Abstract
To test the hypothesis that higher antioxidant potential of hemiparasitic plants is due to sequestration of phenolic compounds from the host plants, samples of Dendrophthoe falcata, a hemiparasite collected from different hosts, were investigated for total phenolics, total flavonoids and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity. The hosts significantly influenced the phenolic content of the hemiparasite. However, similar influence was not detected on radical scavenging activity and no correlation was found in the phenolics and free radical scavenging activities. Further investigation on transfer of constituents revealed that D. falcata sample obtained from a host, Mangifera indica, contained mangiferin, a C-glucosyl xanthone, and some unidentified flavonoids as confirmed by HPTLC flavonoid patterns. The data indicated that the hosts significantly affected total phenolics and total flavonoids in a hemiparasite. This is the first report of transfer of mangiferin from M. indica to a hemiparasite. The present report points towards the need of further investigations on the possible role of transferred phenolics either as mediators of host defense, host defense compounds utilized as cues of identification of the host by the hemiparasite or compounds taken up by the parasites to support their defense against rejection by the hosts.
Introduction
Plant hemiparasites are known to obtain a wide range of primary compounds such as carbon, water and ions as well as secondary compounds from their hosts (Schneider & Stermitz Citation1990, Boros et al. Citation1991, Stermitz & Pomeroy Citation1992, Press et al. Citation1999). Numerous hemiparasites do not synthesize or modify the secondary compounds taken up from their hosts (Simms Citation1992), thus the presence of certain secondary compounds varies within and among the populations of hemiparasitic plants, depending on the host association of individual parasites (Stermitz & Harris Citation1987). The uptake of secondary compounds by a parasite from its host plant is known to increase resistance of parasite towards herbivores. The parasite may also attract pollinators due to such host-derived secondary compounds. Although chemical ecology of interaction between parasitic plants and their hosts is poorly understood, such transfer of compounds to a parasite from its host plant is known to sustain the existence of parasites (Adler et al. Citation2001, Adler Citation2002).
There are 20 different species belonging to the genus Dendrophthoe found all over the world, seven of which are found in India. The hemiparasite, Dendrophthoe falcata (L. f.) Etting. var. falcata (Loranthaceae) is one of the seven species present in India. Hemiparasites have been reported to exist on more than 300 host plants (Sampathkumar & Selvaraj Citation1981). They are also known as potential pests, due to the severe damage which they cause to many economically important plants. In a quest of the natural antioxidant compounds, we investigated the parasitic plants for their content of phenolics and their oxidative radical scavenging activities in vitro. We found that, in the case of samples of D. falcata collected by us, the antioxidant potential of the hemiparasite was consistently higher when compared to the related hosts. This observation is in line with an earlier report stating that a high concentration of phenolics appears to be a general feature of parasitic angiosperms (Khanna et al. Citation1968). It has been reported that the phenolic content of a parasite plant is often independent of host phenolics (Khanna et al. Citation1968). There are reports stating effects of hosts on phenolics of parasitic plants, both in case of hemiparasites and holoparasites, still the higher phenolic content has rarely been correlated with host-parasite interactions. Dendrophthoe falcata bark has been reported to contain phenolic compounds, the majority of which are flavonoids. Phytochemical investigations have also revealed the presence of quercitrin (quercetin-3-O-rhamnoside) as a major flavonoid in this hemiparasite grown on different host plants (Ramchandran & Krishanakumary Citation1990). The same study further states that the flavonoid pattern of the hemiparasite samples collected from different host plants remains the same and there is no influence of the host plants on the flavonoids of D. facalta.
In light of such claims and owing to the lack of reports about the degree of influence of host on secondary chemicals of a parasite, especially phenolics, we investigated the hypothesis that higher anti-oxidant potential of hemiparasitic plants is due to sequestration of phenolic compounds from their host. Further investigation was also carried out to determine the nature of transferred secondary chemicals to the stems of D. falcata obtained from a host, Mangifera indica.
