Abstract
The effect of endophytic Pseudomonas fluorescens isolates Endo2 and Endo35 on induced systemic disease protection against dry root rot of black gram (Vigna mungo L. Hepper) caused by Macrophomina phaseolina was investigated under glasshouse conditions. When the bacterized black gram plants were inoculated with dry root rot pathogen, the activities of peroxidase (PO), polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL) were stimulated in addition to accumulation of phenolics and lignin. Activity of phenylalanine ammonia-lyase (PAL) reached the maximum 24 h after pathogen challenge inoculation, whereas the activities of PO and PPO reached the maximum at 72 h and 48 h, respectively. Isoform analysis revealed that a unique PPO3 isozyme was induced in bacterized black gram tissues inoculated with the pathogen. Phenolics were found to accumulate in bacterized black gram tissues challenged with M. phaseolina one day after pathogen challenge. The accumulation of phenolics reached maximum at the third day after pathogen inoculation. Similar observation was found in the lignin content of black gram plants. In untreated control plants, the accumulation of defence enzymes and chemicals started at the first day and drastically decreased 3 days after pathogen inoculation. These results suggest that induction of defense enzymes involved in phenylpropanoid pathway and accumulation of phenolics and PR-proteins might have contributed to restricting invasion of Macrophomina phaseolina in black gram roots.
Introduction
Dry root rot caused by Macrophomina phaseolina (Tassi) Goid is the major constraint to black gram (Vigna mungo L. Hepper) production (Beckman Citation1987, Karthikeyan et al. Citation2003). Among different management practices available, the biological control methods using antagonistic microorganisms have been proved to be a potential alternative or complementary approach to chemical fungicides to combat the disease effectively (Karthikeyan et al. Citation2003, Saravanakumar et al. Citation2003). Biological control employing indigenous endophytic bacterial flora seems to hold a great promise (Chen et al. Citation1995, Nowak et al. Citation1995, Pleban et al. Citation1995, Rangeshwaran & Prasad Citation2000a Citationb). The internal tissues of plants provide a uniform and safe environment for endophytic bacteria. These advantages envisage the use of endophytic bacteria for more successful biological control of plant diseases (Sturtz & Christie Citation1995, Rajappan & Ramaraj Citation1999, Nejed & Johnson Citation2000). Induction of a plant's defence genes by prior application of inducing agents is called induced resistance (Hammerschmidt and Kuc Citation1995). The defence gene products include phenylalanine ammonia-lyase (PAL), peroxidase (PO) and polyphenol oxidase (PPO) that catalyze the formation of phenolics and lignin. Exploitation of the concepts of systemic acquired resistance (SAR) or induced systemic resistance (ISR) to activate the host defence mechanisms may be another potential disease management strategy. Several soil-borne rhizosphere bacteria and fungi have been shown to induce systemic resistance in plants against pathogens (Van Loon et al. Citation1998). Plants are able to defend themselves successfully with a complex set of preformed structures and inducible reactions. The inducible reactions require the perception of either plant-derived (endogenous) or pathogen-derived (exogenous) signal molecules. These elicitors are of diverse chemical nature and include proteins, peptides, glycoproteins, lipids, and oligosaccharides (Nürnberger Citation1999). Elicitors-trigger plant defense responses are part of the basic or non-host resistance of plants (Nürnberger Citation1999). Defence is often associated with localized hypersensitive cell death (Mittler et al. Citation1997) and the de novo formation of antimicrobial compounds called phytoalexins (Hammond-Kosack & Jones Citation1996). The reinforcement of cell wall constituents is also part of the defence response (Bruce & West Citation1989). The structural and cultivar specificity of elicitors and their ability to trigger plant defence responses at very low concentrations strongly suggest the existence of receptors at the plasma membrane and a downstream signal transduction cascade (Ebel & Cosio Citation1994). Various signaling molecules mediate induction of pathogenesis related (PR) gene expression during pathogen infection.
The present study was carried out to evaluate the effectiveness of endophytic Pseudomonas fluorescens isolates in protection of black gram against dry root rot and to assess the induction of defence enzymes involved in the phenylpropanoid pathway and accumulation of PR-proteins in black gram in response to treatment with endophytic P. fluorescens and challenge inoculation with M. phaseolina.
