ABSTRACT
Botanicals are widely consumed as dietary supplements, medicines, and consumer products. Their chemical complexity and variability present challenges in safety assessment, including potential neurotoxicity. The Botanical Safety Consortium (BSC)‘s Neurotoxicity Working Group is exploring new approach methodologies (NAMs) for the screening of botanical extracts for neurotoxic effects.
Areas covered
This paper outlines the selection of NAMs, including in vitro assays using primary rat cortical neurons, zebrafish embryos, and Caenorhabditis elegans. These assays aim to assess neurotoxic endpoints such as neuronal activity and behavioral responses. Microelectrode array recordings of rat cortical neurons provide insights into the impact of botanical extracts on neuronal function, while the zebrafish embryos and C. elegans assays evaluate neurobehavioral responses. The paper also provides an account of the selection of botanical case studies based on expert judgment and existing neuroactivity/toxicity information. The proposed battery of assays will be tested with these case studies to evaluate their utility for neurotoxicity screening.
Expert opinion
The complexity of botanicals necessitates the use of multiple NAMs for effective neurotoxicity screening. This paper discusses the evaluation of methodologies to develop a robust framework for evaluating botanical safety, including complex neuronal models and key neurodevelopmental process assays. It aims to establish a comprehensive screening framework.
1. Introduction
Botanical dietary supplements, herbal medicines, and related natural products are consumed worldwide to improve health and prevent/treat disease. Botanicals are also common components of various consumer products, including cosmetics. In the United States, the sales of botanical dietary supplements alone reached an estimated $12.3 billion in 2021, marking an almost 10% rise from the previous year [Citation1]. Botanicals, which we here include products derived from plants, algae, and fungi, are chemically complex and can be comprised of hundreds to thousands of chemical constituents [Citation2,Citation3]. The chemical composition of botanical-based products can vary depending on cultivation, processing, and manufacturing conditions; combinations with other botanicals; and product/extract contamination or adulteration. This complexity complicates the assessment of the potential toxicity of products in the market.
Although many global regulatory frameworks mandate toxicity testing for botanicals, registrants often rely on the botanical’s history-of-use information to assess safety. Regulatory actions are typically prompted by adverse event reports after these products have entered the consumer market. The ability to evaluate botanical safety rapidly and cost-effectively is valuable for public health. Owing to the inherent variability of botanical extracts, even when derived from the same species, selecting a single representative botanical sample for traditional in vivo testing is difficult, and testing multiple samples requires significant resources. Therefore, screening assays that predict the potential toxicity of botanicals as complex mixtures are needed.
Neurotoxicity, or a neurotoxic effect, is characterized by adverse changes in the chemistry, structure, or function of the nervous system as a result of exposure to a substance [Citation4]. Neurotoxicity may involve direct or indirect effects on neural cells, including factors such as oxidative stress, neuroinflammation, disruption of neurotransmission, protein aggregation, autophagic flux disruption, and/or interference with key enzymes [Citation5]. These effects may be on the peripheral or central nervous system. Case reports of botanical-induced neurotoxicity are uncommon, and the risk of damage to the nervous system is presumed to be low for most marketed herbal dietary supplements. However, most botanicals are not routinely evaluated for neurotoxic potential, and despite the presumed lack of risk, some botanicals have been shown to adversely affect the central nervous system [Citation6–9]. In severe instances, these effects can result in permanent harm and even fatalities. For example, lychee fruit (Litchi chinensis Sonn., Sapindaceae) can induce acute hypoglycemic encephalopathy in undernourished individuals, and raw cassava (Manihot esculenta Crantz, Euphorbiaceae) can trigger sub-acute spastic paraparesis [Citation10]. These demonstrated cases of the potential for natural products to result in damage to the nervous system raise the concern for evaluating the toxic properties of botanicals.
The Botanical Safety Consortium (BSC) is a global cross-sector group of experts in toxicology, analytical chemistry, pharmacognosy, and in vitro methods working to evaluate the suitability of toxicity assays for botanicals as complex mixtures [Citation11,Citation12]. As a part of this larger effort, the Neurotoxicity Working Group within the BSC is investigating several existing new approach methodologies (NAMs) to assess their suitability for screening botanical extracts. Methods were selected based on a group of cross-sector experts, focusing on methods that were established and reproducible, that represent relevant mechanisms for neurotoxicity, and that were accessible for use by many investigators, and with consideration to costs. To evaluate the selected methods, a series of botanical case studies were established using candidates selected based on suspected neurotoxicity. This manuscript describes the reasoning for the selected NAMs and botanical case studies, including a review of relevant tools available for single chemical toxicity testing that can be adapted to botanicals as complex mixtures, and information on selected botanicals with evidence of neurotoxicity. The NAMs selected by the group are intended to be incorporated into a larger battery of assays to look at multiple endpoints related to botanical safety. While currently limited to these specific assays, other tools and methods e.g. human induced pluripotent stem cell neurons) can be evaluated in the future as decided by the needs of the consortium.
2. Assays for neurotoxicity screening
There are several in vivo assays to assess neurotoxicity in rodents, such as the Organization for Economic Co-operation and Development (OECD) Test Guideline 424 (Neurotoxicity Study in Rodents), OECD Test Guideline 426 (Developmental Neurotoxicity Study), and OECD Test Guideline 443 (Extended One-Generation Reproductive Toxicity Study). Given the reasons outlined previously, the focus of the BSC Neurotoxicity working group is on utilizing NAMs for neurotoxicity screening. Additionally, there is a growing emphasis on identifying NAMs that encompass mechanisms pertinent to neurotoxicity or developmental neurotoxicity as exemplified by the initiatives led by the Division of Translational Toxicity (DTT, formerly the National Toxicology Program) [Citation13].
Given that neurotoxicity can result from effects on many different aspects of neuronal function, a battery of NAMs is needed to assess the potential neurotoxic effects of botanicals on a variety of underlying processes such as ion channel and receptor functions, electrophysiological status, mitochondrial functions, and morphological changes. Models such as zebrafish (Danio rerio) embryos and the nematode (Caenorhabditis elegans) were selected, given their intact nervous system and active metabolism. In the zebrafish embryo, an initial blood-brain barrier is evident by reduced permeability of microvessels as early as 3 days post fertilization showing continued maturation in development [Citation14]. Note that we include zebrafish embryos (until free feeding) and C. elegans in our definition of NAMs, given that both are non-protected animal models. These also support 3 R principles (Reduce, Refine, and Replace) for moving away from testing in mammals [Citation15]. While these two model organisms were selected for the present work, there are other models and assays available for the assessment of neurotoxicity, which may be included in the future. An example would be Drosophila melanogaster, for which there are a number of behavioral assays, as well as more mechanistic assays, that have been developed and used to assess neurotoxicity and developmental neurotoxicity [Citation16,Citation17]. In addition, to understand the effects of botanicals on the spontaneous activity of neuronal networks, microelectrode array (MEA) recordings were used to monitor (changes in) neuronal firing in primary rat cortical cultures ().
As these are screening assays, they primarily investigate acute responses to test materials and acute neurotoxic responses may differ markedly both in mechanism and expression from those associated with chronic and/or delayed-onset effects. These assays are being investigated as a battery, to understand their strengths and weaknesses for detecting known botanical-induced neurotoxicity with the described botanical case studies below.
One challenge in the use of NAMs to support safety decisions is that the effects on neuronal cells detected by a screening assay can indicate a (potentially adverse) neuroactive effect, but not necessarily a neurotoxic effect. In addition, differences in bioavailability between in vitro systems or non-mammalian models and clinical reality may lead to overly conservative assessments of neurotoxicity or false positives; however, such models may provide relevant information and can aid in the prioritization of botanicals for further screening.
2.1. Microelectrode array recordings from in vitro primary rat cortical cultures
Multi-well microelectrode array (MEA) recordings enable the detection of extracellular local field potentials (spikes) at different locations within a neuronal network cultured in vitro, thereby providing a readout of neuronal (network) function and activity () [Citation18]. MEA recordings allow medium-throughput assessment of changes in neuronal network function, thereby taking into account the effects on multiple underlying neurotoxicity endpoints (for review see [Citation19–22]).
Figure 1. Overview of the model system and experimental setup used to determine the suitability of these assays for botanicals. hpf: hours post-fertilization. Created with BioRender.
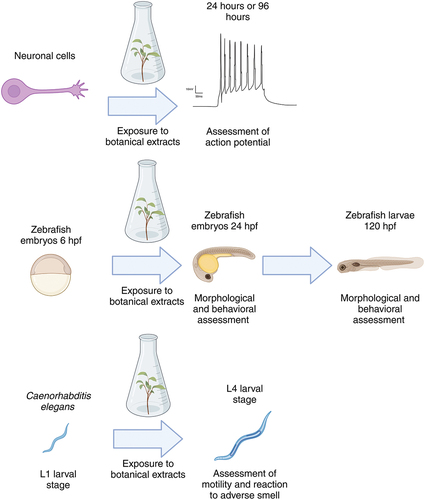
Primary rat cortical neurons – consisting mainly of inhibitory γ-aminobutyric acid (GABA)-ergic and excitatory (glutamatergic) neurons (~55%) and astrocytes (~45%) – are broadly used for in vitro neurotoxicity testing using MEA recordings because they are well characterized, widely accepted, and easily cultured [Citation19,Citation21,Citation23–25]. These cultures resemble many characteristics of the in vivo nervous system (for review see [Citation19,Citation23]), including the development of spontaneous neuronal activity with distinct bursting patterns that are synchronized over the entire network [Citation20,Citation26]. Neuronal networks grown on an MEA are responsive to a variety of compounds, including certain pesticides [Citation27–29], pharmaceuticals [Citation27], natural toxins [Citation24,Citation30], seizureogenic drugs and chemicals [Citation31], and psychoactive substances [Citation32].
Although MEA recordings are valuable screening tools, they have several limitations. For example, primary (rat) cortical neurons have limited metabolic capacity and are not protected by a blood-brain barrier. Additionally, the scarcity of dopaminergic, serotonergic, and cholinergic neurons in cortical cultures results in limited sensitivity to, for example, acetylcholinesterase inhibitors or compounds that affect dopamine/serotonin uptake, such as many recreational drugs. Moreover, the addition of botanical products directly to these neurons may result in the exposure of neurons to compounds or concentrations of compounds that would not have access to the neurons in vivo as a result of either limited bioavailability or metabolism to other forms. Primary neuronal cultures also lack cells responsible for the formation of myelin (oligodendrocytes and Schwann cells), a potential target for substances with neurotoxic potential. Additionally, neurons lack elongated axons, the distal regions of which commonly undergo degeneration in sub-chronic neurotoxic states and neurodegenerative diseases.
2.2. Zebrafish Embryonic/Larval neurobehavioral assays for botanical testing
Zebrafish embryos are a commonly used model for developmental biology and neurotoxicity testing. In the context of NAMs, zebrafish embryos offer a unique advantage due to the fact that (1) they are amenable to high-throughput testing and genetic manipulation as a result of high production (females can lay ~ 200 eggs/week [Citation33]) and external fertilization; (2) they develop rapidly, which allows for assessment of developmental endpoints in a relatively short period; (3) their transparent body during development, which allows for the visualization of neurons and axons either via fluorescent protein-expressing transgenic embryos or through whole-mount immunohistochemistry (for review see [Citation34]); and (4) the evolutionary conservation of cellular and molecular pathways that regulate tissue/organ development and functions [Citation35]. Genetic similarities between zebrafish and humans have also been reported by the zebrafish genome sequencing project, revealing that at least 70% of human protein-coding genes have orthologs in zebrafish [Citation36], suggesting that most human physiology and diseases can be modeled in zebrafish [Citation35]. Zebrafish embryos develop rapidly with most functional organs and organ rudiments present and expression of cytochrome P450 enzymes as early as 24 hours post fertilization (hpf) [Citation37], allowing for high-throughput assessment within a relatively short time. However, when extrapolating findings from zebrafish to humans, it is essential to note the differences. Fish can metabolize chemicals differently than mammals, and are exposed aqueously to the environment rather than orally or intravenously, as in mammalian studies. For these reasons, once a compound is found to have adverse effects on the larval nervous system, further studies are required to determine its applicability to adult zebrafish and mammals. Further systematic comparison of indications of chemical neurotoxicity in zebrafish and mammals is still necessary before zebrafish will be fully accepted as an alternative model for human toxicology [Citation38].
