Abstract
Resistance towards herbivory is expected to influence the competitive ability and ecological success of the resistant plant, but it is unclear how this general knowledge should be incorporated into long-term ecological predictions of plant community dynamics. In order to answer such questions, the long-term ecological effects of density, competition, herbivory and their compound interactions were investigated in a model system of a transgenic herbivore-resistant Arabidopsis thaliana genotype and the isogenic herbivore-sensitive A. thaliana genotype. It was concluded that herbivory had a significant effect on the fecundity of the susceptible genotype at high plant density. The most likely long-term scenario was that the susceptible genotype outcompeted the resistant genotype. But it was also shown that herbivory could down-regulate the equilibrium density of the susceptible genotype and, when the two genotypes were coexisting, up-regulate the equilibrium density of the resistant genotype.
Introduction
Competition and herbivory are two fundamental forces determining the growth, reproduction and survival of plants and they are both believed to have the potential for structuring plant communities (e.g. Crawley Citation1989, Gurevitch et al. Citation2000). Field observations suggest that the effects of competition and herbivory might not be independent of each other; i.e., the effects of a given level of herbivory are expected to depend on the competitive environment of the plant experiencing herbivory (Fowler & Rausher Citation1985, Gurevitch et al. Citation2000; Hambäck & Beckerman Citation2003). It has frequently been asserted that invertebrate herbivores have a larger effect on their host plants when the plants simultaneously are subjected to other forms of stress such as competition or disturbance (Cottam et al. Citation1986, Crawley Citation1989, Rodríguez & Brown Citation1998).
The possible interaction between competition and herbivory may arise due to size-asymmetric competition for limiting resources, e.g. light, among the plants (e.g. Weis & Hochberg Citation2000). At low plant densities individual plants might recover quickly from herbivory, but when growing together in dense mixtures the plants subjected to herbivory would lose their position in the height hierarchy and be quickly overtopped and shaded. Lee and Bazzaz (Citation1980) investigated the effect of defoliation and competition for light on seed and biomass production in velvetleaf, Abutilon theophrasti. In their low-density treatment where each plant was fully exposed to light, even 75% defoliation had no significant effect on reproduction. At high density, however, 75% defoliation reduced fecundity by almost half. They concluded that this supports the hypothesis that defoliation has a greater impact on fecundity when plants are competing for light than when light is no limiting factor.
Additionally, resistance against herbivory has been suggested to be associated with a general cost of resistance (Bergelson & Purrington Citation1996). Theoretically, such a cost of resistance should be more severe in stressful environments and hence cost of plant defence against herbivory should increase when competition is intense (Bergelson & Purrington Citation1996). Cost of herbicide resistance has previously been demonstrated in Arabidopsis thaliana (Mauricio Citation1998, Purrington & Bergelson Citation1997, Purrington & Bergelson Citation1999).
In most studies on the ecological interaction between competition and herbivory, the studied effects are confounded with an unknown genetic variation among the studied plant populations or species. The objective of the present study was to investigate the effect of herbivory on competitive interactions in a model system of a transgenic herbivore-resistant A. thaliana genotype and the near isogenic herbivore-sensitive A. thaliana genotype (Kristensen et al. Citation2005). The transgenic genotype had three genes from Sorghum bicolor inserted that express the biosynthesis of the cyanogenic glucoside dhurrin (Tattersall et al. Citation2001). Cyanogenic glucosides are a group of amino acid-derived metabolites that are widely distributed in the plant kingdom, which confer protection against some, but not all, herbivores. The transgenic A. thaliana plants accumulate dhurrin over time and contain approximately 4 mg g-1 fresh weight in the leaves after four weeks (David Tattersall, personal communication). The presence of dhurrin in the transgenic A. thaliana plants confers resistance to the flea beetle Phyllotreta nemorum, which feed on cruciferous plants (Tattersall et al. Citation2001). The transgenic A. thaliana plants have been observed to germinate later and grow slower than the isogenic plants (David Tattersall, personal communication).
A second objective was to predict the long-term ecological dynamics of the two genotypes as a response to the level of herbivory by modelling the observed competitive interaction. It is expected that the study of the interaction of competition and herbivory in model systems, such as the present, will deliver important insight into the methodology of making quantitative ecological predictions of the influence of herbivory on plant community changes. Such quantitative ecological predictions are needed in applied ecology, e.g. in the risk assessment of genetically modified plants (Damgaard Citation2002, Damgaard & L⊘kke Citation2001), and more generally to advance the scientific field of plant ecology (Cousens Citation2001, Keddy Citation1990). Unfortunately, the complexities of ecological systems are daunting and the notion of making ecological predictions may seem reckless. Just to mention a few of the obstacles: The competitive ability may vary with the physical environment, e.g., temperature, nutrition and water availability (Cahill Jr et al. Citation2005, Clauss & Aarssen Citation1994), species may perform differently in competition with other plant species (Abrams Citation1996, Rees et al. Citation1996) etc, nevertheless, when confronted with a complex system, it is common scientific methodology to reduce the complexity of the system by ignoring some processes to obtain manageable information.