Materials and methods
Plant material and preparation of extracts
The stem samples of non-infected host plants, Mangifera indica (Anacardiaceae), Melia azadirachta (Meliaceae) and Psidium guajava (Myrtaceae) and stems of D. falcata hemiparasitic on the above host plants were collected in August 2005. Collection was done in the morning hours and care was taken to obtain the stems of host and hemiparasite of similar size (2.5–3.2 cm in diameter) and age (2–3 years). All plants specimens were authenticated by a taxonomist, Dr C. B. Salunkhe, Department of Botany, Krishna Mahavidyalaya, Rethare, India. The collected plant material was shade-dried and coarsely powdered; 500 g of each sample was extracted with 80% methanol by cold maceration repetitively (this solvent was used based upon optimization studies in our laboratory showing maximum extraction of flavonoids with higher yields). The extract was concentrated on rotary vacuum evaporator and further dried in vacuum dryer.
Estimation of total phenolics and total flavonoids
Total phenolic content of methanolic extracts of six plant samples were determined by using Folin-Ciocalteu reagent (Singleton & Rossi Citation1965). The blue color formed due to the polyphenol content in the extract was measured at 760 nm using a Shimadzu UV-1601 spectrophotometer and the results were expressed as g/100 g of gallic acid equivalent.
Total flavonoids of methanolic extracts of all six samples were determined using the method of Liu et al. (Citation2005) with some modifications. In brief, the extract was diluted with 80% aqueous ethanol (0.9 ml). Aliquots of 0.5 ml of extract were added to test tube containing 0.1 ml of 10% aluminum nitrate, 0.1 ml 1M aqueous potassium acetate and 4.3 ml of 80% ethanol. The reaction tubes were set aside for 40 min at room temperature. At the end of this time, optical density of each sample was determined at 415 nm using a UV-spectrophotometer. Total flavonoid content was calculated by interpolation on a standard curve established with a reference standard, quercetin. Quercetin and Folin-Ciocalteu reagent were obtained from Sigma-Aldrich, Germany.
Determination of DPPH radical scavenging activity
The free radical scavenging activity of all extracts was investigated using the method of Blois (Citation1958). Solutions of various extract with concentrations ranging from 10–200 µg/ml were prepared in water. A 0.1 mM solution of 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) in ethanol was prepared. In the assay, 1.0 ml of DPPH solution was added to 3.0 ml of each extract solution. Thirty minutes after maintaining at room temperature, the optical density of each reaction mixture was measured at 517 nm. The radical scavenging activity of the extracts was expressed as IC50 values.
HPTLC flavonoid pattern
The flavonoid patterns of methanolic (80%) extract of all samples were recorded using HPTLC (Camag, Switzerland) on pre-coated silica gel plates (Merck) using ethyl acetate: formic acid: glacial acetic acid: water (100:05:10:20) as solvent system. The chromatogram was evaluated densitometrically using winCAT software and the flavonoids were traced after treatment with 2-aminoethyl diphenyl borinate (natural product reagent) at 365 nm.
Extract preparation, isolation and characterization of isolated compound from D. falcata stems
The powdered stem sample of D. falcata hemiparasite on M. indica was extracted for mangiferin detection according to the method of Suleiman et al. (Citation1995). The powdered sample (700 g) was extracted in a soxhlet extractor for 12 h with petroleum ether (60–80°C). The defatted material was extracted with ethanol under reflux for 16 h, and extract obtained was further defatted with petroleum ether and crystallized repeatedly in aqueous ethyl acetate as pale yellow needles.
The crude extract (3.8 g) obtained from the above procedure was processed on column chromatography (150 g silica gel, 60×4 cm) packed in chloroform. Elution was started with chloroform and polarity was gradually increased with methanol. The collected fractions (50 ml each) were monitored by TLC using ethyl acetate: formic acid: glacial acetic acid: water (100:5:10:20) solvent system and 10% methanolic sulphuric acid reagent as visualizing agent. Natural mangiferin (Regional Research Lab., Jammu, India) was used as a reference standard. The Rf value of standard mangiferin was found to be 0.91. The fractions corresponding to this Rf value were combined, evaporated and purified by crystallization with ethyl acetate.