Larval zebrafish behavior is often incorporated in neurotoxicity testing (for review see [Citation39]). Behavioral tests have been used to assess relevant behavioral responses [Citation40]. Altered neurobehavioral results can indicate disruption in neurotransmission, as well as defects in other neural elements, such as neuron or axon development [Citation41]. Common assays for larval zebrafish neurobehavioral testing include embryo photomotor response, larval photomotor response behavior, and larval startle response behavior (; and ).
Figure 2. MicroElectrode Array (MEA) recordings rely on 48-well plates that have a 4 x 4 electrode grid on the bottom of each well, on top of which neuronal cells can be cultured (top left). The spontaneous neuronal activity is recorded as local field potentials (bottom left). The neuroactive potential of botanical extracts can be assessed by comparing the neuronal activity before (baseline recording) and after exposure (right).
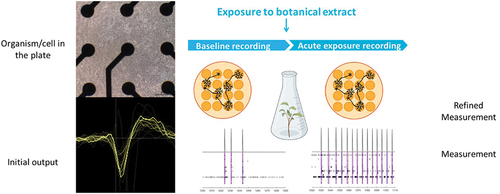
Figure 3. Schematic diagram showing the types of neurobehavioral assays performed in zebrafish larvae, such as larval photomotor response (LPR) assays conducted in light and dark phases, and larval startle response (LSR) assays.
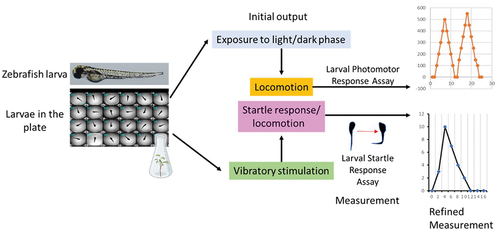
Table 1. Summary of new approach methods initially selected to screen for neurotoxicity.
Zebrafish embryos exhibit tail coiling activity at 17 hpf (for review, see [Citation39]). Upon exposure to light, embryos exhibited increased reflex activity. This response is the embryo photomotor response (EPR), which is mediated by hindbrain regulation [Citation42]. The EPR test was used in ToxCast chemical screens with endpoints showing hypoactivity or hyperactivity that predicted developmental perturbations at 120 hpf [Citation41] (; and ). After 120 hpf, zebrafish show intense locomotor responses to light-to-dark lighting transitions called larval photomotor response behavior (LPR), which is considered a visually mediated reflex. Larvae normally exhibit differential levels of activity depending on lighting conditions with higher levels seen under constant light and lower levels under constant dark. Differential responses can be examined by modulating the pattern of light-dark periods [Citation43]. The LPR test endpoints include distance moved, duration, and speed of movement (for review, see [Citation39]). The larval startle response (LSR) assay is an additional test for larval zebrafish neurobehavior that uses an audible tone to trigger a startle reflex (for review, see [Citation44]). The LSR is mediated by reticulospinal neurons, which are similar to the central elements underlying startle responses in higher-order vertebrates (for review see [Citation45]). Alterations in EPR, LPR, and LSR, as neurobehavioral screening tests can identify potential neuroactive effects of substances.
2.3. Caenorhabditis elegans behavioral assays
Caenorhabditis elegans, a small, free-living nematode, is a model organism used in biological research and for toxicity testing [Citation46,Citation47]. C. elegans is approximately 1 mm in length, transparent, and composed of approximately 1000 cells, all of which are fate-mapped. They have a short lifecycle (2–3 weeks in a laboratory), a sequenced genome, and the ability to reproduce both sexually and via self-fertilization. The species offers many advantages for toxicity testing. C. elegans can be used for neurotoxicity research due to their well-defined nervous system [Citation48]. They are transparent, allowing observation of developmental and reproductive processes affected by substances, and there are transgenic lines that can facilitate in-depth studies on different types of toxicity. In addition, the model can be used to investigate adult-onset neurotoxicity [Citation49]. Their use in behavioral studies ( and ; ), and environmental toxicity assessments underscores their versatility. However, it is important to recognize the limitations of C. elegans in toxicity research, notably the physiological and metabolic differences from humans, and the absence of certain human organs. While C. elegans is invaluable for preliminary toxicity screening and understanding basic toxicological mechanisms, findings often require subsequent testing in more complex organisms.
Figure 4. Schematic diagram showing the types of neurobehavioral assays performed with C. elegans larvae, such as the reaction to adverse smell and body movement assays.
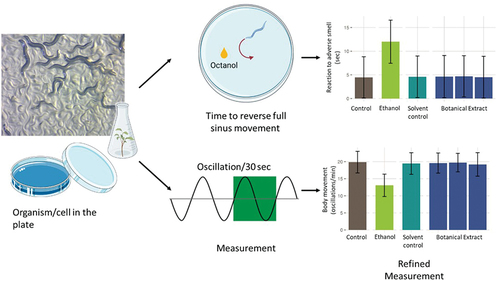
Neurobehavioral assays in C. elegans [Citation50] may provide not only predictions of neurotoxicity but also information on the underlying molecular pathways (for review see [Citation51]). All neurons of C. elegans are mapped and their major interactions well characterized (for review, see [Citation51]). There are 302 neurons in adults, with 118 morphologically distinct neuron types and 56 glial cells together forming over 7600 synapses [Citation52]. The formation, trafficking, and release of synaptic vesicles in C. elegans are analogous to those observed in mammals [Citation53]. While common neurotransmitters such as glutamate, GABA, dopamine, serotonin, and acetylcholine are present [Citation54], the worms lack other neurotransmitters such as epinephrine, norepinephrine, and histamine and do not express voltage-gated sodium channels [Citation55,Citation56].
3. Botanical case studies
To evaluate the selected assays, botanicals were nominated and chosen for testing by the BSC based on existing data. Several of the botanicals selected are not typically taken as dietary supplements (e.g. aconite, aristolochia, comfrey, and oleander), but are useful as case studies given their evidence of toxicity. lists the selected botanicals. Those in bold were specifically selected by the Neurotoxicity Working Group. Other botanicals on the list were selected by other working groups and may have had some or no available data with respect to neurotoxicity/activity. Botanicals were selected based on existing human data (clinical studies or adverse event reporting), animal data (e.g. Good Laboratory Practice compliant rodent studies), mechanistic data (in vitro culture), or a combination of data types. This data was compiled as part of an ongoing nonsystematic search of publicly accessible online databases. When selecting botanicals, studies with some level of chemical quantification and characterization were prioritized as the available literature on botanicals does not always describe chemical characterization.
Table 2. List of botanical extracts used in the BSC, including their standardized common and scientific names, distributed structure-searchable toxicity substance identifier (DTXSID), and part(s) of the plant used to derive the extract. Botanicals with suspected neuroactivity/toxicity are bolded. Table modified from Waidyanatha et al. [Citation52].
After selection, botanicals were sourced from known suppliers with certificates of analysis, and various analytical techniques were used to characterize the constituents [Citation57]. provides an overview of neurotoxicity data available for each botanical.
Table 3. Summary of information with respect to neurotoxicity or activity. Please note this was not a comprehensive literature search and was focused on neurotoxicity and activity and thus may not cover all toxicity data related to the selected botanicals. There may be differences in the preparation of materials in existing literature and the preparation methods described above. The quality of the studies was not evaluated and may vary.
3.1. Aconite
Aconite is a flowering plant found in western and central Europe. Aconite is known to have both cardiotoxic and neurotoxic effects in cases of accidental overdose [Citation58,Citation59]. Aconitine (DTXSID4046319) is a known toxic alkaloidal constituent found throughout the plant, especially in leaves and roots. Aconite was selected for the investigation of suspected neurotoxicity based on its historical use as a poison. There are many modern case reports of fatal, or nearly fatal, poisonings [Citation60–62]. Extracts of aconite plants or roots were used as a remedy for rheumatism and gout and the tincture for neuralgia. Official German and Austrian pharmacopoeias contained monographs for aconite roots and aconite root tinctures until the early 20th century, but those monographs were eliminated due to toxicity concerns [Citation63]. While modern medicinal use of aconite is rare in Europe, a related species, Aconitum carmichaelii Debeaux, which also contains the alkaloid aconitine, is still used in traditional Chinese medicine for its purported benefits on spleen and kidney health [Citation64]. To reduce the toxic effects, aconite has to be subjected to a decoction; however, cases of aconite-induced poisoning are not uncommon in China [Citation65].
Death due to aconite overdose is usually caused by ventricular arrhythmias and asystole. Neurological, cardiovascular, and gastrointestinal symptoms were observed before death, with neurological symptoms (e.g. dizziness, headache, tingling, deterioration of consciousness, and dysarthria) being the most common symptom of aconitine overdose [Citation66]. Patients can be treated with charcoal hemoperfusion to counteract the effects of aconite [Citation67]. Some of aconite’s constituents, including aconitine, target voltage-dependent sodium channels in neurons [Citation68]. This hyperexcitation evokes depolarization and increases neuronal excitability (for review see [Citation69]). Hyperexcitation is also related to reported clinical cardio- and neurotoxic effects (for review see [Citation70,Citation71]).
Zebrafish embryos exposed to aconite displayed coiling behavior mediated by downregulated serotonin (5-HT) receptors [Citation72]. Aconite has been shown to increase dopamine levels in zebrafish larvae by slowing turnover/metabolism and increasing larval mobility [Citation73]. Immortalized mouse hippocampal neuronal cells (HT22 cell line) showed signs of aconitine-induced neurotoxicity via an excitatory process caused by glutamic acid and aspartic acid signaling, which can lead to downstream effects, including intracellular calcium overload and oxidative stress [Citation74]. It has also been reported that aconitine binds to site 2 of the alpha subunit of membrane sodium channels (which are absent in C. elegans) and suppresses their activation [Citation75]
Overall, aconite is known to induce neurotoxicity in humans.
3.2. Aristolochia
Aristolochia fangchi (aka fang ji or guang fang ji, along with other common names that have led to confusion with other plants) is a perennial climbing vine. Infamously, an outbreak of kidney disease in Belgian patients occurred due to A. fangchi in the 1990s. Patients were taking a weight-loss product that was supposed to contain Stephania tetrandra S. Moore, Menispermaceae (stephania, aka fen fang ji) but was made with A. fangchi root extracts apparently due to confusion of the vernacular names. Within 3 years, more than 100 patients mistakenly taking A. fangchi had kidney damage, and 70 of those suffered kidney failure, requiring transplants or dialysis. Some patients developed cancer or urinary tract diseases [Citation76,Citation77]. A. fangchi and other Arisitolochia species contain aristolochic acids (including aristolochic acid I [DTXSID0040969] and II [DTXSID00197166]), which are known to be genotoxic and nephrotoxic. This botanical was selected for its genotoxic property [Citation76,Citation77], which carries the potential not only for carcinogenic potential but also for delayed-onset neurodegeneration.
Little data is available on the effects of A. fangchi on the brain. Given the known genotoxicity of aristolochic acids, neuronal damage is possible if the constituents can reach the target site. In a mouse model, intraperitoneal injection of aristolochic acid I resulted in a negative impact on cognition [Citation78]. Zebrafish embryos exposed to aristolochic acids during development displayed hyperactivity behavioral effects [Citation79]; however, the acute neuromodulatory properties of A. fangchi and aristolochic acid have not been thoroughly investigated.
3.3. Ashwagandha
Ashwagandha is an evergreen shrub that grows in the drier parts of tropical and subtropical zones in Asia, Africa, and the Middle East. The root is used in Ayurveda and other traditional medical systems and people take it to improve their wellbeing as Ashwagandha is purported to have potential application in the treatment of human neuropsychiatric and neurodegenerative disorders [Citation80]. Ashwagandha root extract was selected based on its negative results in a developmental toxicity study. However, there have been reported cases of hepatotoxicity [Citation81,Citation82]. Ashwagandha root contains constituents of biological interest, including the steroidal lactone withaferin A (DTXSID10965459).