Furthermore, the present study was also motivated by the expressed intention of using the transgenic herbivore-resistant A. thaliana genotype for educational purposes in Danish schools. In this connection, the transgenic A. thaliana genotype went through an environmental risk assessment where it was concluded that there was too little knowledge on the possible selective advantage of the transgene to allow the use of the transgene genotype for educational purposes.
Our prior expectation was that without herbivores the isogenic genotype would be a superior competitor since it did not produce dhurrin, and as the level of herbivory increased, the competitive ability of the transgenic genotype would increase due to herbivore resistance. Furthermore, we expected that the most likely long-term ecological scenario would be a function of the level of herbivory. That is, without herbivores the susceptible genotype will outcompete the resistant genotype, at some intermediary level of herbivory the two genotypes will coexist, and at even higher levels of herbivory the resistant genotype will outcompete the susceptible genotype.
Materials and methods
Plants
Seeds of the transgenic dhurrin producing A. thaliana genotype (3x.8.2.2.1 derived from Col-0) and the nearly isogenic genotype Col-0 (Kristensen et al. Citation2005) were kindly provided by the Department of Plant Biology, Royal Veterinary & Agricultural University, Denmark. The line 3x8.2.2.1 contains the CYP79A1, CYP71E1 and sbHMNGT genes from Sorghum bicolor. The construction procedure of the transgenic A. thaliana has been described in Tattersall et al. (Citation2001).
A. thaliana is almost completely self-fertilizing (Abbott & Gomes Citation1989), which means that there is limited genetic exchange between A. thaliana genotypes on an ecological time scale (Miyashita et al. Citation1999). Thus, since there is no sexual transmission between individual plants, the ecological success of different genotypes may be modelled as if the genotypes were separate species. Furthermore, the number of seeds produced has been shown to be a good measure of fitness (Clauss & Aarssen Citation1994) and has been found to correlate well with vegetative biomass (Clauss & Aarssen Citation1994, Damgaard & Jensen Citation2002, Aarssen & Clauss Citation1992).
The plants were grown in a greenhouse with 12-h photoperiods at an average temperature of 20°C (minimum: 14°C, maximum: 37°C), and a relative humidity of 64% (minimum: 48%, maximum: 83%). The soil used was a complete fertilized compost mixture (SM-gr⊘n, Stenr⊘gel Mosebrug a/s). The plants were watered from the bottom with fertilized water (14:3:25/NPK, 100g L−1; ion, 10ml L−1; phosphoric acid, 25ml L−1).
Insects
The flea beetle Phyllotreta nemorum L. (Coleoptera: Chrysomelidae: Alticinae), which generally feeds on plant species belonging to the Brassicaceae and Resedaceae, was used as the herbivore because it is known to be susceptible to dhurrin (Tattersall et al. Citation2001). To our knowledge, P. nemorum has not been reported feeding on A. thaliana in nature (Nielsen et al. Citation2001), but in the laboratory the flea beetle accepts leaves of A. thaliana as a food source (Nielsen et al. Citation2001), and in North America other species of flea beetles (Psylliodes convexior and Phyllotreta zimmermani) have been observed to be the most common herbivores on A. thaliana (Mauricio Citation1998).
The over-wintering beetles emerge and feed for some days before they lay their eggs in the soil around the host plants. The larvae of P. nemorum climb on the plant and enter the mesophyll of one of the lower leaves. They spend the whole feeding period as leaf miners, and molt twice in the mine. The full-grown third instar larvae leave the mine and pupate in the soil. After hatching from the pupae the new generation feeds for some weeks before entering hibernation. There is usually only one generation per year in Denmark (Nielsen Citation1977). The laboratory stock of the larvae was reared on radish, Raphanus sativus and maintained at 24°C and a photoperiod of 18 h light (Nielsen Citation1989).
Insect movement between genotypes
In order to choose an appropriate design of the competition experiment, the response of the insect larvae to the insect-resistant transgenic plants was investigated, by observing whether there was any herbivore migration between the two A. thaliana genotypes. Susceptible and resistant plants were established in 1:1 mixtures with plants two, two and a half or three weeks old. Within each age class, insects were applied to the resistant plants in densities of two, four or eight larvae per plant. The mixtures each contained eight susceptible and eight resistant plants. The experiment was made at one planting density, i.e., 0.09 plants cm−2. The experiment was terminated 24 days after the first application of insects.
When the larvae were placed on the resistant plants in a mixture of resistant plants and susceptible plants, the larvae migrated to the susceptible plants. Some larvae started to mine on the resistant leaves, but after a few days they migrated to the susceptible plants. The resistant plants appeared unaffected by the larvae. It was impossible to count the number of larvae that migrated from the resistant plants to the susceptible plants, but it was assessed that almost all of them did. Consequently, insects were only applied to the susceptible genotype in the competition experiment.