The pure compound (750 mg) obtained from column chromatography was subjected to UV, IR, HPTLC, HPLC and MS studies. The UV absorption spectrum of the compound in methanol was recorded in the range of 200–500 nm on Shimadzu UV-1601 spectrophotometer at 1 cm path length. The IR spectrum of the compound was recorded on FT-IR 8400S by press pellet technique with AR grade KBr. The Rf and retention time data for the isolated compound and mangiferin were obtained by using HPTLC and HPLC, respectively. The Rf of the isolated compound and mangiferin was recorded on HPTLC (Camag, Switzerland) with Linomet V applicator and scanner III (WinCAT software) using as a solvent system ethyl acetate: formic acid: glacial acetic acid: water (100:5:10:20). The isolated compound was further analysed on HPLC (Shimadzu, LC10A) using reverse phase C18 column (25 cm×4.6 i.d., 5 µ particle size) in isocratic mode with acetonitrile: acetic acid (16:84) at a flow rate of 1.0 ml/min as eluent. The column was purged with the mobile phase for 5 min, followed by equilibrium for 4 h; the total analytical run time was 30 min. The spectral data was obtained using SPD-M 10AVP photo-diode array detector in combination with class VP software. The identity of compound isolated was further confirmed using MS spectra. The MS spectra were obtained using, turbo spray ionization source, at electron energy of 70V, at source temperature 190°C.
Statistical analysis
For total phenolics, total flavonoids and radical scavenging activity of all samples at least four readings were considered and data were expressed as mean±SD. Comparison was made between samples of the hemiparasite collected from different host plants by one way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test using GraphPad Prism (version 4) software, by considering P values ≤ 0.05 as significant.
Results
It is evident from results that the methanolic extracts of three host plants have correlation and a clear pattern in total phenolics, total flavonoids and DPPH free radical scavenging activity with highest value for M. indica followed by P. guajava and M. azadirachta ().
Table I Total phenolics, total flavonoids and DPPH radical scavenging activity of methanolic extracts of Dendrophthoe falcata and corresponding host plants.
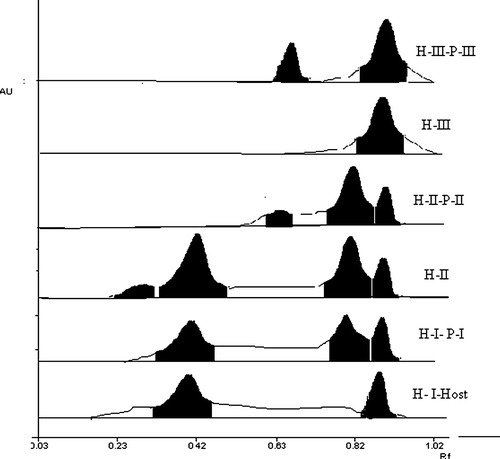
The hemiparasite samples obtained from these plants showed significant influence of host on total phenolics (p < 0.001) and total flavonoids (p<0.05). Significant influence, however, was not detected in case of radical scavenging activity (p>0.05). It is also evident that values for total flavonoids of the hemiparasite are either very close or higher than their corresponding host plants, which is also reflected in lower IC50 values for DPPH radical scavenging activity. This trend, in turn, also reproduced in HPTLC flavonoid pattern wherein, at least one additional peak, not found in host plant, was observed in corresponding parasitic plant ().
Figure 1. HPTLC chromatograms of flavonoid pattern of Dendrophthoe falcata parasitic on different host plants, recorded at 365 nm after treatment with NP reagent.H-I- Psidium guajava, (Rf 0.38, 0.89), H-I-P-I – D. falcata parasitic on P. guajava, (Rf 0.4, 0.8, 0.89), H-II- Mangifera indica, (Rf 0.25, 0.41, 0.81, 0.89), H-II-P-II- D. falcata parasitic on M. indica,( Rf 0.66, 0.8, 0.89) H-III – Melia azadirachta, (Rf 0.89) H-III-P-III- D. falcata parasitic on M. azadirachta, (Rf 0.69, 0.89).