While a PubMed search (8 February 2024) found no reports of neurotoxicity associated with ashwagandha root, there is evidence in the literature of ashwagandha having neuroactive properties. Reviews of rodent studies have reported trends in changes in brain oxidative stress markers consistent with antioxidant activity [Citation83] and mitigation of behavioral effects (e.g. changes in gait or muscle coordination) resulting from challenges such as hypoxia or restraint stress [Citation83,Citation84]. However, they did not report whether the included literature was assessed for publication bias. A meta-analysis of both human and animal studies noted that ashwagandha was associated with decreased stress or anxiety-induced changes in rodent performance in behavioral assays (e.g. the forced swim test and the elevated maze test) [Citation80].
3.4. Asian Ginseng
Asian ginseng is a perennial herb with branched roots, which is grown ideally for 6–10 years before harvest. It is native to Asia, including China and Korea. The root has been used for health-related purposes for thousands of years. The U.S. National Center for Complementary and Integrative Health suggests that short-term oral use (up to six months) is safe for most people [Citation85]. However, its effect on sensitive populations (e.g. pregnant people) has been understudied. The root extract of this botanical was selected for its reported lack of toxic effects across endpoints relevant to botanical safety.
With respect to neuronal activity, studies have suggested that Asian ginseng root increases dopamine and acetylcholine levels in the rat brain (for review see [Citation86]) and increased gap junction intercellular communication in rat astrocytes [Citation87]. Also in rats, Asian ginseng’s effects on learning and memory were attributed to antioxidant activity [Citation88]. The National Toxicology Program’s U.S. NTP) 2-year studies of Asian ginseng root in rats and mice found no significant effects with result to the nervous system [Citation89]. According to a study based on publicly accessible online databases, predictions regarding Asian ginseng’s effects on genes related to Alzheimer’s disease suggest that it may potentially influence key regulatory pathways, specifically calcium signaling and the serotonergic synapse. While this was in the context of treatment, dysregulated calcium signaling (inhibition or activation), if sustained, can result in neurotoxic effects.
High concentrations of certain constituents of Asian ginseng such as β-N-oxalyl-l-α,β-diaminopropionic acid (β-ODAP), and γ-aminobutyric acid (GABA; DTXSID6035106) have been found in the seed and 1–3 years old plants of Asian ginseng [Citation90]. β-ODAP is a neuroactive amino acid and GABA is an inhibitory neurotransmitter. However, it should be noted that we are focused on an extract prepared from the mature root in our studies, which has not been shown to contain high concentrations of β-ODAP or GABA [Citation91], and neither constituent was identified in our samples [Citation92]. Neither seeds nor young plants are used as ingredients in dietary supplements or herbal medicine.
3.5. Blue cohosh
Blue cohosh is a flowering plant in the barberry family of plants. It is grown in eastern North America. The root has been used in combination with other botanicals for abortive or contraceptive purposes. Blue cohosh root is also used by midwives to help induce labor, but it has been associated with perinatal stroke, congestive heart failure, and neonatal shock [Citation93]. It is known to induce teratogenic effects in animals and was therefore selected for the developmental endpoint. Blue cohosh should not be confused with black cohosh (Actaea racemosa L., Ranunculaceae).
Blue cohosh contains alkaloids, including the quinolizidine alkaloids N-methylcytisine (DTXSID80876866), anagryne (DTXSID40964091), and baptifoline, and the aporphine alkaloid magnoflorine. N-methylcytisine can have nicotinic-like effects (for review see [Citation94]). Both N-methylcytisine and another constituent, anagyrine (DTXSID40964091) can bind to neuronal nicotinic-acetylcholine receptors as an agonist [Citation95,Citation96]. Chronic exposure to low-dose nicotine (e.g. that which tobacco smokers are exposed to) can cause central sympathetic activation by affecting peripheral chemoreceptors or the autonomic or vasomotor centers of the brain stem (for review see [Citation94]). A blue cohosh methanol extract showed mitochondriotoxic activity in a cell-based hypoxia-inducible factor reporter assay that was used to screen natural products for mitochondrial poisons [Citation93]. While there is some evidence here, to understand the potential neurotoxic effects of blue cohosh further studies would be required.
3.6. Comfrey
Comfrey is a native plant to Asia and Europe, with oval to lance-shaped leaves and bell-like flowers. While the root has been used medicinally for over 2000 years, especially topically for wound healing, it is known to contain genotoxic constituents, namely pyrrolizidine alkaloids (PAs) [Citation97]. PAs are heterocyclic organic compounds made by plants as a defense mechanism from predators [Citation98]. In comfrey, some of the known PAs are lycopsamine (DTXSID60145542) and 7-acetyllycopsamine (DTXSID50223742).
Alkaloid extracts of comfrey have been shown to inhibit the activity of sodium-hydrogen exchangers (NHEs) in mammalian cells [Citation99]. NHEs play a crucial role in maintaining pH homeostasis in cells and tissues. Alterations in pH can affect nerve excitability and are thought to be involved in many dysfunctions of the nervous system [Citation100–102]. On the other hand, studies on animal models of hypoxia-ischemia show the neuroprotective effects of NHE inhibitors [Citation103]. There needs to be further investigation of the effects of comfrey on the nervous system.
3.7. Ephedra
Ephedra also known as ma huang, is an herbaceous perennial plant native to China [Citation104]. It has been used to treat various conditions, such as colds, fever, headache, etc. in China and India, and has been purported to promote weight loss. Constituents of biological interest include alkaloids like ephedrine (DTXSID0022985), pseudoephedrine (DTXSID0023537), and N-methylephedrine (DTXSID401021166) [Citation104].
The US Food and Drug Administration banned all dietary supplements containing ephedrine alkaloids from being sold in the US in 2004 [Citation105,Citation106]. The ban was a result of adverse effects, including cardiovascular effects and neurological effects (e.g. heart attack, seizure, stroke, and death) [Citation107,Citation108]. The modest short-term effect it had on weight loss was heavily outweighed by the serious risk these products posed. A case report showed transient blindness occurred in a patient due to posterior reversible encephalopathy syndrome following ingestion of an ephedra-based supplement [Citation109]. Additionally, people taking ephedra may experience dizziness, headache, nausea, insomnia, and other behavioral changes. These effects are enhanced when taken with caffeine, an ingredient that was commonly sold in combination with ephedra in weight loss and athletic performance-enhancing products before 2004 [Citation110].
In a study in rats, ephedra induced oxidative damage in the prefrontal cortex, and hyperactivity [Citation111], indicating that ephedra possesses neurotoxic potential. Ephedra’s neurotoxic potential in rats is attributed to its ability to increase the levels of amino acid neurotransmitters in the prefrontal cortex [Citation112]. Ephedrine can inhibit the dopamine and norepinephrine membrane transporters (DAT and NET), although it is mainly known for its agonistic effects on α- and β-adrenergic receptors [Citation113–115]. One study did report beneficial effects in rats with subarachnoid hemorrhage treated with ephedra [Citation116], pointing to questions on efficacy versus toxicity, and dose and exposure patterns. Based on the variety of evidence described above, ephedra was anticipated to elicit a neurotoxic/active effect in the selected assays.
3.8. Green tea dry decaffeinated extract
Green tea is one of the most common beverages in the world and has numerous studies purporting effects and benefits [Citation117]. Green tea as a beverage is believed to be safe (with natural levels of caffeine) at doses up to eight cups per day. Green tea is generally considered neuroprotective due to the high level of antioxidant polyphenols (for a review see [Citation118]).
While the tea is considered safe, the BSC focused on concentrated green tea extracts which contain high amounts of catechins. These are commonly used as dietary supplement ingredients [Citation119]. In this project, decaffeinated concentrated green tea extract was selected by the Hepatotoxicity Working Group due to reported adverse event reports and by the Genotoxicity Working Group due to U.S. NTP studies [Citation119,Citation120].
The U.S. NTP conducted a 2-year study and a 3-month study with both rats and mice testing a green tea extract containing 48% epigallocatechin gallate (EGCG, DTXSID70925231). Neither study identified evidence of carcinogenic activity in rats or mice (both males and females in both groups). However, there were nonneoplastic lesions of the liver, stomach, and intestine in male and female rats. In both the 3-month and 2-year studies, there were no reported effects on the nervous system, including the brain [Citation119]. The United States Pharmacopeia (USP) did an extensive review of green tea extracts due to reported liver toxicity and issued the following statement ‘Do not take on an empty stomach … Do not use if you have a liver problem and discontinue use and consult a healthcare practitioner if you develop symptoms of liver trouble’ [Citation120]. One clinical study that investigated the use of concentrated green tea extract for neuroprotection in multiple sclerosis was terminated due to hepatotoxicity in the form of elevated liver enzymes, though a purported marker of neuronal metabolic health did increase after 6 months of treatment [Citation121].
While there is an extensive amount of literature on green tea, this section focused on concentrated extracts of decaffeinated green tea. The effect these extracts have on the nervous system is not fully understood.
3.9. Goldenseal
Goldenseal is a perennial herb native to the eastern United States and southeastern Canada. It has a long history of use for various medicinal purposes. Traditionally, goldenseal has been purported to treat skin disorders, digestive issues, and urinary tract infections, among other reported uses. Currently, it is marketed in dietary supplements for the cold and flu, allergies, and gastrointestinal complaints, often in combination with echinacea (Echinacea spp., Asteraceae)
The U.S. NTP completed feed studies in rats and mice for goldenseal root powder, for 2 weeks, 3 months, and 2 years. In the 2-year study, there was increased liver cancer in the high-dose male and female rats, and in the high-dose male mice. There were no reported effects on the nervous system in any of the groups [Citation122].
Some evidence of the neurotoxic effects of goldenseal’s constituents has been reported (for review see [Citation123]). Hydrastine [DTXSID9025409], an alkaloidal constituent of goldenseal, has been observed to induce apoptosis in PC12 cells, a type of pheochromocytoma cell line with neuronal characteristics [Citation124]. Additionally, hydrastine reduced dopamine biosynthesis in PC12 cells, potentially by inhibiting tyrosine hydroxylase [Citation125]. Berberine [DTXSID9043857], another alkaloidal constituent in goldenseal, induced rapid mitochondria-related toxicity in primary neurons derived from rodents, including mitochondrial swelling, decreased mitochondrial membrane potential, and depletion of ATP content accompanied by elevated oxidative stress [Citation126]. Treatment with N-methyl-D-aspartate (NMDA) receptor antagonists such as MK-801 and memantine completely prevented berberine-induced toxicities [Citation126]. In addition, there is evidence that several alkaloids from goldenseal, including hydrastine, berberine, palmatine [DTXSID9048065], and canadine [DTXSID5022724], are effective acetylcholinesterase inhibitors [Citation127].
There have been many studies that specifically looked at berberine. Preclinical studies show that berberine can modulate neurotransmitters and their receptors (e.g. acting as D1 dopamine receptor agonist and D2 dopamine receptor antagonist) and report it to exert beneficial effects against several nervous system disorders (for review see [Citation128]). Through its antioxidant and anti-apoptotic effects, berberine was able to ameliorate Amyloid-beta (Aβ)-induced neurotoxicity in vitro in HT22 (immortalized mouse hippocampal neuronal cell line) cells [Citation129]. It has also been reported that berberine has the potential to dislodge bilirubin from its bond with serum albumin. This is potentially dangerous, particularly for newborn infants (for review see [Citation130]), since infants with high bilirubin concentrations are at risk of bilirubin passing the blood-brain barrier, which can lead to damage to the brain and spinal cord.
Given the mix of information, it is not clear how goldenseal seal root extract and its constituents affect the nervous system.