Herbivore timing and density
Simultaneously, the effect of the herbivore under various experimental conditions was investigated in a second pilot experiment. In this experiment susceptible plants were grown in monoculture of nine plants with plants of two, two-and-a-half or three weeks of age. Within each age class, insects were applied to the plants in densities of two, four or eight larvae per plant. Visual assessment of the plants made it clear that only two-week-old plants were affected by application of insect larvae. If larvae were applied to older plants the effects were apparently much less. This experiment demonstrated that two and four larvae per plant were not enough to cause significant damage to the plants. It was therefore decided to use 0, 4 and 8 larvae per plant in the competition experiment.
Germination
The germination probability of the two A. thaliana genotypes were studied in monocultures at five densities; 0.041, 0.45, 1.23, 2.0 and 4.08 plants cm−2 corresponding to box sizes of 70×70 cm, 18×24.5 cm, 12×13.5 cm, 10×10 cm, and 7×7 cm, respectively. The densities were chosen so as to span the range from crowded conditions to ample plant space. Two hundred seeds were added to each planting frame. To aid an even distribution of the seeds in the planting boxes they were mixed with sand (Wilson Citation2000). The sand was sieved trough a 425 µm sieve and then through a 250 µm sieve. Since the seeds of A. thaliana only are a few hundred micrometers in their longest dimension, the sand that ran through the 425 µm sieve but not through the 250 µm sieve was used. To optimize the distribution of seeds at each of the five plant densities, they were mixed with 100, 9, 3, 2 and 1 gram of sand respectively. After the weighing, the sand was heat treated for 24 h at 80°C to minimize the risk of infections. Finally, the sand was moistened and mixed with the seeds in ten small Petri dishes. The Petri dishes were covered with black plastic and placed at 5°C. After a week, the Petri dishes with sand and seeds were dispersed over the soil in the planting boxes. The number of seedlings was counted regularly until no more seedlings emerged within a period of five days.
The germination experiment was carried out twice. The first experiment was terminated after 28 days. In the second experiment susceptible plants were harvested after 33 days, whereas resistant plants were harvested after 36 days.
The Jonckheere test for ordered alternatives (Siegel & Castellan Citation1988), which tests whether the samples are ordered in a specific a priori sequence, was used to test for any significant effect of plant density.
The number of emerged seedlings was assumed to be mixed binomial distributed with a variable germination probability, which was assumed to be beta distributed. Possible effects of genotype and experiments were tested using likelihood ratio tests.
Competition and herbivory
A response surface competition experiment (Inouye Citation2001) was conducted between susceptible and resistant A. thaliana at a variable degree of herbivory. In the experiment, plants were grown either in monoculture or in a 1:1 mixture at three densities. In all treatments involving the susceptible A. thaliana a parallel series of treatments were established with 4 and 8 larvae of the leaf beetle P. nemorum, respectively. Simultaneously, the relationships between biomass and seed production were established in order to calculate seed production on the basis of biomass of the harvested plants.
Relationship between shoot biomass and seed production
It was not feasible to count the seeds of competing A. thaliana plants. Instead a relationship between biomass and fecundity was established. This was done by harvesting 50 susceptible plants at three plant densities, i.e., 0.2, 0.069 and 0.031 plants cm−2 and 50 resistant plants at the same densities. The plants were treated as the plants in the competition experiment (see below), but before weighing the plants the number of seeds was counted from 236 siliques (107 susceptible and 129 resistant) and the corresponding silique length was measured. This was done in order to get a relationship between the number of seeds and the length of the siliques. The 236 siliques were from 24 different plants (9 susceptible and 15 resistant). This regression was then used to estimate the total number of seeds on 251 plants (120 susceptible and 131 resistant) on which the length of each silique were measured. The 251 plants were weighed in order to get a relationship between plant dry weigh and seed number.
Possible effects of genotype or plant density on the relationships between silique length and number of seed and between plant dry weight and number of seed respectively, was tested using the GLM procedure (SAS Citation2001).
Competition experiment
The two genotypes were grown either in monoculture or in mixture at three densities (for monocultures: 0.20, 0.069, 0.031 plants cm−2, corresponding to box sizes of 14.5×14.5 cm2, 28.7×28.7 cm2, and 40×40 cm2, and for mixtures: 0.24, 0.069, 0.031 plants cm−2, corresponding to box sizes of 20.2×20.2 cm2, 38×38 cm2, 57×57 cm2). The densities were chosen so as to span the range from crowded conditions to ample plant space. Each box with only one genotype included 50 plants and each box with both genotypes included 100 plants (50 susceptible and 50 resistant). Three levels of herbivory were applied to all treatments including the susceptible genotype. At all the above-mentioned densities either 0, 4 or 8 larvae were placed on all the susceptible plants. Due to low productivity of the insect stock the larvae were applied over 4 days. Every day one larvae was transferred to each plant at the low herbivore treatment and two larvae to the plants assigned to the high herbivore treatment.