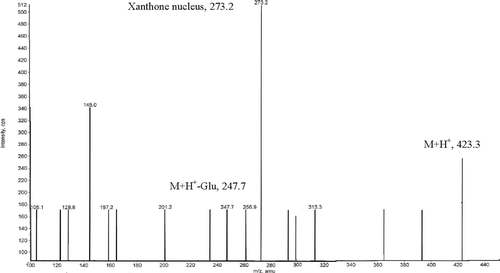
The compound isolated was a pale yellow crystalline powder, UV (methanol) λmax (log ε) 365 (0.0763), 316 (0.2202), 258.69 (0.4435), 241.6 (0.4020), IR (KBr), νmax 3384, 3269.12, 1059, 1164.5, 1701.10, 1608.02, 1448.44, 1280.6 cm−1. The Rf=0.91, HPLC retention time was 5.035 (). The turbo spray MS shown molecular ion peak at m/z 423(M + H+) and characteristic loss of glucose moiety (M + H+- Glu) at m/z 247.7, and peak of basic xanthone nucleus at m/z at 273.2 ()
Figure 2. HPLC chromatograms of reference standard, mangiferin (A) and compound isolated from Dendrophthoe falcata L. parasitic on Mangifera indica (B). Scaling of Figure B has been expanded to better visualize the mangiferin peak.
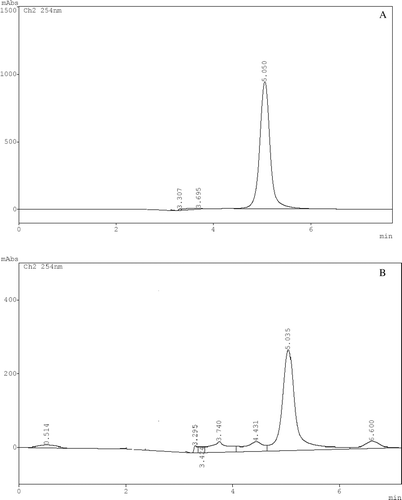
Figure 3. MS spectra of compound isolated from stems of Dendrophthoe falcata parasitic on Mangifera indica.
The analytical data on the compound isolated from D. falcata confirms that it is mangiferin. In addition to the comparison made between stems of host and hemiparasite, leaves of hemiparasite were also investigated for the presence of mangiferin. It is interesting to note that mangiferin was not detected (data not shown) in leaves of D. falcata obtained from M. indica, whereas, leaves of M. indica have been reported to contain mangiferin (Desai et al. Citation1966).
Discussion
The production of reactive oxygen species (ROS) in plants is well documented. ROS are the key components participating in plant defensive responses (Lamb & Dixon Citation1997). Most plants constitutively synthesize phenylpropanoids including flavonoids and hydroxycinnamic acids. However, accumulation of phenolics in plants can be induced by abiotic and biotic stresses (Dixon & Paiva Citation1995, Yamasaki et al. Citation1995, Bary et al. Citation2000). Since the reduced forms of phytophenolics are powerful antioxidants equivalent to ascorbate, plants are a logical starting point for the search of antioxidant compounds. While studying in vitro antioxidant potential of D. falcata, obtained from different host plants, we found that this hemiparasite has higher antioxidant potential than the corresponding host. High concentration of phenolics appears to be a general feature of parasitic angiosperms (Khanna et al. Citation1968). However, the significance of such higher phenolic content is rarely corroborated in host-parasite interaction studies. It is well known that a correlation exists between phenolic content of a plant and its antioxidant activity (Naik et al. Citation2006). The phenolic content of hemiparasites is often independent of host phenolics, although significant host influence does occur (Khanna et al. Citation1968). A study on D. falcata reports that flavonoid pattern of hemiparasites collected from different host plants was found to be same and no influence of the host plant on the flavonoids of D. falcata was observed (Ramchandran & Krishanakumary Citation1990). This study further shows that in the case of D. falcata barks, the flavonoids constitute a major part of phenolics and quercitrin (quercetin-3-O-rhamnoside) is a major flavonoid irrespective of the host plants.