3.10. Kratom
Kratom is an evergreen tree native to Southeast Asia. Its leaves have been used medically or recreationally. Some of its constituents can act as partial opioid agonists [Citation131], making it popular among opioid users. Increasingly, people use kratom for pain and to alleviate symptoms of opioid withdrawal due to its psychoactive effects [Citation132]. Based on determinations by the US FDA, kratom cannot be legally marketed as a dietary supplement and cannot be lawfully added to conventional foods [Citation133]. The US FDA has warned that kratom users can experience serious adverse events, including liver toxicity, seizures, and substance use disorder. The adverse event potential of kratom is a subject of much debate, as many of the case reports of adverse events in the literature include patients with a history of drug abuse [Citation134,Citation135].
Rats exposed orally to kratom for 28 days had hippocampal neuron death in their brains, which can potentially lead to memory impairment [Citation136]. In another study, adolescent rats treated with 30 mg/kg of kratom during postnatal days (PND) 31–45 exhibited impaired long-term object recognition memory in adulthood indicating vulnerability of the developing brain to kratom [Citation137]. Kratom blocks both L-type and T-type Ca2+ channels in neuroblastoma cells [Citation138]. Changes in Ca2+ signaling are attributed to the impeded hippocampal transmission and plasticity causing impaired memory observed with kratom administration [Citation139].
The inhibitory properties of kratom extract described above can be explained by its alkaloidal constituents mitragynine [DTXSID701032140] and 7-OH-mitragynine [DTXSID20903988], which act as µ-opioid receptor partial agonists (for review see [Citation140,Citation141]). Mitragynine exposure induced apoptosis and cell cycle arrest at the G2/M phase in HT22 (immortalized mouse hippocampal neuron cell lines) cells [Citation142]. Adolescent (PND 31–45) male rats given mitragynine orally for 15 days showed impaired long-term object recognition memory during adulthood (PND 70–84), while social behavior and spatial learning remained unaffected; however, both mitragynine and lyophilized kratom decoction orally for 15 days caused impaired reference memory [Citation140]. Mitragynine adversely affected hippocampal synaptic plasticity in rats that showed significantly impaired basal synaptic transmission and long-term potentiation suggesting mitragynine to be a weak AMPA- and NMDA receptor antagonist [Citation143].
Given this evidence, kratom is expected to exhibit activity in the selected neurotoxicity assays.
3.11. Kava
Kava (also known as kava kava), is a psychoactive plant native to the Pacific Islands. The root of P. methysticum has been historically used as a ceremonial beverage but modernly is used as an herbal medicinal product. Kava has purported anti-depressant and anxiolytic effects (for review see [Citation144]).
The U.S. NTP completed 2-week, 3-month, and 2-year studies in rats and mice treated by gavage daily 5 days/week with extracts of kava root in corn oil for kava extract. There were increases in liver cancers in male and female mice. There were increased incidences of liver lesions in male and female rats, and slightly increased testicular tumor rates in males [Citation145]. There were no reported effects on the nervous system in any of the groups [Citation145].
Kavalactones and flavokawains in kava root extract have reported hepatotoxic properties, depending on preparation [Citation146], but these constituents have been reported to also modulate GABA receptors and inhibit voltage-dependent Na+ and Ca2+ channels (for review see [Citation147]). Exposure of C. elegans to a kavalactone supplement resulted in alterations in the behavior of L4 larvae, with organisms exposed to increasing concentrations of kavalactones being more likely to display convulsed or paralyzed behavior [Citation148]. The study’s authors hypothesized that the behavior changes were a result of cholinergic-enhancing effects.
Kava has been shown to inhibit monoamine oxidase B (MAO-B) activity in mice [Citation149]. MAO metabolizes neurotransmitters, such as dopamine, serotonin, norepinephrine, and tyramine (for review see [Citation150]). MAO-B is found in glial cells of the brain near dopaminergic synapses [Citation151] and plays a role in the regulation of monoamine storage and release in the synaptic cleft [Citation152]. A case report on patients ingesting kava preparations showed that the patients developed symptoms indicative of kava having dopamine-antagonistic properties [Citation153].
While there is evidence of neuroactivity, there has been little reported neurotoxicity resulting from kava consumption.
3.12. Milk thistle
Milk thistle is an annual plant of the daisy family native to Southern Europe, Russia, Asia Minor, and Northern Africa, and naturalized in North/South America and Australia [Citation154]. Milk thistle fruit is used by western herbalists and naturopathic physicians to treat dyspeptic complaints, while formulations made from the plant are used by physicians to treat liver damage [Citation155]. Milk thistle is thought to be safe. It was selected by the BSC for its reported lack of toxicity, especially given the results of an NTP 2-year study in rats and mice [Citation156].
It has been reported that silymarin, which is a mixture of flavonolignans including silybin A and B [DTXSID30858697], improves methamphetamine-induced memory dysfunction by decreasing the level of dopamine in the prefrontal cortex and serotonin in the hippocampus of mice [Citation157,Citation158]. In rats, silymarin increased serotonin levels in the hippocampus and amygdala, while inhibiting a single prolonged stress-induced decrease in dopamine levels in the hippocampus [Citation157]. Another study reported that silibyn (unclear which exact constituent or mix thereof) reduced acetylcholinesterase (AChE) activity in mice and showed beneficial effects on Alzheimer’s disease-like symptoms [Citation159].
Milk thistle has been suggested to be neuroprotective (for review see [Citation160]). The neuroprotective properties of milk thistle are primarily ascribed to silymarin (for review see [Citation161,Citation162]). However, there are indications that acute exposure of rat cortical synaptosomes to silymarin can inhibit calcium influx and glutamate release [Citation157], which could result in the inhibition of neuronal activity.
It is not expected that milk thistle extract will induce neurotoxicity.
3.13. Oleander
Oleander is an evergreen shrub native to the Mediterranean region but cultivated worldwide as an ornamental plant [Citation163]. The primary constituent of biological interest is oleandrin (DTXSID40861950), a steroidal glycoside. Oleandrin, which occurs in all parts of the plant, has been used in ethnomedicine as a treatment for a wide spectrum of diseases including asthma, eczema, ringworm, congestive heart failure, indigestion, and neurodegenerative diseases [Citation164].
However, oleander poisoning is well documented [Citation165–168]. Its toxic effects on the lung, liver, and heart have been reported, which may be linked to its inhibition of the Na+/K+-ATPase pump in the cell membrane (for review see [Citation169]). Inhibition of Na+/K+-ATPase (which plays a critical role in synaptic transmission [Citation170]) and the subsequent increased intracellular Ca2+ concentrations are likely due to oleandrin and other cardiac glycosides present in oleander [Citation171]. There is evidence showing oleandrin may be a strong inducer of brain derived neurotrophic factor (BDNF) and therefore promoting brain health [Citation157,Citation172,Citation173].
Reduced cerebral Na+/K+-ATPase activity was associated with changes in locomotion and altered behavior in silver catfish [Citation174]. Rats and mice acutely exposed to oleander extract had increased troponin (a marker of cardiotoxicity) and creatine kinase (an enzyme present in skeletal muscle, myocardium, and brain), as well as mild histopathological lesions and moderate coagulative necrosis in sampled brains [Citation175].
3.14. Tripterygium
Root extracts of tripterygium (aka thunder god vine) have been used in traditional Chinese medicine to treat a variety of inflammatory and autoimmune disorders [Citation176]. Constituents that have shown activity include the diterpenoid triptolide (DTXSID5041144) and the nor-triterpenoid celastrol (DTXSID2040993) [Citation177].
Serious adverse effects associated with tripterygium overdose, such as vomiting, abdominal pain, difficulty breathing, heart failure, and death, have been reported [Citation178,Citation179]. There is a case report of a young man who exhibited profuse vomiting, diarrhea, and other serious symptoms following the ingestion of tripterygium extract [Citation179]. While it was discovered he had evidence of coexisting cardiac damage, he died 3 days later. A meta-analysis of 46 clinical trials found that tripterygium included intestinal, reproductive, liver, kidney, and other types of toxicity [Citation178]. A study in rats found that in 1- and 3-month studies, tripterygium caused reduced body and testes weights, in addition to reduced testosterone levels [Citation178].
Epicatechin isolated from tripterygium was reported to have neuroprotective effects against tau-GFP-induced neuronal death in zebrafish embryos [Citation180]. Triptolide, celastrol, demethylzeylasteral, and wilforgine derived from tripterygium extract may inhibit voltage-gated sodium channels [Citation181], which could result in inhibition of neuronal activity.
Based on the evidence in humans, we expect to see neurotoxic responses in the assays.
3.15. Usnea
Usnea is a lichen genus found around the world with over 350 species in the genus. The species commonly utilized in traditional medicine are referred to as ‘beard lichen’ due to their filamentous strands that grow on tree branches [Citation182]. In traditional Chinese medicine, Usnea spp. have been used as a treatment for many ailments including headache, ocular irritation, malaria, and snake bites [Citation183]. The sample used by the BSC was wild sourced in North America and therefore likely consists of U. cavernosa Tuck., U. filipendula Stirt., U. longissima Ach. (currently accepted name Dolichousnea longissima (Ach.) Arcticus), or U. scabrata Nyl [Citation182]. The primary constituent of biological interest is usnic acid (DTXSID0040123), a dibenzofuran which is found in many Asian, European, and North American usnea species [Citation182]. This botanical was selected based on evidence of development and reproductive toxicity.
In the U.S. NTP study, rats exposed to Usnea lichen containing ± usnic acid in feed for 3 months had severe toxicity. Males had significant hepatotoxicity at multiple dose levels, and females at the high dose. Mice also exhibited hepatotoxicity, and in female mice, there was ovarian atrophy and extended estrous cycle [Citation182]. There were no reported effects on the nervous system [Citation182].
In additional studies, usnea lichen has been reported to have hepatotoxic effects (for review see [Citation184]). In rat hepatocytes, usnic acid caused significant depletion of cellular ATP levels and cell death [Citation185]. However, anti-inflammatory, neuroprotective, and cognition-enhancing effects of usnic acid have been reported in rodent models of Alzheimer’s disease and Parkinson’s disease [Citation186–188]. Although no neurotoxicity by usnea/usnic acid has been reported, its ability to deplete ATP content through mitochondrial dysfunction in hepatocytes [Citation185] raises concern about its potential to affect the nervous system, especially when the brain depends on ATP produced elsewhere in the body as it does not produce enough ATP for its functioning (for review see [Citation189]). In human (SH-SY5Y) neuroblastoma cells, usnic acid induced the production of reactive oxygen species [Citation190], suggesting that usnic acid may have neurotoxic potential. In a study on rats relating to hepatic metabolism, it has been suggested that usnic acid may be dangerous to the brain in the fasting stage due to its ability to reduce the output of hepatic glucose and ketone bodies and increase ammonia production [Citation191].
Based on this mixed evidence, neurotoxicity is not expected, however there may be neuroactivity or other effects in the assays.
3.16. Yohimbe
Yohimbe is an evergreen of the coffee family native to tropical regions of the African west coast. The bark has traditionally been taken as a treatment for fever, leprosy, and cough, as well as a treatment for erectile dysfunction, and as an aphrodisiac in West Africa [Citation192,Citation193]. More recently, yohimbe has been sold as an aphrodisiac and as an athletic performance enhancer [Citation194,Citation195]. Constituents of biological relevance include indole alkaloids, such as yohimbine (DTXSID9040130) [Citation192].
Yohimbine has been reported to induce neurotoxic effects, such as anxiety, tremors, incoordination, dissociative reaction, retrograde amnesia, and seizures when ingested [Citation6]. In a case report, a male bodybuilder experienced severe acute effects, including vomiting, loss of consciousness, and seizures after ingesting 5 grams of yohimbine. He recovered twelve hours later [Citation6]. There also have been reports of yohimbine being found at high blood levels in two individuals who died unexpectedly [Citation196].
Yohimbine reduced acetylcholine in rat striatum and showed anti-dopaminergic properties [Citation197]. Dose-dependent bimodal response of acetylcholine levels to yohimbine poses a challenge to categorizing this botanical either as a stimulant or a suppressant of the cholinergic system. Yohimbine has also been shown to inhibit calcium channels [Citation198], thereby possibly reducing neuronal activity.