The seeds were sown in watered potting-soil in planting-trays, covered with black plastic and kept at 5°C to ensure synchronized germination. After four days the planting-trays were placed in the greenhouse and the cover was removed. Eight to 12-day-old seedlings were used in the experiment. The plants were randomly assigned to a specific position in the experimental boxes in order to minimize the spatial covariance of the genotypes (Damgaard Citation2004c). The boxes were placed close together in order to minimize any edge effect. According to the results of the first experiment, larvae were transferred to two weeks after transplantation. The larvae were placed on the lower rosette leaves.
The experiment was terminated when the majority of the plants had reached the late reproductive stage (50 days). The shoot biomass of all plants was harvested, dried at 80°C for 24 h and weighed individually. The roots were not harvested, because the root biomass of A. thaliana is very small relative to shoot biomass.
The observed variation in biomass and fecundity may arise from three different sources: Among plants within a plot, among plots and among treatments. However, based on our experience with observing individual competing plants, which often display a skewed size-distribution due to size-asymmetric growth and a sizeable border effect, and since there were logistic constraints on the number of experimental plants, the number of plants within a plot was augmented in the experimental design at the price of measuring a possible among-plot effect. That is, the among-plot variation was a priori assumed to be negligible compared to the two other sources of variation. This assumption was validated by subjective assessments of the plots during the course of the experiment. Furthermore, since the mean treatment effects were analysed in a regression-based response surface competition model, where the primary goal is to determine the shape of the response surface, a possible among-plot effect among “neighbouring” treatment points partly will be “averaged out” (Inouye Citation2005).
Competition model
The effect of herbivory on the estimated fecundity (seed production) of the two competing genotypes of A. thaliana was modelled by a generalization of a discrete hyperbolic competition model (Damgaard Citation2003). We assume that within the limited domain of the conducted competition experiment, herbivory affects the competitive interactions and the fecundity of the susceptible plant species linearly. Furthermore, since the flea beetle die when exposed to the resistant A. thaliana (Tattersall et al. Citation2001), it is assumed that the fecundity of the resistant genotype is unaffected by the flea beetle, i.e.,
The model was fitted to the mean treatment effects after both the data and the competition model were log-transformed. After transformation, the residuals were approximately normally distributed with a homogeneous variance. To avoid negative parameter values, the parameters a i , b i , d i and f i were transformed with the exponential function during the fitting procedure.
The competition model (1) is quite flexible and in many cases the model will be over-parameterized. Such a possible over-parameterization generally reduces the testing power of the model. Consequently, it was tested whether the competition model could be simplified by setting d i and f i equal to one using a likelihood ratio test. Likewise, different biological hypotheses on the parameters were also tested using likelihood ratio tests.
The joint Bayesian posterior distribution of the parameters in the competition model was sampled using the Metropolis-Hastings algorithm with a multinomial candidate distribution (100,000 iterations with a burn-in period of 1000) assuming uniform prior distributions of the location parameters. The sampling procedure was checked by visual inspections of the sampling chains as well as computing the autocorrelation and acceptance ratio (Carlin & Louis Citation1996).
Equilibrium properties of the competition model
Even though it would be naive to think that a plant community has a precisely defined long-term ecological equilibrium, knowledge on the equilibrium properties may provide valuable information in predicting the future states of the plant community. Assuming a constant and density-independent probability of seed germination and seedling establishment (p i ) the recursive equations for the density of the two annual plant species in the year t+1 are:
Results
Germination
There was no significant effect of plant density on germination for the two genotypes. It was tested whether the emergence rate increased with increasing density, which was not the case (Jonckheere tests: susceptible plants: p>0.1, resistant plants: p>0.1), or whether the emergence rate decreased with increasing plant density, which also was not the case (Jonckheere tests: susceptible plants: p>0.1, resistant plants: p>0.1).
The germination probability of the susceptible and resistant genotypes was in both experiments significantly different (likelihood ratio tests: first experiment: p<0.0005, second experiment: p<0.01), but there were no significant differences between the two experiments (likelihood ratio tests: susceptible: p=0.23; resistant: p=0.057) and the two experiments were pooled. The estimated germination rate of the susceptible plants was 0.58 and the estimated germination rate of the resistant plants was 0.20, i.e., the germination rate of the susceptible plants is approximately three times higher than the germination rate of the resistant plants.
Relationship between shoot biomass and seed production
On average there were 37.19 seeds in a silique and the mean silique length was 1.23 cm. The relationship between silique length and seed number per silique did neither depend on genotype (F-test: p=0.11) nor plant density (F-test: p=0.93), hence all siliques were treated as one pooled sample. A non-linear model was fitted to the data (number of seed per silique = 25.8086×silique length 1,6793 (r2=0.77)) and used to estimate the number of seeds on 251 sampled plants.