The present data on D. falcata show that the hosts significantly determine total phenolics and total flavonoids of this hemiparasite. The transfer of flavonoids was evident from total flavonoid content as well as from HPTLC pattern, wherein at least one additional peak was recorded which was not found in corresponding host plant. However similar relationship was not observed between phenolics of the hemiparasite and their radical scavenging activity. This has been proposed to occur due to transfer of other non-flavonoid phenolics and/or compounds transferred to the hemiparasite are in minute quantity. Even in case of hosts, despite higher total phenolic content, higher IC50 values were observed in DPPH radical scavenging activity. Correlation in phenolic content of plants and their antioxidant activity has been emphasized in earlier reports (Naik et al. Citation2006). However, the results of the present investigation suggest that radical scavenging activity may not be a correct indicator for total phenolics and total flavonoid content. Other studies agree with our observations. The interference may be due to ascorbic acid which frequently occurs as a component of the plant extracts under study (Pellati et al. Citation2004). The inconsistency observed in our study can also be explained considering the fact that different flavonoids vary in their oxidative radical scavenging activity (Pietta Citation2000, Dugas et al. Citation2000). Moreover, the total amount of flavonoids that is being transferred to the hemiparasite may not be sufficient to accrue its radical scavenging activity. However, while investigating the effects of host plant on secondary chemicals of parasites it must be considered that secondary compounds may be generally transferred in very minute quantities, making difficult to study secondary chemicals transferred in early host-parasite associations. Due to such possibilities, we selected mangiferin, a major phenolic of M. indica and performed investigation on stems of hemiparasite because the woody stems are usually less prone to environmental fluctuations for their secondary metabolite content than leaves.
Mangiferin, C-glucosyl xanthone, is known for its antibacterial, antifungal, antitumor (Guha et al. Citation1996), antiviral (Zheng & Lu Citation1990, Yoosook et al. Citation2000 ) and antioxidant activities (Sanchez et al. Citation2000). The transfer of mangiferin from M. indica to Cuscuta reflexa has also been reported (Subramanian & Nair Citation1966). However, C. reflexa is a holoparasite and is known to sequester majority of the constituents as well as other resources from host plant.
The transfer of mangiferin to a hemiparasite, D. falcata is an unusual observation. To our knowledge this is the first report stating that mangiferin, a C-glucosyl xanthone, can be transferred from a host plant to a hemiparasite. Interestingly, mangiferin was detected only in stem samples but was found to be absent in leaves of D. falcata (data not shown). This may be due to sequestration of mangiferin in earlier stages of association or as a result of initial host defense response against biotic stress caused by the hemiparasite. Alternatively, this might be a host defense and a host recognition compound transferred from the host to the hemiparasite in the initial stages of association. In case of vascular plants, the phenolics synthesized in response to one stress are likely to play multiple protective roles against other stresses. Also, polyphenols function as antioxidants to support the primary ascorbate-dependent detoxification system as a backup defense in plants (Yamasaki et al. Citation1995). Generally, the host defense and host recognition is clearly associated with certain chemicals which are transferred by the host as defense molecules while are used by the hemiparasite as host recognition cues. The representative examples are quinones and phenolics. These are among the most commonly described classes of allelopathic phytotoxins (Inderjit Citation1996) and are known to exert genotoxic and mutagenic effects as a result of their pro-oxidant properties (Flowers et al. Citation1997).
In some plants, mangiferin co-occurs with C-glucosyl flavones rather than with other xanthones. It has also been established that the biosynthetic pathway leading to mangiferin is more related to that of flavones rather than other xanthones (Dar et al. Citation2005). Hence, there is a possibility that flavonoids may be transferred to the hemiparasite from the host along with mangiferin. Although, direct evidence has not been generated in our study regarding such transfer, HPTLC flavonoid pattern indicates the possibility that such transfer might occur in the case of D. falcata. This claim is also based on earlier observations by other authors showing that flavonoids are known to promote haustoria formation in some root parasites (Albrecht et al. Citation1999). Hence, we propose that along with mangiferin some flavonoids may be transferred to D. falcata, either in the form of stress response compounds of the host or as host defense molecules which are further utilized by the hemiparasites as recognition cues. However, further studies are needed to confirm the possibility of such co-transfer.