Based on the evidence in humans and mechanistically, neurotoxic responses are expected to be seen in the assays.
4. Conclusions
Botanicals are being used worldwide by consumers and there is a need to screen such products for toxicity, including neurotoxicity, to ensure products are safe. As such, the BSC is investigating the suitability of NAMs to aid in the screening of botanical products. The NAMs described in this review could present a more cost-effective approach to traditional toxicity methods for screening of endpoints of interest including neurotoxicity. Multiple assays have been selected by a cross-sector team of experts to test their suitability for botanicals as complex mixtures, using well-characterized, data-rich botanicals as case studies. The assays will include neurons in culture, in an invertebrate (C. elegans), and in zebrafish embryos.
This manuscript describes the assays and the selected botanicals relevant for the screening of neuroactivity/toxicity. Immediate next steps are to test the botanicals described in the assays (for initial results see [Citation199]), evaluate the data, compare the results to the existing literature, and develop a toolkit of assays to screen for neurotoxicity.
While this effort is currently focused on screening and hazard identification, there is the potential for future work that could increase the predictivity of NAMs to human responses. Information related to toxicokinetics needs to be considered, including for metabolism of chemical constituents, distribution to target sites (including crossing the blood-brain barrier), and absorption differences between humans and in vitro exposures. In vitro to in vivo exploration could help with dose setting and testing human-relevant concentrations. More complex neuronal models with multiple cell types and neurons would help identify more specific effects and mechanisms of actions that these functional and whole organism assays do not provide.
This work demonstrates a strategy for assessing assays to screen for the neurotoxicity of botanicals. These novel studies planned to improve the botanical safety toolkit and give researchers tools to better understand neurotoxicity, prioritize and plan future testing as needed, and better understand the botanical being tested.
5. Expert opinion
As discussed, safety evaluation of botanicals is difficult compared to single chemicals due to the intricate and variable chemistry inherent in botanicals which complicates the selection of a single representative botanical sample for traditional in vivo rodent testing challenging. This results in the potential for needing to test/evaluate of multiple samples, which demands substantial resources [Citation189]. Using multiple NAMs for hazard screening of botanicals offers a way of increasing the coverage of neurological screening assessments, as they are typically less resource intensive than traditional in vivo toxicology models. This advancement would allow for more accessible and rapid screening of botanical and natural products and aid in protecting public health. The BSC is not alone in its interest in furthering the implementation of NAMs for the use of neurotoxicity screening. As discussed previously, there is a growing emphasis on identifying NAMs pertinent to neurotoxicity or developmental neurotoxicity as exemplified by the initiatives led by the DDT [Citation11], and the emphasis on investigating the integration of NAMs for food safety assessment within EFSA [Citation190]. Given the prominence of work focused on the use of NAMs for assessment of neurotoxicity, it would appear that the future of neurotoxicity screening lies with NAMs.
There is much ongoing work in this field; however, it has largely been focused on the use of NAMs with discrete chemicals and small molecules [Citation191]. The work outlined in the present paper represents a first step in the systematic evaluation of in vitro neurotoxicity screening methods for use with botanicals. While the efforts of the BSC are currently focused on screening and hazard identification, the ultimate goal of the consortium is to develop a framework that can facilitate the evaluation of botanicals. The assays currently selected present a first assessment of suitable methods. In the future, the inclusion of more complex neuronal models with multiple cell types and neurons would help identify more specific effects and mechanisms of actions that these functional and whole organism assays do not provide, and aid in developing a more robust framework. In addition, the inclusion of in vitro assays that cover key neurodevelopmental processes such as neuronal migration and differentiation, and neurite outgrowth would aid in describing potential mechanisms of action of botanicals identified as potential developmental neurotoxicant in the whole organism assays evaluated.
While evaluation of the assays is a key component, there are many steps between current knowledge and the ultimate goal of developing a screening framework, both for the BSC and for the field of NAMs in general. A key area is a better understanding of toxicokinetics, absorption, distribution (including the potential to cross the blood-brain barrier), metabolism, and excretion of chemical constituents is also key. Such an understanding would aid in in vitro to in vivo extrapolation which would help with ensuring doses utilized in testing are at human-relevant concentrations and could aid in expanding NAM assessments of botanicals beyond the hazard/screening level.
This work demonstrates a strategy for assessing assays to screen for the neurotoxicity of botanicals. These novel studies will expand knowledge regarding the botanical safety toolkit and provide researchers with additional tools to better understand neurotoxicity, prioritize and plan future testing as needed, and better understand the botanical being tested. It is the view of the authors that in the next five years in that we will have an increased understanding of NAMs for neurotoxicity, potentially including novel or more metabolically capable methods, and we will have an increased understanding of how these tools can be used to screen for neurotoxicity, especially with botanicals as complex mixtures.
Article highlights
The complexity of botanicals can complicate the assessment of potential neurotoxic/active effects.
This manuscript describes the assays and the selected botanicals relevant for the screening of neuroactivity/toxicity.
The selected assays include neurons in culture, in an invertebrate (C. elegans), and in zebrafish embryos.
This paper non-systematically summarizes available data related to neuroactivity and neurotoxic potential for the 16 selected botanicals.
These planned novel studies will expand the botanical safety toolkit and give researchers tools to better understand neurotoxicity, prioritize and plan future testing as needed.
Declaration of interest
P Spencer – Consultant, Shenzhen Center for Disease Control & Prevention, Shenzhen, PRC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We would like to thank Robin Marles for reviewing this paper. We also acknowledge the committee members for their support and helpful feedback during the development of this document.
The opinions expressed in this article are the authors’ own and do not reflect the views of the National Institutes of Health, the Food and Drug Administration, the Department of Health and Human Services, or the United States government. The inclusion of a product in this paper does not imply endorsement, certification, or recommendation.
Additional information
Funding
References
- Smith T, Resetar H, Morton C. US sales of herbal supplements increase by 9.7% in 2021. J Am Bot Counc. 2022:42–69. https://openurl.ebsco.com/EPDB%3Agcd%3A3%3A10824531/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A161226031&crl=c
- Sorkin BC, Kuszak AJ, Bloss G, et al. Improving natural product research translation: from source to clinical trial. Faseb J. 2020;34(1):41–65. doi: 10.1096/fj.201902143R
- Houriet J, Vidar WS, Manwill PK, et al. How low can you go? Selecting intensity thresholds for untargeted metabolomics data preprocessing. Anal Chem. 2022;94(51):17964–17971. doi: 10.1021/acs.analchem.2c04088
- Klaassen CD, Casarett LJ, Doull J, editors. Casarett and Doull’s toxicology: the basic science of poisons. 8th ed. NY: McGraw-Hill Education; 2013.
- Programme international sur la sécurité des substances chimiques, editor. Neurotoxicity risk assessment for human health: principles and approaches. Geneva: World health organization; 2001.
- Giampreti A, Lonati D, Locatelli C, et al. Acute neurotoxicity after yohimbine ingestion by a body builder. Clin Toxicol. 2009;47(8):827–829. doi: 10.1080/15563650903081601
- Guo J, Zhang J, Liu Q, et al. Research progress on components and mechanisms of neurotoxicity induced by traditional Chinese medicine. J Appl Toxicol. 2023;43(3):338–349. doi: 10.1002/jat.4396
- Höllerhage M, Rösler TW, Berjas M, et al. Neurotoxicity of dietary supplements from annonaceae species. Int J Toxicol. 2015;34(6):543–550. doi: 10.1177/1091581815602252
- Lüde S, Vecchio S, Sinno-Tellier S, et al. Adverse effects of plant food supplements and plants consumed as food: results from the poisons centres-based PlantLIBRA study. Phytother Res. 2016;30(6):988–996. doi: 10.1002/ptr.5604
- Palmer VS, Tshala-Katumbay DD, Spencer PS. Plants with neurotoxic potential in undernourished subjects. Rev Neurol (Paris). 2019;175(10):631–640. doi: 10.1016/j.neurol.2019.07.015
- Mitchell CA, Dever JT, Gafner S, et al. The botanical safety consortium: a public-private partnership to enhance the botanical safety toolkit. Regul Toxicol Pharmacol. 2022;128:105090. doi: 10.1016/j.yrtph.2021.105090
- Botanical safety consortium – a public-private partnership to improve botanical safety [Internet]. [cited 2024 May 30]. Available from: https://botanicalsafetyconsortium.org/
- Behl M, Ryan K, Hsieh J-H, et al. Screening for developmental neurotoxicity at the national toxicology program: the future is here. Toxicological Sci. 2019;167(1):6–14. doi: 10.1093/toxsci/kfy278
- Quiñonez-Silvero C, Hübner K, Herzog W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev Biol. 2020;457(2):181–190. doi: 10.1016/j.ydbio.2019.03.005
- Bauer B, Mally A, Liedtke D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. IJMS. 2021;22(24):13417. doi: 10.3390/ijms222413417
- Cabrita A, Medeiros AM, Pereira T, et al. Motor dysfunction in drosophila melanogaster as a biomarker for developmental neurotoxicity. iScience. 2022;25(7):104541. doi: 10.1016/j.isci.2022.104541
- Rand MD. Drosophotoxicology: the growing potential for drosophila in neurotoxicology. Neurotoxicol Teratol. 2010;32:74–83. doi: 10.1016/j.ntt.2009.06.004
- Wallace K, Strickland JD, Valdivia P, et al. A multiplexed assay for determination of neurotoxicant effects on spontaneous network activity and viability from microelectrode arrays. Neurotoxicology. 2015;49:79–85. doi: 10.1016/j.neuro.2015.05.007
- Johnstone AFM, Gross GW, Weiss DG, et al. Microelectrode arrays: a physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology. 2010;31(4):331–350. doi: 10.1016/j.neuro.2010.04.001
- Robinette BL, Harrill JA, Mundy WR, et al. In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Front Neuroeng. 2011;4:1. doi: 10.3389/fneng.2011.00001
- McConnell ER, McClain MA, Ross J, et al. Evaluation of multi-well microelectrode arrays for neurotoxicity screening using a chemical training set. Neurotoxicology. 2012;33(5):1048–1057. doi: 10.1016/j.neuro.2012.05.001
- Spira ME, Hai A. Multi-electrode array technologies for neuroscience and cardiology. Nat Nanotech. 2013;8(2):83–94. doi: 10.1038/nnano.2012.265
- De Groot MWGDM, Westerink RHS, Dingemans MML. Don’t judge a neuron only by its cover: neuronal function in in vitro developmental neurotoxicity testing. Toxicological Sci. 2013;132:1–7. doi: 10.1093/toxsci/kfs269
- Hogberg HT, Sobanski T, Novellino A, et al. Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons. Neurotoxicology. 2011;32(1):158–168. doi: 10.1016/j.neuro.2010.10.007
- Valdivia P, Martin M, LeFew WR, et al. Multi-well microelectrode array recordings detect neuroactivity of ToxCast compounds. Neurotoxicology. 2014;44:204–217. doi: 10.1016/j.neuro.2014.06.012
- Cotterill E, Hall D, Wallace K, et al. Characterization of early cortical neural network development in multi-well microelectrode array plates. SLAS Discov. 2016;21(5):510–519. doi: 10.1177/1087057116640520
- Mack CM, Lin BJ, Turner JD, et al. Burst and principal components analyses of MEA data for 16 chemicals describe at least three effects classes. Neurotoxicology. 2014;40:75–85. doi: 10.1016/j.neuro.2013.11.008
- Dingemans MML, Schütte MG, Wiersma DMM, et al. Chronic 14-day exposure to insecticides or methylmercury modulates neuronal activity in primary rat cortical cultures. Neurotoxicology. 2016;57:194–202. doi: 10.1016/j.neuro.2016.10.002
- Richardson JR, Fitsanakis V, Westerink RHS, et al. Neurotoxicity of pesticides. Acta Neuropathol. 2019;138(3):343–362. doi: 10.1007/s00401-019-02033-9
- Kasteel EEJ, Westerink RHS. Comparison of the acute inhibitory effects of tetrodotoxin (TTX) in rat and human neuronal networks for risk assessment purposes. Toxicol Lett. 2017;270:12–16. doi: 10.1016/j.toxlet.2017.02.014
- Tukker AM, Wijnolts FMJ, de Groot A, et al. Applicability of hiPSC-derived neuronal cocultures and rodent primary cortical cultures for in vitro seizure liability assessment. Toxicological Sci. 2020;178(1):71–87. doi: 10.1093/toxsci/kfaa136
- Zwartsen A, Hondebrink L, Westerink RH. Neurotoxicity screening of new psychoactive substances (NPS): effects on neuronal activity in rat cortical cultures using microelectrode arrays (MEA). Neurotoxicology. 2018;66:87–97. doi: 10.1016/j.neuro.2018.03.007
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (danio rerio). 4th ed. Eugene (OR): University of Oregon Press; 2000.