The regression analysis between seed production and plant dry weight of the 251 sampled plants showed a significant difference between genotypes (F-test: p=0.0016). The susceptible plants were not affected by plant density (F-test: p=0.97), whereas the resistant plants did show a significant density dependent relationship between shoot biomass and seed production (F-test: p<0.0001). None of the genotypes were significantly affected by herbivory. Hence, for the susceptible plants only a single linear regression model was constructed, whereas for the resistant plants a linear regression model was constructed for each plant density (). These regression models were used to estimate the fecundity of all the plants.
Table I The linear regression models of the number of seed as a function of plant dry weight in grams.
Competition and herbivory
The susceptible genotype showed, as expected, a decreasing fecundity with increasing herbivory at intermediate and high density (see ). At low density and grown in mixture with the resistant genotype there was no effect of herbivory on the fecundity of the susceptible genotype. However, when grown in monoculture, the fecundity increased with increasing herbivory. Such a positive response of herbivory may seem surprising, but has been demonstrated in several cases (Hawkes & Sullivan Citation2001, Trumble et al. Citation1993). As expected, the fecundity decreased with density and there was a significant interaction between density and whether the genotypes where grown in monoculture or in mixed cultures (see ).
Figure 1. Estimated mean number of seeds produced by the susceptible and resistant plants in monoculture and mixtures at the three different levels of herbivory (0, 4 or 8 larvae plant−1 on the susceptible plants) and three planting densities, i.e., low, intermediate and high plant density. The standard errors are shown.
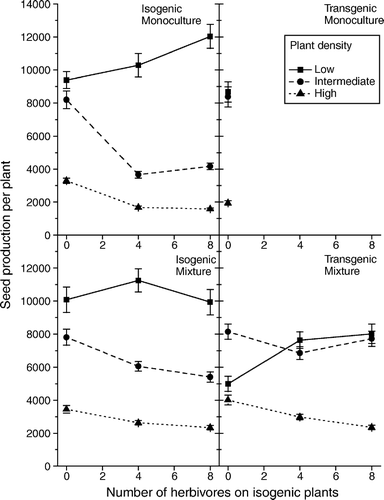
Table II ANOVA of estimated fecundity. The non-significant interaction terms are removed.
The estimated fecundity data was analysed using the competition model (1). The shape parameters f res and f sus were not significantly different from one (likelihood ratio test: p=0.39), and the competition model was consequently simplified by setting f res =f sus =1. The shape parameters d res and d sus were significantly different from one (likelihood ratio test: p=0.01). One of the parameters in the simplified model β sus , were significantly different from zero (likelihood ratio test: p=0.02), i.e., the herbivore had an effect on the fecundity of the susceptible genotype at high plant density, α sus γ sus , γ res was not significantly different from zero (likelihood ratio tests: p=0.18, 0.17, and 0.10, respectively), i.e., the herbivores did not have a significant effect on the fecundity of the susceptible genotype at low plant density and the competitive ability of either genotype. The model was consequently simplified by setting α sus γ sus and γ res to zero.
The fact that d res and d sus are significantly different from one complicates the biological interpretation of the estimated parameters in the competition model considerable and only quite general tests as the above-mentioned can be made. Instead we will look at the predicted equilibrium properties of the competition model.
Equilibrium properties
The equilibrium properties of the fitted model were analysed at different probabilities of germination and establishment under the strong and biologically unrealistic assumptions that the two genotypes of A. thaliana are the two only genotypes in the A. thaliana population, that the density of A. thaliana is not controlled by other plant species, and that the level of herbivory do not vary among plants and years.
The probability of germination of the two A. thaliana genotypes was obtained from the germination experiment (resistant genotype: 0.20, susceptible genotype 0.58), but there was no prior information on the probability of establishment. However, seed size is often considered to be positively correlated with the probability of establishment (Rees et al. Citation2001) and, since the seeds of A. thaliana are extraordinarily small, it was assumed that the probability of establishment of A. thaliana is relatively low, and different values in a biological realistic range were tried.
Initially, it was assumed that the probability of establishment was the same for the two genotypes (=0.001) and that the probability of establishment of the susceptible plants was independent of the herbivore level. Inserting the maximum likelihood estimates from the simplified competition model (1) into the recursive equation (2) and solving for stable equilibrium points, we find that the most likely scenario is that the two genotypes will coexist at equilibrium. As expected the most likely predicted equilibrium density of the susceptible genotype decreases with the level of herbivory, and the predicted equilibrium density of the resistant genotype increases with the level of herbivory (see ).
Figure 2. Predicted stable equilibrium densities of the susceptible genotype (dash line) and the resistant genotype (full line), when the maximum likelihood estimates of the simplified model (1) were inserted into equation (2) and the probabilities of establishment were assumed to be 0.001 for both genotypes.