Materials and methods
Plant, pathogen and bacterial isolates
The pathogen Macrophomina phaseolina (Tassi) Goid. was isolated in the laboratory from root rot infected black gram plants (Vigna mungo L. Hepper) using potato dextrose agar (PDA) medium. The infected portions were cut into small pieces (about 2 mm) with a flamed scalpel and two pieces were placed in a dish containing PDA medium and then incubated at 25°C for 3 d. The organism was sub-cultured to obtain a pure culture. Pure cultures were maintained in PDA until needed. Endophytic bacteria Endo2 and Endo35 were isolated from healthy roots black gram cv. Co-5 according to Bhowmik et al. (Citation2002). The strains were identified as Pseudomonas fluorescens according to Bergey's Manual of Systematic Bacteriology (Krieg & Holt Citation1984). The bacterial cultures were maintained in King's B broth (KBB) (King et al. Citation1954) in 30% glycerol at −70°C. From the stock, fresh cultures were prepared on plates of King's B medium (KBM).
Glasshouse trials
The efficacy of endophytic bacterial strains of P. fluorescens in inducing the defence enzymes against dry root rot in black gram was assessed under greenhouse condition. The seeds of the susceptible black gram (cv. Co5) obtained from the Department of Pulses, Tamil Nadu Agricultural University, Coimbatore, were surface sterilized with 2% sodium hypochlorite, rinsed in sterile distilled water and dried overnight under a sterile air stream. Ten milliliters of bacterial inoculum containing 108 cfu ml−1 was taken in a Petri dish. To this, 100 mg of carboxymethylcellulose was added as an adhesive material. One gram of seeds were soaked in 10 ml of bacterial suspension for 2 h and dried overnight in a sterile Petri dish. The potting soil (red soil: sand: decomposed cow dung manure at 1:1:1 w/w/w; available N, P, K, Ca and Mg of red soil were 140, 20, 260, 0.26 and 0.17 kg ha-1, respectively, and pH was 7.1) was sterilized by autoclaving for 1 h on two consecutive days and filled in earthen pots (0.35 m diameter, 0.50 m height). The bacterized seeds of black gram were sown in pots filled with 6 kg of potting soil at a rate of 20 seeds/pot. The pots were placed on the glasshouse benches and maintained at 22–28°C and 60–90% relative humidity (RH). The pathogen was mass multiplied in sand-maize medium (Riker & Riker Citation1936) and inoculated at the rate of 70 g/kg of soil and mixed thoroughly. Plants were watered regularly. Control plants without bacterial treatment were also maintained. Dry root rot incidence was recorded 30 days after sowing using the formula
The experiment was carried out using a completely randomized block design with three replications (five pots per replication; 20 seeds in each pot). The experiment was repeated once.
Sample collection
The roots of Pseudomonas treated black gram plants subjected to fungal infection were collected at 0, 24, 48, 72, 96 and 120 h intervals and various analyses were made. Roots from uninoculated plants, maintained under the same conditions as inoculated plants were collected at same intervals and used as controls. Samples were stored at -80°C until analysed. Samples were analysed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time.
Assay of PO
Root samples (1 g) were homogenized in 2 ml of 0.1 M phosphate buffer, pH 7.0 at 4°C. The homogenate was centrifuged at 16,000 g at 4°C for 15 min and the supernatant was used as enzyme source. The reaction mixture consisted of 1.5 ml of 0.05 M pyrogallol, 0.5 ml of enzyme extract and 0.5 ml of 1% H2O2. The reaction mixture was incubated at room temperature (28±2°C). The changes in absorbance at 420 nm were recorded at 30 sec intervals for 3 min. The enzyme activity was expressed as changes in the absorbance min−1 mg−1 protein (Hammerschmidt et al. Citation1982).
Assay of PPO
PPO activity was determined as per the procedure given by Mayer et al. (Citation1965). Root samples (1 g) were homogenized in 2 ml of 0.1 M sodium phosphate buffer (pH 6.5) and centrifuged at 16,000 g for 15 min at 4°C. The supernatant was used as enzyme source. The reaction mixture consisted of 200 µl of the enzyme extract and 1.5 ml of 0.1 M sodium phosphate buffer (pH 6.5). To start the reaction, 200 µl of 0.01 M catechol was added and the activity was expressed as changes in absorbance at 495 nm min−1 mg−1 protein.