In conclusion, our findings suggest that higher anti-oxidant potential of the hemiparasite is due to its own flavonoid content and transferred compounds have less influence on radical scavenging activity. Here we showed a new report revealing the transfer of mangiferin, a C-glucosyl xanthone, from M. indica to a hemiparasite, D. falcata. The evident lack of correlation between the phenolics content and the free radical scavenging activity however requires further investigation. Similar studies on transfer of phenolics are solicited to uncover the nature of their recruitment as host defense compounds, compounds sequestered by hemiparasite for their own defense or host defense molecules utilized by hemiparasite as recognition cues.
References
- Adler , LS , Karban , R and Strauss , SY . 2001 . Direct and indirect effects of alkaloids on plant fitness via herbivory and pollination . Ecol , 82 : 2032 – 2044 .
- Adler , LS . 2002 . Host effects on herbivory and pollination in a hemiparasitic plant . Ecol , 83 : 2700 – 2710 .
- Albrecht , H , Yoder , JI and Phillips , DA . 1999 . Flavonoids promote haustoria formation in the root parasite Triphysaria . Plant Physio , 119 : 585 – 591 .
- Bary , AE , Baley-Jeeres , J and Weretilnyk , E . 2000 . “ Response to abiotic stresses ” . In Biochemistry and molecular biology plants , Edited by: Buchanin , BB , Gruissem , W and Jones , RL . 1158 – 1203 . New Delhi : IK International .
- Blois , MS . 1958 . Antioxidant determinations by the use of a stable free radical . Nature , 26 : 1199 – 1200 .
- Boros , CA , Marshall , DR , Caterino , CR and Stermitz , FR . 1991 . Iridoid and phenylpropanoid glycosides from Orthocarpus spp.: Alkaloid content as a consequence of parasitism on Lupinus . J Nat Prod (Lloydia) , 54 : 506 – 513 .
- Dar , A , Faizi , S , Naqvi , S , Roome , T , Zikr-ur-Rehman , S , Ali , M , Firdus , S and Moin , ST . 2005 . Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship . Biol Pharm Bull , 24 : 596 – 600 .
- Desai , PD , Ganguly , AK , Govindachari , TR , Joshi , BS , Kamat , VN , Manmade , AH , Mohamed , PA , Nagle , SK , Nayak , RH , Saksena , AK , Sathe , SS and Vishwanathan , N . 1966 . Chemical investigation of some Indian plants: Part II . Indian J Chem , 4 : 457 – 459 .
- Dixon , RA and Paiva , N . 1995 . Stress-induced phenylpropanoid metabolism . Plant Cell , 7 : 1085 – 1097 .
- Dugas , AJ , Jose , Castaneda-Acosta , Gloria , CB , Kimberly , LP , Nikolaus , HF and Witson , GW . 2000 . Evaluation of the total peroxy radical scavenging capacity of flavonoids: Structure-activity relationships . J Nat Prod , 63 : 327 – 331 .
- Flowers , L , Oshishi , T and Penning , TM . 1997 . DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: Role of reactive oxygen species, Cu (II)/Cu (I) redox cycling and o-semiquinone anion radicals . Biochemistry , 36 : 8640 – 8648 .
- Guha , S , Ghoshal , S and Chattopadhyay , U . 1996 . Antitumour, immunomodulatory and anti-HIV effects of mangiferin a naturally occurring glucosyl xanthone . Chemotherapy , 42 : 443 – 451 .
- Inderjit . 1996 . Plant phenolics in allelopathy . Botanical Rev , 62 : 186 – 202 .
- Khanna , SK , Viswanathan , PN , Tewari , CP , Krishnan , PS and Sanwal , GG . 1968 . Biochemical aspects of parasitism by angiosperm parasites: phenolics in parasites and hosts . Plant Physiol , 21 : 949 – 959 .
- Lamb , C and Dixon , RA . 1997 . The oxidative burst in plant disease resistance . Annu Rev Plant Physiol Plant Mol Biol , 48 : 251 – 275 .