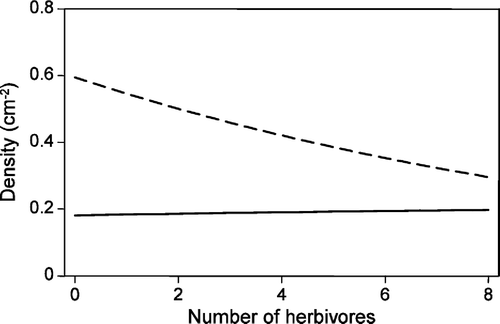
A sensitivity analysis, where the probability of establishment was varied, showed that the above conclusion, i.e., that the predicted density of the susceptible genotype decreases and the density of the resistant increases with the level of herbivory is quite robust. It is only the equilibrium densities and whether or not the two genotypes may coexist at equilibrium which varies.
However, there is a considerable degree of uncertainty that is not reflected in the maximum likelihood estimates of the simplified competition model. If the joint Bayesian posterior distribution of the parameters was used to predict the long-term fate of the two genotypes, the different scenarios were almost independent of the level of herbivory. When the probability of establishment was 0.001, the most likely scenario was that the susceptible genotype outcompeted the resistant genotype at all examined levels of herbivory (p=[0.70, 0.726, 0.74], when the number of herbivores were 0, 4 and 8, respectively). But there was also a relative large probability that the two genotypes would coexist (p=[0.21, 0.12, 0.12]) at equilibrium.
Using the methodology described in (Damgaard Citation2004a) it was demonstrated that the population dynamics of the resistant species and the coexisting two species system tended to be unstable, since the cumulative fecundity per unit area reaches a maximum at an intermediary density (over-compensation) and it has been shown theoretically that over-compensation may lead to periodic oscillations or chaotic dynamics when the fecundity is sufficiently high (Damgaard Citation2004a, May Citation1975, May & Oster Citation1976). The possible effect of unstable population dynamics on the risk assessment of genetically modified plants is discussed elsewhere (Damgaard & Borksted Citation2004).
Discussion
Germination
The germination experiment showed no effect of sowing density on the germination rates of A. thaliana, although it has previously been found that germination may either increase or decrease with seed density (Inouye Citation1980, Lortie & Turkington Citation2002). It is often speculated that a possible negative response to density involves germination inhibitors released from the seeds (Linhart Citation1976). Possible explanations for the positive response to the presence of other seeds nearby are that groups of seeds retain moisture better than single seeds and that germinating seeds secrete substances, which increase the germination rates of other seeds (Linhart Citation1976).
The number of emerging susceptible and resistant plants differed significantly. The susceptible plants germinated almost three times better than the resistant plants. Furthermore, the resistant plants emerged later than the susceptible plants. The synthesis of dhurrin could be the reason why the resistant plants had a lower germination rate than the susceptible plants; if the emerging seedlings are allocating resources to dhurrin synthesis, fewer resources are available to growth. This implies that the resistant plants synthesize dhurrin already as emerging seedling. Another explanation might be that the resistant plants, possibly due to the dhurrin synthesis, allocate fewer resources to the seed.
Competition and herbivory
The objective of this study is ambitious in investigating the long-term ecological effects of density, competition, herbivory and their compound interactions at the same time. The chosen design and analysis of the competition experiment is adapted for testing rather general hypotheses on the effect of herbivory on plant community dynamics. In a regression-based analysis using competition model (1), we found that herbivory had a significant effect on the fecundity of the susceptible genotype at high plant density. The most likely long-term ecological scenario was that the susceptible genotype outcompeted the resistant genotype. But it was also shown that herbivory could down-regulate the equilibrium density of the susceptible genotype and, when the two genotypes were coexisting, up-regulate the equilibrium density of the resistant genotype. The results of the competition experiment did meet our prior expectations in a certain way, i.e., increasing level of herbivory did favour the resistant genotype over the susceptible genotype. However, possibly due to a low level of herbivory our prior expectation, that the resistant genotype would outcompete the susceptible genotype at the highest level of herbivory, was not met.
It would be valuable to study the occurrence of P. nemorum in A. thaliana populations and to investigate whether an early attack of the herbivore has an effect on seedling mortality and the probability of establishment. In the unlikely event that A. thaliana is the main feeding source in the habitat, any increase in the frequency of the herbivore-resistant genotype may lead to a feedback mechanism reducing the degree of herbivory in the plant population. In theory the resistant plants might even show up to be beneficial to the herbivore-sensitive plants, e.g., if repeated landings on the herbivore-resistant genotype cause the insect to move away from the local area while successive landings on host plants induce egg-laying behaviour (Hambäck & Beckerman Citation2003). Additionally, it is to be expected that dhurrin might confer resistance towards other herbivores on A. thaliana besides P. nemorum and the effect of those herbivores on the competitive ability of the resistant genotype needs to be studied in order to give a complete picture of the likely ecological fate of the resistant genotype in a natural habitat.
