J, Djukaj S, et al. Accelerated reactive dissolution model of drug release from long-acting injectable formulations. Eur J Pharm Biopharma. 2023.
- Chen Y-A, Ou S-M, Lin C-C. Influence of dialysis membranes on clinical outcomes: from history to innovation. Membranes (Basel). 2022;12(2):152. doi: 10.3390/membranes12020152
- Cascone S Modeling and comparison of release profiles: Effect of the dissolution method. Eur J Pharm Sci. 2017;106:352–361. 10.1016/j.ejps.2017.06.021
- Burke MD, Koetting MC. Development of a Clinically Relevant Dissolution Approach to Simulate Physiological Forces with a USP 2 Apparatus: “Peristaltic Dissolution”. J Pharm Innov. 20202020/08 /22;16(4):699–714.
- Wolf K, Te Lindert M, Krause M, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013 Jun 24;201(7):1069–1084. 10.1083/jcb.201210152
- Haas GJ, Dunn AJ, Marcinczyk M, et al. Biomimetic sponges for regeneration of skeletal muscle following trauma. J Biomed Mater Res A. 2019 Jan;107(1):92–103.10.1002/jbm.a.36535
- Fischer KM, Scott TE, Browe DP, et al. Hydrogels for Skeletal Muscle Regeneration. Regen Eng Trans Med. 2020:1–9.
- Purslow PP The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front Physiol. 2020;11:495. 10.3389/fphys.2020.00495
- Naomi R, Ridzuan PM, Bahari H. Current Insights into Collagen Type I. Polymers (Basel). 2021 Aug 9;13(16). 2642 10.3390/polym13162642
- Karpakka J, Virtanen P, Väänänen K, et al. Collagen synthesis in rat skeletal muscle during immobilization and remobilization. J Appl Physiol (1985). 1991Apr;70(4):1775–80.
- Wiig H, Reed RK, Tenstad O Interstitial fluid pressure, composition of interstitium, and interstitial exclusion of albumin in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2000 May;278(5):H1627–39. 10.1152/ajpheart.2000.278.5.H1627
- Byrne MP, Smith TJ, Montgomery VA, et al. Purification, potency, and efficacy of the botulinum neurotoxin type A binding domain from Pichia pastoris as a recombinant vaccine candidate. Infect Immun. 1998 Oct;66(10):4817–4822. 10.1128/IAI.66.10.4817-4822.1998
- Beyer S, Xie L, Schmidt M, et al. Optimizing novel implant formulations for the prolonged release of biopharmaceuticals using in vitro and in vivo imaging techniques. J Control Release. 2016 Aug 10;235:352–364.10.1016/j.jconrel.2016.06.013
- Goyon A, Excoffier M, Janin-Bussat MC, et al. Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Oct 15;1065-1066:119–128.10.1016/j.jchromb.2017.09.033
- Safarpour Y, Jabbari B. Botulinum Toxin Treatment of Movement Disorders. Curr Treat Options Neurol. 2018 Feb 24;20(2):4.10.1007/s11940-018-0488-3
- Veronese FM, Pasut G PEGylation, successful approach to drug delivery. Drug Discov Today. 2005 Nov 1;10(21):1451–1458.10.1016/S1359-6446(05)03575-0
- Vogel AM, Lennon DR, Broadbent R, et al. Palivizumab prophylaxis of respiratory syncytial virus infection in high-risk infants. J Paediatr Child Health. 2002 Dec;38(6):550–554.10.1046/j.1440-1754.2002.00057.x
- Markowitz CE. Interferon-beta: mechanism of action and dosing issues. Neurology. 2007 Jun 12;68(24 Suppl 4):S8–11.
- Evidente VG, Pappert EJ. Botulinum toxin therapy for cervical dystonia: the science of dosing. Tremor Other Hyperkinet Mov (N Y). 2014;4:273.
- Howard JB, Carpenter FH L-asparaginase from Erwinia carotovora. Substrate specificity and enzymatic properties. J Biol Chem. 1972 Feb 25;247(4):1020–1030. 10.1016/S0021-9258(19)45610-X
- Graham ML Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev. 2003 Sep 26;55(10):1293–1302. 10.1016/S0169-409X(03)00110-8
- Nicoli S, Santi P, Couvreur P, et al. Design of triptorelin loaded nanospheres for transdermal iontophoretic administration. Int J Pharm. 2001 Feb 19;214(1–2):31–35. 10.1016/S0378-5173(00)00632-3
- Park K, Skidmore S, Hadar J, et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J Control Release. 2019 Jun 28;304:125–134.10.1016/j.jconrel.2019.05.003
- Nishiuchi Y, Inui T, Nishio H, et al. Chemical synthesis of the precursor molecule of the Aequorea green fluorescent protein, subsequent folding, and development of fluorescence. Proc Natl Acad Sci USA. 1998 Nov 10;95(23):13549–54.