- Kanungo J, Cuevas E, Ali S, et al. Zebrafish model in drug safety assessment. CPD. 2014;20(34):5416–5429. doi: 10.2174/1381612820666140205145658
- Ablain J, Zon LI. Fish and men: using zebrafish to fight human diseases. Trends Cell Biol. 2013;23(12):584–586. doi: 10.1016/j.tcb.2013.09.009
- Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111
- Nawaji T, Yamashita N, Umeda H, et al. Cytochrome P450 expression and chemical metabolic activity before full liver development in zebrafish. Pharmaceuticals. 2020;13(12):456. doi: 10.3390/ph13120456
- d’Amora M, Giordani S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front Neurosci. 2018;12:976. doi: 10.3389/fnins.2018.00976
- Horzmann KA, Freeman JL. Making waves: new developments in toxicology with the zebrafish. Toxicological Sci. 2018;163(1):5–12. doi: 10.1093/toxsci/kfy044
- Kalueff AV, editor. The rights and wrongs of zebrafish: behavioral phenotyping of zebrafish [internet]. Cham: Springer International Publishing; 2017 [cited 2023 Oct 19]. doi: 10.1007/978-3-319-33774-6
- Reif DM, Truong L, Mandrell D, et al. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2016;90(6):1459–1470. doi: 10.1007/s00204-015-1554-1
- Kalueff AV, Gebhardt M, Stewart AM, et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 2013;10(1):70–86. doi: 10.1089/zeb.2012.0861
- MacPhail RC, Brooks J, Hunter DL, et al. Locomotion in larval zebrafish: influence of time of day, lighting and ethanol. Neurotoxicology. 2009;30(1):52–58. doi: 10.1016/j.neuro.2008.09.011
- López-Schier H. Neuroplasticity in the acoustic startle reflex in larval zebrafish. Curr Opin Neurobiol. 2019;54:134–139. doi: 10.1016/j.conb.2018.10.004
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007
- Zhang S, Li F, Zhou T, et al. Caenorhabditis elegans as a useful model for studying aging mutations. Front Endocrinol. 2020;11:554994. doi: 10.3389/fendo.2020.554994
- Leung MCK, Williams PL, Benedetto A, et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological Sci. 2008;106(1):5–28. doi: 10.1093/toxsci/kfn121
- Sammi SR, Jameson LE, Conrow KD, et al. Caenorhabditis elegans neurotoxicity testing: novel applications in the adverse outcome pathway framework. Front Toxicol. 2022;4:826488. doi: 10.3389/ftox.2022.826488
- Martins AC, Gubert P, Li J, et al. Caenorhabditis elegans as a model to study manganese-induced neurotoxicity. Biomolecules. 2022;12(10):1396. doi: 10.3390/biom12101396
- Hart AB. WormBook: the online review of C elegans biology [internet] [internet]. WormBook. 2006 [cited 2023 Oct 19]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK19734/
- Ruszkiewicz JA, Pinkas A, Miah MR, et al. C. elegans as a model in developmental neurotoxicology. Toxicol Appl Pharmacol. 2018;354:126–135. doi: 10.1016/j.taap.2018.03.016
- White JG, Southgate E, Thomson JN, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340.
- Barclay JW, Morgan A, Burgoyne RD. Neurotransmitter release mechanisms studied in Caenorhabditis elegans. Cell Calcium. 2012;52(3–4):289–295. doi: 10.1016/j.ceca.2012.03.005
- Brownlee DJA, Fairweather I. Exploring the neurotransmitter labyrinth in nematodes. Trends Neurosci. 1999;22(1):16–24. doi: 10.1016/S0166-2236(98)01281-8
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282(5396):2028–2033. doi: 10.1126/science.282.5396.2028
- Goodman MB, Hall DH, Avery L, et al. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20(4):763–772. doi: 10.1016/S0896-6273(00)81014-4
- Waidyanatha S, Collins BJ, Cristy T, et al. Advancing botanical safety: a strategy for selecting, sourcing, and characterizing botanicals for developing toxicological tools. Food Chem Toxicol. 2024;186:114537. doi: 10.1016/j.fct.2024.114537
- Li S, Yu L, Shi Q, et al. An insight into current advances on pharmacology, pharmacokinetics, toxicity and detoxification of aconitine. Biomed Pharmacother. 2022;151:113115. doi: 10.1016/j.biopha.2022.113115
- Gao X, Hu J, Zhang X, et al. Research progress of aconitine toxicity and forensic analysis of aconitine poisoning. Forensic Sci Res. 2020;5(1):25–31. doi: 10.1080/20961790.2018.1452346
- Pullela R, Young L, Gallagher B, et al. A case of fatal aconitine poisoning by monkshood ingestion. J Forensic Sci. 2008;53(2):491–494. doi: 10.1111/j.1556-4029.2007.00647.x
- Strzelecki A, Pichon N, Gaulier JM, et al. Acute toxic herbal intake in a suicide attempt and fatal refractory ventricular arrhythmia. Basic Clin Pharmacol Toxicol. 2010;107(2):698–699. doi: 10.1111/j.1742-7843.2010.00566.x
- Feldkamp A, Köster B, Weber HP. [Fatal poisoning caused by aconite monk’s hood (Aconitum napellus)]. Monatsschr Kinderheilkd. 1991;139(6):366–367.
- Povšnar M, Koželj G, Kreft S, et al. Rare tradition of the folk medicinal use of Aconitum spp. is kept alive in Solčavsko, Slovenia. J Ethnobiol Ethnomed. 2017;13(1):45. doi: 10.1186/s13002-017-0171-x
- Zhao X, Ni S, Liang N, et al. Clinical application of Aconitum carmichaelii Debx. (Fu Zi in Chinese) by traditional Chinese medicine physicians--A cross-sectional questionnaire survey in Beijing. J Tradit Chin Med Sci. 2021;8(4):302–308. doi: 10.1016/j.jtcms.2021.10.008
- Sun W, Yan B, Wang R, et al. In vivo acute toxicity of detoxified Fuzi (lateral root of Aconitum carmichaeli) after a traditional detoxification process. EXCLI J. 2018 [cited 2024 Feb 7];17:Doc889. [Internet]. Available from: https://www.excli.de/vol17/Huang_31082018_proof.pdf
- Chung JY, Lee SJ, Lee HJ, et al. Aconitine neurotoxicity according to administration methods. JCM. 2021;10(10):2149. doi: 10.3390/jcm10102149
- Lin C-C, Chan TYK, Deng J-F. Clinical features and management of herb-induced aconitine poisoning. Ann Emerg Med. 2004;43(5):574–579. doi: 10.1016/j.annemergmed.2003.10.046
- Li T-F, Fan H, Wang Y-X. Aconitum-derived bulleyaconitine a exhibits antihypersensitivity through direct stimulating dynorphin a expression in spinal microglia. The J Pain. 2016;17(5):530–548. doi: 10.1016/j.jpain.2015.12.015
- Wang S. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15(2):151–159. doi: 10.1016/S0898-6568(02)00085-2
- Chan TYK. Aconite poisoning. Clin Toxicol. 2009;47(4):279–285. doi: 10.1080/15563650902904407
- Nyirimigabo E, Xu Y, Li Y, et al. A review on phytochemistry, pharmacology and toxicology studies of Aconitum. J Pharm and Pharmacol. 2014;67(1):1–19. doi: 10.1111/jphp.12310
- Chen H, Wang F, Ni X, et al. Aconitine disrupts serotonin neurotransmission via 5‐hydroxytryptamine receptor in zebrafish embryo. J Appl Toxicol. 2021;41(3):483–492. doi: 10.1002/jat.4059
- Zhou J, Peng C, Li Q, et al. Dopamine homeostasis imbalance and dopamine receptors-mediated AC/cAMP/PKA pathway activation are involved in aconitine-induced neurological impairment in zebrafish and SH-SY5Y cells. Front Pharmacol. 2022;13:837810. doi: 10.3389/fphar.2022.837810
- Zhang Y, Chen S, Fan F, et al. Neurotoxicity mechanism of aconitine in HT22 cells studied by microfluidic chip-mass spectrometry. J Pharm Anal. 2023;13(1):88–98. doi: 10.1016/j.jpha.2022.11.007
- Ameri A. The effects of aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998;56(2):211–235. doi: 10.1016/S0301-0082(98)00037-9
- Debelle FD, Vanherweghem J-L, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74(2):158–169. doi: 10.1038/ki.2008.129
- Grady D. Chinese herb is suspected in cancer. The New York Times [Internet]. 2000 Jun 8 [cited 2023 Oct 19]. Available from: https://www.nytimes.com/2000/06/08/us/chinese-herb-is-suspected-in-cancer.html
- Shang X, You C, Li X, et al. Involvement of 5-HT2 serotonin receptors in cognitive defects induced by aristolochic acid I in mice. Toxicology. 2021;447:152624. doi: 10.1016/j.tox.2020.152624
- Chen J, Kong A, Shelton D, et al. Early life stage transient aristolochic acid exposure induces behavioral hyperactivity but not nephrotoxicity in larval zebrafish. Aquat Toxicol. 2021;238:105916. doi: 10.1016/j.aquatox.2021.105916
- Speers AB, Cabey KA, Soumyanath A, et al. Effects of Withania somnifera (Ashwagandha) on stress and the stress- related neuropsychiatric disorders anxiety, depression, and insomnia. CN. 2021;19(9):1468–1495. doi: 10.2174/1570159X19666210712151556
- Lubarska M, Hałasiński P, Hryhorowicz S, et al. Liver dangers of herbal products: a case report of Ashwagandha-induced liver injury. Int J Environ Res Public Health. 2023;20(5):3921. doi: 10.3390/ijerph20053921
- Siddiqui S, Ahmed N, Goswami M, et al. DNA damage by Withanone as a potential cause of liver toxicity observed for herbal products of Withania somnifera (Ashwagandha). Curr Res Toxicol. 2021;2:72–81. doi: 10.1016/j.crtox.2021.02.002
- Durg S, Dhadde SB, Vandal R, et al. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: a systematic review and meta-analysis. J Pharm and Pharmacol. 2015;67(7):879–899. doi: 10.1111/jphp.12398
- Zahiruddin S, Basist P, Parveen A, et al. Ashwagandha in brain disorders: A review of recent developments. J Ethnopharmacol. 2020;257:112876. doi: 10.1016/j.jep.2020.112876
- Asian Ginseng. U.S. National Center for Complementary and Integrative Health. [Internet]. [cited 2023 Oct 19]. Available from: https://www.nccih.nih.gov/health/asian-ginseng
- Jin T-Y, Rong P-Q, Liang H-Y, et al. Clinical and preclinical systematic review of Panax ginseng C. A. Mey and its compounds for fatigue. Front Pharmacol. 2020;11:1031. doi: 10.3389/fphar.2020.01031
- Zheng Q-L, Zhu H-Y, Xu X, et al. Korean red ginseng alleviate depressive disorder by improving astrocyte gap junction function. J Ethnopharmacol. 2021;281:114466. doi: 10.1016/j.jep.2021.114466
- Petkov VD, Belcheva S, Petkov VV. Behavioral effects of Ginkgo biloba L. Panax ginseng C.A. Mey. and Gincosan®. Am J Chin Med. 2003;31(6):841–855. doi: 10.1142/S0192415X03001533
- National Toxicology Program. Toxicology and carcinogenesis studies of ginseng (CAS No. 50647-08-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2011:1–149.