One of the driving factors to explain the interaction between competition and herbivory is size-asymmetric competition (e.g., Weis & Hochberg Citation2000), i.e., plants that are attacked by herbivores will, when competition is intense, lose the battle for e.g., light. A. thaliana is a thin plant with limited shading capability and not expected to be a plant species that shows strong size-asymmetric competition. Consequently, the interaction between competition and herbivory in the slender A. thaliana, which is expected to show a limited level of size-asymmetric growth, may be relatively small compared to plant species with a larger shading capability. Nevertheless, it has previously been shown that pathogens could change the balance of competition between two genotypes of A. thaliana (Damgaard & Jensen Citation2002). In that study the susceptible genotype of A. thaliana was a better competitor than the resistant when the pathogen was not present. However, when the plants were infected with the pathogen, the competitive ability of the resistant genotype would increase and the predicted probability that the resistant genotype outcompete the susceptible genotypes increased (Damgaard & Jensen Citation2002).
Thanks to Jens Kvist Nielsen and Peter de Jong for providing the Phyllotreta nemorum and to Birger Lindberg M⊘ller, S⊘ren Bak and David B. Tattersall for providing the two genotypes of Arabidopsis thaliana. Also thanks to Birgitte Gudmand, Birgit Nielsen, John-Geert Rytter, and Trine Guldager S⊘rensen for their help.
References
- Aarssen , LW and Clauss , MJ. 1992 . Genotypic variation in fecundity allocation in Arabidopsis thaliana . J Ecol , 80 : 109 – 114 .
- Abbott , RJ and Gomes , MF. 1989 . Population genetic structure and outcrossing rate of Arabidopsis thaliana . Heredity , 62 : 411 – 418 .
- Abrams , PA. 1996 . Evolution and the consequences of species introduction and deletions . Ecology , 77 : 1321 – 1328 .
- Bergelson , J and Purrington , CB. 1996 . Surveying patterns in the cost of resistance in plants . Am Naturalist , 148 : 536 – 558 .
- Cahill , JF Jr , Kembel , SW and Gustafson , DJ. 2005 . Differential genetic influences on competitive effect and response in Arabidopsis thaliana . J Ecol , 93 : 958 – 967 .
- Carlin , BP and Louis , TA. 1996 . Bayes and empirical Bayes methods for data analysis , London : Chapman & Hall .
- Clauss , MJ and Aarssen , LW. 1994 . Phenotypic plasticity of size-fecundity relationships in Arabidopsis thaliana . J Ecol , 82 : 447 – 455 .
- Cottam , DA , Whittaker , JB and Malloch , ACJ. 1986 . The effects of Chrysomelid beetle grazing and plant competition on the growth of Rumex obtusifolius . Oecologia , 70 : 452 – 456 .
- Cousens , R. 2001 . My view . Weed Science , 49 : 579 – 580 .
- Crawley , MJ. 1989 . Insect herbivores and plant population dynamics . Ann Rev Entomol , 34 : 531 – 564 .
- Damgaard , C. 1998 . Plant competition experiments: Testing hypotheses and estimating the probability of coexistence . Ecology , 79 : 1760 – 1767 .
- Damgaard C. 2002 . Quantifying the invasion probability of genetically modified plants . BioSafety, 7: Paper 1 (BY02001) Online Journal – URL: http://www.bioline.org.br/by .
- Damgaard , C. 2003 . Modelling plant competition along an environmental gradient . Ecolog Modelling , 170 : 45 – 53 .
- Damgaard , C. 2004a . Dynamics in a discrete two-species competition model: Coexistence and over-compensation . J Theoretical Biol , 227 : 197 – 203 .
- Damgaard , C. 2004b . Evolutionary ecology of plant-plant interactions – an empirical modelling approach , Aarhus, , Denmark : Aarhus University Press .
- Damgaard , C. 2004c . Inference from plant competition experiments: The effect of spatial covariance . Oikos , 107 : 225 – 230 .
- Damgaard , C and Borksted , B. 2004 . Transgenic insect resistant Arabidopsis may show chaotic population dynamic . Ecological Complexity , 1 : 261 – 265 .
- Damgaard , C and Jensen , BD. 2002 . Disease resistance in Arabidopsis thaliana increases the competitive ability and the predicted probability of long-term ecological success under disease pressure . Oikos , 98 : 459 – 466 .
- Damgaard C , L⊘kke H . 2001 . A critique of the “concept of familiarity” as used in the ecological risk assessment of genetically modified plants . BioSafety , 6 : Paper 1 (BY01001) Online Journal – URL: http://www.bioline.org.br/by .
- Fowler , NL and Rausher , MD. 1985 . The joint effect of competitors and herbivores on growth and reproduction in Aristlochia retculata . Ecology , 66 : 1580 – 1587 .
- Gurevitch , J , Morrison , JA and Hedges , LV. 2000 . The interaction between competition and predation: A meta-analysis of field experiments . Am Naturalist , 155 : 435 – 453 .