- Rogge MC, Charenkavanich S, DiBiase M, et al. Impaired bioavailability of interferon beta-1a when administered intramuscularly by needle-free injection. Drug Deliv. 1998;5(4):275–280. doi: 10.3109/10717549809065758
- Probst M, Schmidt M, Weitschies W, et al. In vitro simulation of distribution processes following intramuscular injection. CDBME. 2016;2(1):383–386.
- Calderan L, Carton F, Andreana I, et al. An ex vivo experimental system to track fluorescent nanoparticles inside skeletal muscle. Eur J Histochem. 2023 Jan 2;67(1).10.4081/ejh.2023.3596
- Kanazawa Y, Nagano M, Koinuma S, et al. Effects of aging on basement membrane-related gene expression of the skeletal muscle in rats. Biomed Res. 2021;42(3):115–119. doi: 10.2220/biomedres.42.115
- Hill RL, Wilmot JG, Belluscio BA, et al. Comparison of drug delivery with autoinjector versus manual prefilled syringe and between three different autoinjector devices administered in pig thigh. Medical Devices: Evidence and Research. 2016:257–266..
- Turner PJ, Muraro A, Roberts G Pharmacokinetics of adrenaline autoinjectors. Clin Exp Allergy. 2022 Jan;52(1):18–28.10.1111/cea.14055
- Dreborg S, Kim H The pharmacokinetics of epinephrine/adrenaline autoinjectors. Allergy Asthma Clin Immunol. 2021 Mar 8;17(1):25.10.1186/s13223-021-00511-y
- Whyte A, Parker C. A review of the efficacy and tolerability of antipsychotic long-acting injections. Prog Neurol Psychiatry. 2016 Jul-Aug;20(4):22–28.
- Khanolkar A, White S, Margerison E. A Novel Method to Optimize Drug Delivery for Parenteral Products Involving New Therapies and Unmet Needs. Pharm Res. 2023:1–13.
- Bettonte S, Berton M, Battegay M, et al. Development of a physiologically‐based pharmacokinetic model to simulate the pharmacokinetics of intramuscular antiretroviral drugs. CPT: Pharmacometrics & Systems Pharmacology. 2024..
- Di J, Hou P, Corpstein CD, et al. Multiphysics modeling and simulation of local transport and absorption kinetics of intramuscularly injected lipid nanoparticles. J Control Release. 2023;359:234–243.10.1016/j.jconrel.2023.05.048
- Atoyebi S, Bunglawala F, Cottura N, et al. Physiologically‐based pharmacokinetic modelling of long‐acting injectable cabotegravir and rilpivirine in pregnancy. Br J Clin Pharmacol. 2024. 10.1111/bcp.16006
- Siemons M, Schroyen B, Darville N, et al. Role of modeling and simulation in preclinical and clinical long-acting injectable drug development. Aaps J. 2023;25(6):99. doi: 10.1208/s12248-023-00864-9
- Saldanha L, Langel Ü, Vale N. In silico studies to support vaccine development. Pharmaceutics. 2023;15(2):654.
- Dubbelboer IR, Sjögren E Physiological based pharmacokinetic and biopharmaceutics modelling of subcutaneously administered compounds–An overview of in silico models. Int J Pharm. 2022;621:121808. 10.1016/j.ijpharm.2022.121808
- Micallef J, Arutiunian A, Dubrowski A. The development of an intramuscular injection simulation for nursing students. Cureus. 2020;12(12). doi: 10.7759/cureus.12366
- Micallef J, Lin AC, Arutiunian A, et al. Design of an Intramuscular Injection Simulator: Accommodating Cultural Differences. Cureus. 2021;13(10).10.7759/cureus.18980
- Tantacharoenrat C, Precharattana M. An Electronic-based Simulator for Intramuscular Injection in Newborns. Int J Nurs Educ. 2023;15(2):1–6.
- Mc Crudden MTC, Larraneta E, Clark A, et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release. 2018 Dec 28;292:119–129.10.1016/j.jconrel.2018.11.002