- Kuo Y-H, Ikegami F, Lambein F. Neuroactive and other free amino acids in seed and young plants of panax ginseng. Phytochemistry. 2003;62(7):1087–1091. doi: 10.1016/S0031-9422(02)00658-1
- Long Y, Ye Y, Xing Q. Studies on the neuroexcitotoxin β‐ N ‐oxalo‐L‐α, β‐diaminopropionic acid and its isomer α‐ N ‐oxalo‐L‐α, βdiaminopropionic acid from the root of Panax species. Int J Peptide and Protein Res. 1996;47(1–2):42–46. doi: 10.1111/j.1399-3011.1996.tb00808.x
- National Toxicology Program (NTP). Botanical Safety Consortium – Chemical Analysis [Internet]. 2023 [cited 2024 Feb 7]. p. 500–007–001–000–003. Available from: https://cebs.niehs.nih.gov/cebs/paper/15717
- Datta S, Mahdi F, Ali Z, et al. Toxins in botanical dietary supplements: blue cohosh components disrupt cellular respiration and mitochondrial membrane potential. J Nat Prod. 2014;77(1):111–117. doi: 10.1021/np400758t
- Schep LJ, Slaughter RJ, Beasley DMG. Nicotinic plant poisoning. Clin Toxicol. 2009;47(8):771–781. doi: 10.1080/15563650903252186
- Slater Y. Halogenated cytisine derivatives as agonists at human neuronal nicotinic acetylcholine receptor subtypes. Neuropharmacology. 2003;44(4):503–515. doi: 10.1016/S0028-3908(03)00025-X
- Green BT, Lee ST, Panter KE, et al. Actions of piperidine alkaloid teratogens at fetal nicotinic acetylcholine receptors. Neurotoxicol Teratol. 2010;32(3):383–390. doi: 10.1016/j.ntt.2010.01.011
- Wei X, Ruan W, Vrieling K. Current knowledge and perspectives of pyrrolizidine alkaloids in pharmacological applications: a mini-review. Molecules. 2021;26(7):1970. doi: 10.3390/molecules26071970
- Schramm S, Köhler N, Rozhon W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules. 2019;24:498. doi: 10.3390/molecules24030498
- Verma V, Singh N, Jaggi AS. Sodium-hydrogen exchanger inhibitory potential of Malus domestica , Musa × paradisiaca , Daucus carota , and Symphytum officinale. J Basic Clin Physiol Pharmacol [Internet]. 2014 [cited 2023 Oct 19];25(1):99–108. Available from: https://www.degruyter.com/document/doi/10.1515/jbcpp-2013-0088/html
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83(4):1183–1221. doi: 10.1152/physrev.00010.2003
- Hwang S-M, Koo N-Y, Jin M, et al. Intracellular acidification is associated with changes in free cytosolic calcium and inhibition of action potentials in rat trigeminal ganglion. J Biol Chem. 2011;286(3):1719–1729. doi: 10.1074/jbc.M109.090951
- Pavlov I, Kaila K, Kullmann DM, et al. Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions?: cortical inhibition, pH and cell excitability in epilepsy. J Physiol. 2013;591(4):765–774. doi: 10.1113/jphysiol.2012.237958
- Uria-Avellanal C, Robertson NJ. Na+/H+ exchangers and intracellular ph in perinatal brain injury. Transl Stroke Res. 2014;5(1):79–98. doi: 10.1007/s12975-013-0322-x
- LiverTox: Clinical and research Information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012 [cited 2023 Oct 19]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK547852/
- Ephedra. NCCIH. [Internet]. [cited 2023 Oct 19]. Available from: https://www.nccih.nih.gov/health/ephedra
- Food, Drug Administration HHS. Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk. Final rule Fed Regist. 2004;69:6787–6854.
- Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289(12):1537–1545. doi: 10.1001/jama.289.12.1537
- Office of Dietary Supplements - Ephedra and Ephedrine Alkaloids for Weight Loss and Athletic Performance [Internet]. [cited 2023 Nov 30]. Available from: https://ods.od.nih.gov/factsheets/EphedraandEphedrine-HealthProfessional/
- Moawad FJ, Hartzell JD, Biega TJ, et al. Transient blindness due to posterior reversible encephalopathy syndrome following ephedra overdose. South Med J. 2006;99(5):511–514. doi: 10.1097/01.smj.0000215739.90211.3b
- Vukovich MD, Schoorman R, Heilman C, et al. Caffeine–herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clin Exp Pharmacol Physiol. 2005;32(1–2):47–53. doi: 10.1111/j.1440-1681.2005.04152.x
- Zheng F, Wei P, Huo H, et al. Neuroprotective effect of gui zhi (Ramulus Cinnamomi) on ma huang- (Herb Ephedra-) Induced toxicity in rats treated with a ma huang-gui zhi herb pair. Evidence-Based Complementary and Alternative Med. 2015;2015:1–9. doi: 10.1155/2015/913461
- Niu B, Zheng F, Xu J. Protective effect of gui zhi (Ramulus Cinnamomi) on abnormal levels of four amino acid neurotransmitters by chronically ma huang (Herb Ephedra) intoxicated prefrontal cortex in rats treated with a ma huang-gui zhi herb pair. J Ethnopharmacol. 2020;249:112408. doi: 10.1016/j.jep.2019.112408
- Dawson P, Moffatt JD. Cardiovascular toxicity of novel psychoactive drugs: Lessons from the past. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39(2):244–252. doi: 10.1016/j.pnpbp.2012.05.003
- Bowyer JF. An evaluation of l-ephedrine neurotoxicity with respect to hyperthermia and caudate/putamen microdialysate levels of ephedrine, dopamine, serotonin, and glutamate. Toxicological Sci. 2000;55(1):133–142. doi: 10.1093/toxsci/55.1.133
- Calvert R, Vohra S, Ferguson M, et al. A beating heart cell model to predict cardiotoxicity: Effects of the dietary supplement ingredients higenamine, phenylethylamine, ephedrine and caffeine. Food Chem Toxicol. 2015;78:207–213. doi: 10.1016/j.fct.2015.01.022
- Zuo S, Li W, Li Q, et al. Protective effects of Ephedra sinica extract on blood–brain barrier integrity and neurological function correlate with complement C3 reduction after subarachnoid hemorrhage in rats. Neurosci Lett. 2015;609:216–222. doi: 10.1016/j.neulet.2015.10.056
- Green Tea. U.S. National Center for Complementary and Integrative Health. [Internet]. [cited 2023 Oct 20]. Available from: https://www.nccih.nih.gov/health/green-tea
- Prasanth M, Sivamaruthi B, Chaiyasut C, et al. A review of the role of green tea (camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients. 2019;11(2):474. doi: 10.3390/nu11020474
- National Toxicology Program. Toxicology studies of green tea extract in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of green tea extract in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2016:1–238.
- Oketch-Rabah HA, Roe AL, Rider CV, et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep. 2020;7:386–402. doi: 10.1016/j.toxrep.2020.02.008
- Lovera J, Ramos A, Devier D, et al. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: phase I single group and phase II randomized placebo-controlled studies. J Neurol Sci. 2015;358(1–2):46–52. doi: 10.1016/j.jns.2015.08.006
- National Toxicology Program. Toxicology and carcinogenesis studies of goldenseal root powder (Hydrastis canadensis) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser. 2010:1–188.
- Mandal SK, Maji AK, Mishra SK, et al. Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues. Pharmacol Res. 2020;160:105085. doi: 10.1016/j.phrs.2020.105085
- Yin SY, Lee JJ, Kim YM, et al. Effects of (1R,9S)-β-hydrastine on l-DOPA-induced cytotoxicity in PC12 cells. Eur J Pharmacol. 2004;488(1–3):71–77. doi: 10.1016/j.ejphar.2004.02.021
- Kim SH, Shin JS, Lee JJ, et al. Effects of hydrastine derivatives on dopamine biosynthesis in PC12 cells. Planta Med. 2001;67(7):609–613. doi: 10.1055/s-2001-17356
- Kysenius K, Brunello CA, Huttunen HJ, et al. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. Tang S-J, editor. PLOS ONE. 2014;9(9):e107129. doi: 10.1371/journal.pone.0107129
- Senol Deniz FS, Ekhteiari Salmas R, Emerce E, et al. Cholinesterase inhibitory and in silico toxicity assessment of thirty-four isoquinoline alkaloids - berberine as the lead compound. CNS Neurol Disord Drug Targets. 2023;23(6):773–783. doi: 10.2174/1871527322666230417083053
- Kulkarni SK, Dhir A. Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24(3):317–324. doi: 10.1002/ptr.2968
- Zhang R, Lei B, Wu G, et al. Protective effects of berberine against β-amyloid-induced neurotoxicity in HT22 cells via the Nrf2/HO‐1 pathway. Bioorg Chem. 2023;133:106210. doi: 10.1016/j.bioorg.2022.106210
- Goldenseal. Drugs and Lactation Database (LactMed®). [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006 [cited 2023 Oct 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK501866/
- Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin Toxicol (Phila). 2008;46:146–152. doi: 10.1080/15563650701241795
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi: 10.1016/j.drugalcdep.2020.107849
- Office of the Commissioner. FDA and Kratom [Internet]. FDA. 2023 [cited 2023 Nov 29]. Available from: https://www.fda.gov/news-events/public-health-focus/fda-and-kratom
- Anand A, Hosanagar A. The addictive potential and challenges with use of the “herbal supplement” kratom: a case report and literature review. Pain Med. 2022;23(1):4–9. doi: 10.1093/pm/pnab126
- Hossain R, Sultana A, Nuinoon M, et al. A critical review of the neuropharmacological effects of kratom: an insight from the functional array of identified natural compounds. Molecules. 2023;28(21):7372. doi: 10.3390/molecules28217372
- Hassan Z, Singh D, Suhaimi FW, et al. Evaluation of toxicity profile of kratom (Mitragyna speciosa Korth) decoction in rats. Regul Toxicol Pharmacol. 2023;143:105466. doi: 10.1016/j.yrtph.2023.105466
- Suhaimi FW, Zul Aznal AN, Mohamad nor Hazalin NA, et al. Kratom (M. speciosa) exposure during adolescence caused long-lasting cognitive behavioural deficits associated with perturbated brain metabolism pathways in adult rats. Behav Brain Res. 2023;446:114411. doi: 10.1016/j.bbr.2023.114411
- Matsumoto K, Horie S, Takayama H, et al. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005;78(1):2–7. doi: 10.1016/j.lfs.2004.10.086
- Basheer M, Hassan Z, Gam L-H. Upregulation of brain’s calcium binding proteins in mitragynine dependence: a potential cellular mechanism to addiction. Int J Med Sci. 2023;20(1):102–113. doi: 10.7150/ijms.78861
- Suhaimi FW, Yusoff NHM, Hassan R, et al. Neurobiology of Kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126:29–40. doi: 10.1016/j.brainresbull.2016.03.015
- Karunakaran T, Ngew KZ, Zailan AAD, et al. The chemical and pharmacological properties of mitragynine and its diastereomers: An insight review. Front Pharmacol. 2022;13:805986. doi: 10.3389/fphar.2022.805986
- Viwatpinyo K, Mukda S, Warinhomhoun S. Effects of mitragynine on viability, proliferation, and migration of C6 rat glioma, SH-SY5Y human neuroblastoma, and HT22 immortalized mouse hippocampal neuron cell lines. Biomed Pharmacother. 2023;166:115364. doi: 10.1016/j.biopha.2023.115364
- Effendy MA, Yunusa S, Mat NH, et al. The role of AMPA and NMDA receptors in mitragynine effects on hippocampal synaptic plasticity. Behav Brain Res. 2023;438:114169. doi: 10.1016/j.bbr.2022.114169
- Moragrega I, Ríos JL. Medicinal plants in the treatment of depression. II: evidence from clinical trials. Planta Med. 2022;88(12):1092–1110. doi: 10.1055/a-1517-6882
- National Toxicology Program. Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser. 2012:1–186.