- Hambäck , PA and Beckerman , AP. 2003 . Herbivory and plant resource competition: A review of two interacting interactions . Oikos , 101 : 26 – 37 .
- Hawkes , CV and Sullivan , JJ. 2001 . The impact of herbivory on plants in different resource conditions: A meta-analysis . Ecology , 82 : 2045 – 2058 .
- Inouye , B. 2001 . Response surface experimental designs for investigating interspecific competition . Ecology , 82 : 2696 – 2706 .
- Inouye , BD. 2005 . The importance of the variance around the mean effect size of ecological processes: comment . Ecology , 86 : 262 – 265 .
- Inouye , RS. 1980 . Density-dependent germination response by seeds of desert annuals . Oecologia , 46 : 235 – 238 .
- Keddy , PA. 1990 . “ Competitive hierarchies and centrifugal organisation in plant communities ” . In Perspectives on plant competition , Edited by: Grace , JB and Tilman , D . London : Academic Press .
- Kristensen , C , Morant , M and Olsen , CE . 2005 . Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome . PNAS , 102 : 1779 – 1784 .
- Lee , TD and Bazzaz , FA. 1980 . Effects of defoliation and competition on growth and reproduction in the annual plant Abutilon theophrasti . J Ecol , 75 : 871 – 886 .
- Linhart , YB. 1976 . Density-dependent seed germination strategies in colonising plant species . Journal of Ecology , 64 : 375 – 380 .
- Lortie , C and Turkington , R. 2002 . The effect of initial seed density on the structure of a desert annual plant community . J Ecol , 90 : 435 – 445 .
- Mauricio , R. 1998 . Cost of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana . Am Naturalist , 151 : 20 – 28 .
- May , RM. 1975 . Biological populations obeying difference equations: Stable points, stable cycles, and chaos . J Theoret Biol , 51 : 511 – 524 .
- May , RM and Oster , GF. 1976 . Bifurcations and dynamic complexity in simple ecological models . Am Naturalist , 110 : 573 – 599 .
- Miyashita , NT , Kawabe , A and Innan , H. 1999 . DNA variation in the wild plant Arabidopsis thaliana revealed by amplified fragment length polymorphism analysis . Genetics , 152 : 1723 – 1731 .
- Nielsen , JK. 1977 . Host plant relations of Phyllotreta nemorum L. (Coleoptera Chrysomelidae). I Field studies . Zeitschrift für Angewante Entomologie , 84 : 396 – 407 .
- Nielsen , JK. 1989 . The effect of glucosinolates on responses of young Phyllotreta nemorum larvae to non-host plants . Entomol Exp Appl , 51 : 249 – 259 .
- Nielsen , JK , Hansen , ML , Agerbirk , N , Petersen , BL and Halkier , BA. 2001 . Responses of the flea beetle Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile . Chemoecology , 11 : 75 – 83 .
- Purrington , CB and Bergelson , J. 1997 . Fitness consequences of genetically engineered herbicide and antibiotic resistance in A. thaliana . Genetics , 145 : 807 – 814 .
- Purrington , CB and Bergelson , J. 1999 . Exploring the phsiological basis of cost of herbicide and antibiotic resistance in A. thaliana . Am Naturalist , 154 : S82 – S91 .
- Rees , M , Condit , R , Crawley , M , Pacala , S and Tilman , D. 2001 . Long-term studies of vegetation dynamics . Science , 293 : 650 – 655 .
- Rees , M , Grubb , PJ and Kelly , D. 1996 . Quantifying the impact of competition and spatial heterogeneity on the structure and dynamics of a four-species guild of winter annuals . Am Naturalist , 147 : 1 – 32 .
- Rodríguez , MA and Brown , VK. 1998 . Plant competition and slug herbivory: Effects on the yield and biomass allocation patterns of Poa annua L . Acta Oecologica , 19 : 37 – 46 .
- SAS 2001 . SAS/STAT version 8.02 . SAS Institute Inc. , Cary, , NC, USA .
- Siegel , S and Castellan , NJ. 1988 . Nonparametric statistics for the behavioral sciences , New York : McGraw-Hill .
- Tattersall , DB , Bak , S , Jones , PR , Olsen , CE , Nielsen , JK and Hansen , ML. 2001 . Resistance to an herbivore through engineered cyanogenic glucoside synthesis . Science , 293 : 1826 – 1828 .
- Trumble , JT , Kolodny-Hirsch , DM and Ting , IP. 1993 . Plant compensation for arthropod herbivory . Ann Rev Entomol , 38 : 93 – 119 .
- Weis , AE and Hochberg , ME. 2000 . The diverse effects of intraspecific competition on the selective advantage to resistance: A model and its predictions . Am Naturalist , 156 : 276 – 292 .
- Wilson , ZA. 2000 . Arabidopsis, a practical approach , Oxford, , UK : Oxford University Press .