- Liu , CT , Ching , YW , Yih , MW and Chin , YT . 2005 . Ultrasound-assisted extraction methodology as a tool to improve the antioxidant properties of herbal drug Xiao-chia-hu-tang . J Ethnopharmacol , 99 : 293 – 300 .
- Naik , GH , Priyadarsini , KI and Mohan , H . 2006 . Free radical scavenging reactions and phytochemical analysis of triphala, an ayurvedic formulation . Curr Sci , 90 : 1100 – 1105 .
- Pellati , F , Benvenuti , S , Margo , L , Melegari , M and Soragni , F . 2004 . Analysis of phenolic compounds and radical scavenging activity of Echinacea spp . J Pharm Biomed Anal , 35 : 289 – 301 .
- Pietta , PG . 2000 . Flavonoids as antioxidants . J Nat Prod , 63 : 1035 – 1042 .
- Press , MC , Scholes , JD and Watting , JR . 1999 . “ Parasitic plants: Physiological and ecological response interaction with their hosts ” . In Physiology and plant ecology , Edited by: Press , MC , Scholes , JD and Varker , MA . 175 – 197 . Oxford : Blackwell Sciences .
- Ramchandran , AG and Krishanakumary , P . 1990 . Flavonoids of Dendrophthoe falcata Ettingsh growing on different host plants . Ind J Chem , 29 : 584 – 585 .
- Sampathkumar , R and Selvaraj , R . 1981 . Some new host for Dendrophthoe falcata (Linn. f.) Ettingh. (Loranthus longiflorus Desr.) . J Bombay Nat Hist Soc , 78 : 200 – 203 .
- Sanchez , GM , Re , L , Giuliani , A , Nunez-Selles , AJ , Davison , GP and Leon-Fernandez , OS . 2000 . Protective effects of Mangifera indica L. extract mangiferin and selected antioxidant against TPA-induced biomolecule oxidation and peritoneal macrophages activation in mice . Pharmacol Res , 42 : 565 – 573 .
- Schneider , MJ and Stermitz , FR . 1990 . Uptake of host plant alkaloids by root parasitic Pedicularis spp . Phytochemistry , 29 : 1811 – 1814 .
- Simms , E. 1992 . “ Costs of plant resistance to herbivory ” . In Plant resistance to herbivores and pathogens , Edited by: Fritz , R and Simms , E . 392 – 425 . Chicago : University of Chicago Press .
- Singleton , VL and Rossi , JA . 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . Am J Enol Viticult , 16 : 144 – 158 .
- Stermitz , FR and Harris , GH . 1987 . Transfer of pyrrolizidine and quinolizidine alkaloids to Castilleja (Scrophulariaceae) hemiparasites from composite and legume host plants . J Chem Ecol , 13 : 1917 – 1925 .
- Stermitz , FR and Pomeroy , M . 1992 . Iridoid glycosides from Castilleja purpurea and C. indivisa, and quinolizidines alkaloid transfer from Lupinus texensis to C. indivisa via root parasitism . Biochem Syst Ecol , 20 : 473 – 475 .
- Subramanian , SSG and Nair , R. 1966 . Occurrence of mangiferin in Cuscuta reflexa growing on Mangifera indica . Ind J Chem , 4 : 335 – 336 .
- Suleiman , AK , Hideki , T and Munekazu , I. 1995 . A Xanthone C-Glucoside from Iris nigricans . Phytochemistry , 38 ( 3 ) : 729 – 731 .
- Yamasaki , H , Heshiki , R and Ikehara , N. 1995 . Leaf-goldening induced by high light in Ficus microcarpa L. f., a tropical fig . J Plant Res , 108 : 171 – 180 .
- Yoosook , C , Bunyapraphatsara , N , Boonyakait , Y and Kantasuk , C. 2000 . Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants . Phytomed , 6 : 411 – 419 .
- Zheng , MS and Lu , ZY. 1990 . Antiviral effect of mangiferin and isolated mangiferin on herpes simplex virus . Chinese Med J , 103 : 160 – 165 .