- Wang Y, Su C, Zhang B, et al. Biological activity, hepatotoxicity, and structure-activity relationship of kavalactones and flavokavins, the two main bioactive components in Kava (Piper methysticum). Evid Based Complement Alternat Med. 2021;2021:1–14. doi: 10.1155/2021/6851798
- Romm AJ, Kava K. Botanical medicine for women’s health. St Louis (Mo): Churchill Livingstone/Elsevier; 2010. p. 539–541.
- Kautu BB, Phillips J, Steele K, et al. A behavioral survey of the effects of kavalactones on Caenorhabditis elegans neuromuscular transmission. J Exp Neurosci. 2017;11:117906951770538. doi: 10.1177/1179069517705384
- Krum BN, de Freitas CM, Busanello A, et al. Ex vivo and in vitro inhibitory potential of kava extract on monoamine oxidase B activity in mice. J Tradit Complement Med. 2022;12(2):115–122. doi: 10.1016/j.jtcme.2021.07.002
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Oct 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK539848/
- Konradi C, Riederer P, Jellinger K, et al. Cellular action of MAO inhibitors. J Neural Transm Suppl. 1987;25:15–25.
- Riederer P, Laux G. MAO-inhibitors in Parkinson’s disease. Exp Neurobiol. 2011;20(1):1–17. doi: 10.5607/en.2011.20.1.1
- Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg & Psychiatry. 1995;58(5):639–640. doi: 10.1136/jnnp.58.5.639
- Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules. 2017;22(11):1942. doi: 10.3390/molecules22111942
- Blumenthal M, Busse WR; Bundesinstitut für Arzneimittel und Medizinprodukte (Germany), editors. The complete German Commission E monographs. Therapeutic guide to herbal medicines. Austin (TX): boston: American Botanical Council; Integrative Medicine Communications; 1998.
- National Toxicology Program. Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser. 2011:1–177.
- Lu C-W, Lin T-Y, Chiu K-M, et al. Silymarin inhibits glutamate release and prevents against kainic acid-induced excitotoxic injury in rats. Biomedicines. 2020;8(11):486. doi: 10.3390/biomedicines8110486
- Xie Z, Ding S, Shen Y. Silibinin activates AMP-activated protein kinase to protect neuronal cells from oxygen and glucose deprivation-re-oxygenation. Biochem Biophys Res Commun. 2014;454(2):313–319. doi: 10.1016/j.bbrc.2014.10.080
- Duan S, Guan X, Lin R, et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: a dual-target drug for the treatment of Alzheimer’s disease. Neurobiol Aging. 2015;36(5):1792–1807. doi: 10.1016/j.neurobiolaging.2015.02.002
- Fanoudi S, Alavi MS, Karimi G, et al. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem Toxicol. 2020;43(3):240–254. doi: 10.1080/01480545.2018.1485687
- Haddadi R, Shahidi Z, Eyvari-Brooshghalan S. Silymarin and neurodegenerative diseases: Therapeutic potential and basic molecular mechanisms. Phytomedicine. 2020;79:153320. doi: 10.1016/j.phymed.2020.153320
- Devi KP, Malar DS, Braidy N, et al. A mini review on the chemistry and neuroprotective effects of silymarin. Curr Drug Targets. 2017;18(13):1529–1536. doi: 10.2174/1389450117666161227125121
- Nerium oleander L.(PIM 366) [Internet]. [cited 2023 Oct 20]. Available from: https://inchem.org/documents/pims/plant/pim366.htm#SectionTitle:1.3%20%20Common%20name(s)%20and%20synonyms
- Zhai J, Dong X, Yan F, et al. Oleandrin: A systematic review of its natural sources, structural properties, detection methods, pharmacokinetics and toxicology. Front Pharmacol. 2022;13:822726. doi: 10.3389/fphar.2022.822726
- Azzalini E, Bernini M, Vezzoli S, et al. A fatal case of self-poisoning through the ingestion of oleander leaves. J Forensic Leg Med. 2019;65:133–136. doi: 10.1016/j.jflm.2019.05.016
- Pietsch J, Oertel R, Trautmann S, et al. A non-fatal oleander poisoning. Int J Legal Med. 2005;119(4):236–240. doi: 10.1007/s00414-005-0548-6
- Carfora A, Petrella R, Borriello R, et al. Fatal poisoning by ingestion of a self-prepared oleander leaf infusion. Forensic Sci Med Pathol. 2021;17(1):120–125. doi: 10.1007/s12024-020-00338-w
- Bavunoglu I, Balta M, Turkmen Z. Oleander poisoning as an example of self-medication attempt. Balkan Med J. 2016;33(5):559–562. doi: 10.5152/balkanmedj.2016.150307
- Farkhondeh T, Kianmehr M, Kazemi T, et al. Toxicity effects of Nerium oleander, basic and clinical evidence: a comprehensive review. Hum Exp Toxicol. 2020;39(6):773–784. doi: 10.1177/0960327120901571
- Moseley AE, Williams MT, Schaefer TL, et al. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27(3):616–626. doi: 10.1523/JNEUROSCI.4464-06.2007
- Botelho AFM, Pierezan F, Soto-Blanco B, et al. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon. 2019;158:63–68. doi: 10.1016/j.toxicon.2018.11.429
- van Kanegan MJ, He DN, Dunn DE, et al. BDNF mediates neuroprotection against oxygen-glucose deprivation by the cardiac glycoside oleandrin. J Neurosci. 2014;34(3):963–968. doi: 10.1523/JNEUROSCI.2700-13.2014
- Garofalo S, Grimaldi A, Chece G, et al. The glycoside oleandrin reduces glioma growth with direct and indirect effects on tumor cells. J Neurosci. 2017;37(14):3926–3939. doi: 10.1523/JNEUROSCI.2296-16.2017
- Serafini S, de Freitas Souza C, Baldissera MD, et al. Fish exposed to water contaminated with eprinomectin show inhibition of the activities of AChE and Na+/K±ATPase in the brain, and changes in natural behavior. Chemosphere. 2019;223:124–130. doi: 10.1016/j.chemosphere.2019.02.026
- Khordadmehr M, Nazifi S. Study of troponin, creatine kinase biomarkers, and histopathological lesions in experimental Nerium oleander toxicity in rats and mice. J Vet Res. 2018;62(1):97–102. doi: 10.2478/jvetres-2018-0013
- Dinesh P, Rasool M. Herbal formulations and their bioactive components as dietary supplements for treating rheumatoid arthritis. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases [Internet]; Elsevier. 2019 [cited 2023 Oct 20]. p. 385–399. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128138205000222
- Liu R, Li X, Huang N, et al. Toxicity of traditional Chinese medicine herbal and mineral products. Advances in Pharmacology [Internet]. Elsevier; 2020 [cited 2023 Oct 20]. p. 301–346. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054358919300638
- Ru Y, Luo Y, Zhou Y, et al. Adverse events associated with treatment of Tripterygium wilfordii Hook F: A quantitative evidence synthesis. Front Pharmacol. 2019;10:1250. doi: 10.3389/fphar.2019.01250
- Chou W-C, Wu C-C, Yang P-C, et al. Hypovolemic shock and mortality after ingestion of tripterygium wilfordii hook F.: a case report. Int J Cardiol. 1995;49(2):173–177. doi: 10.1016/0167-5273(95)02282-2
- Wu B-K, Yuan R-Y, Chang Y-P, et al. Epicatechin isolated from tripterygium wilfordii extract reduces tau-GFP-induced neurotoxicity in zebrafish embryo through the activation of Nrf2. Biochem Biophys Res Commun. 2016;477(2):283–289. doi: 10.1016/j.bbrc.2016.06.058
- Xu Y, Li W, Wen R, et al. Voltage-gated sodium channels, potential targets of Tripterygium wilfordii Hook. f. to exert activity and produce toxicity. J Ethnopharmacol. 2023;311:116448. doi: 10.1016/j.jep.2023.116448
- National Toxicology Program. Toxicity studies of Usnea lichens containing (±) Usnic acid (CASRN 125-46-2) administered in feed to F344/N NCTR Rats and B6C3F1/NCTR Mice. Natl Toxicol Program Tech Rep Ser. 2022:1–143.
- Crawford SD. Lichens used in Traditional Medicine. In: Ranković B, editor. Lichen Secondary Metabolites [Internet]. Cham: Springer International Publishing; 2015 [cited 2023 Oct 20]. p. 27–80. Available from: https://doi.org/10.1007/978-3-319-13374-4_2
- Kwong SP, Wang C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ Toxicol Pharmacol. 2020;80:103493. doi: 10.1016/j.etap.2020.103493
- Guo L, Shi Q, Fang J-L, et al. Review of usnic acid and Usnea Barbata Toxicity. J Environ Sci Health Part C. 2008;26(4):317–338. doi: 10.1080/10590500802533392
- Cazarin CA, Dalmagro AP, Gonçalves AE, et al. Usnic acid enantiomers restore cognitive deficits and neurochemical alterations induced by Aβ1–42 in mice. Behav Brain Res. 2021;397:112945. doi: 10.1016/j.bbr.2020.112945
- Erfani S, Valadbeigi T, Aboutaleb N, et al. Usnic acid improves memory impairment after cerebral ischemia/reperfusion injuries by anti-neuroinflammatory, anti-oxidant, and anti-apoptotic properties. Iran J Basic Med Sci [Internet]. 2020 [cited 2023 Oct 20];23. doi: 10.22038/ijbms.2020.43280.10165
- Lee S, Lee Y, Ha S, et al. Anti-inflammatory effects of usnic acid in an MPTP-induced mouse model of Parkinson’s disease. Brain Res. 2020;1730:146642. doi: 10.1016/j.brainres.2019.146642
- Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front Mol Neurosci. 2018;11:216. doi: 10.3389/fnmol.2018.00216
- Rabelo TK, Zeidán-Chuliá F, Vasques LM, et al. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol Vitro. 2012;26(2):304–314. doi: 10.1016/j.tiv.2011.12.003
- Moreira CT, Oliveira AL, Comar JF, et al. Harmful effects of usnic acid on hepatic metabolism. Chem Biol Interact. 2013;203(2):502–511. doi: 10.1016/j.cbi.2013.02.001
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the evaluation of the safety in use of Yohimbe (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille). EFS2 [Internet]. 2013 [cited 2023 Oct 19]. Available from: https://data.europa.eu/doi/10.2903/j.efsa.2013.3302
- Lo Faro AF, di Trana A, La Maida N, et al. Biomedical analysis of New Psychoactive Substances (NPS) of natural origin. J Pharm Biomed Anal. 2020;179:112945. doi: 10.1016/j.jpba.2019.112945
- Clark JT, Smith ER, Davidson JM. Enhancement of sexual motivation in male rats by yohimbine. Science. 1984;225(4664):847–849. doi: 10.1126/science.6474156
- Grunewald KK, Bailey RS. Commercially marketed supplements for bodybuilding athletes. Sports Med. 1993;15(2):90–103. doi: 10.2165/00007256-199315020-00003
- Anderson C, Anderson D, Harre N, et al. Case study: two fatal case reports of acute yohimbine intoxication. J Anal Toxicol. 2013;37(8):611–614. doi: 10.1093/jat/bkt057
- Scatton B, Zivkovic B, Dedek J. Antidopaminergic properties of yohimbine. J Pharmacol Exp Ther. 1980;215(2):494–499.
- Watanabe K, Yano S, Horiuchi H, et al. Ca2+ channel-blocking effect of the yohimbine derivatives, 14β-benzoyloxyyohimbine and 14β- p -nitrobenzoyloxyyohimbine. J Pharm and Pharmacol. 2011;39(6):439–443. doi: 10.1111/j.2042-7158.1987.tb03416.x
- Kleef Rgdm V, Embry MR, Mitchell CA, et al. Neuroactivity screening of botanical extracts using microelectrode array (MEA) recordings. Food Chem Toxicol. 2024;184:114438. doi: 10.1016/j.fct.2024.114438