Activity gel electrophoresis for PO and PPO
To study the expression pattern of different isoforms of peroxidases in different treatments, activity gel electrophoresis was carried out by using samples collected 72 h after challenge inoculation. Root samples were homogenized with liquid nitrogen and 1 g of powdered sample was extracted with 2 ml of 0.1 M sodium phosphate buffer, pH 7.0 at 4°C. The homogenate was centrifuged for 20 min at 10,000 rpm. For native anionic polyacrylamide gel electrophoresis, resolving gel of 8% acrylamide concentration and stacking gel of 4% acrylamide concentration were prepared. A total of 100 micrograms equivalent of protein was loaded on each lane. After electrophoresis, the gels were incubated in the solution containing 0.15% benzidine in 6% NH4Cl for 30 min in dark and then drops of 30% H2O2 was added with constant shaking till the bands of peroxidase appear (Sindhu et al. Citation1984). For PPO, activity gel electrophoresis was carried out by using samples collected 48 h after challenge inoculation. One hundred micrograms equivalent of protein was loaded on each lane. After electrophoresis the gel was equilibrated for 30 min in 0.1% p-phenylene diamine in 0.1M potassium phosphate buffer (pH 7.0) followed by 10 mM catechol in the same buffer. The addition of catechol followed by a gentle shaking resulted in appearance of dark brown discrete protein bands (Jayaraman et al. Citation1987). After staining, the gel was washed with distilled water and photographed.
Protein determination
The protein content was determined by Bradford method (Bradford Citation1976) using bovine serum albumin as the standard.
Estimation of phenylalanine ammonia-lyase, phenol and lignin
The PAL assay was conducted as per the method described by Ross and Sederoff (Citation1992) using L-phenylalanine as substrate. Standard curve was drawn with graded amounts of trans-cinnamic acid in toluene as described earlier. The enzyme activity was expressed as ηmoles of trans-cinnamic acid/min/g fresh tissue. Phenol and lignin content of black gram roots were estimated according to Zieslin and Ben-Zaken (Citation1993) and Chesson (Citation1978) respectively. The content of the total soluble phenols was calculated from a standard curve obtained from a Folin-Ciocalteau reagent with a phenol solution (C6H6O) and expressed as catechol equivalents g−1 tissue weight while the lignin content was expressed in terms of percentage on dry weight basis of the tissues.
Statistical analysis
The data were statistically analysed using the IRRISTAT version 92-1 programme developed by biometrics unit at the International Rice Research Institute, Philippines. Values obtained for each variable were analysed by analysis of variance (ANOVA). Mean comparisons were conduced with Fisher's protected least significant difference (LSD, p < 0.05) test.
Results
Dry root rot incidence
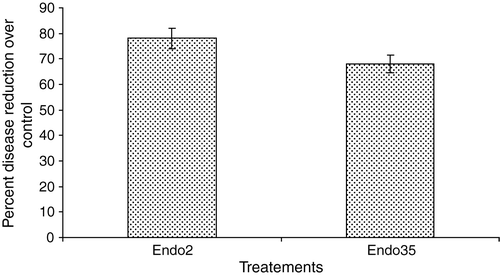
It was generally noticed that dry root rot disease was maximum in the control plants than in the bacterized plants. Application of endophytic P. fluorescens strains Endo2 and Endo35 significantly reduced the incidence of root rot disease and recorded percentage reduction of 78 and 68, respectively. In the control, 97% of the plants were infected with dry root rot pathogen (). When the black gram seeds were treated with endophytic P. fluorescens and sown, both the strains multiplied well in the rhizosphere and the population increased form 10×106 g−1 of soil (on the 15th day) to 13.7×106 g−1 of soil (on the 30th day).
PO and PPO activity
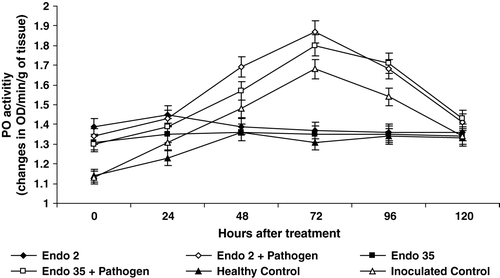
Seed bacterization with P. fluorescens significantly induced the activity of PO in black gram. Among the two strains, P. fluorescens Endo2 induced more PO activity compared to Endo35. Significantly higher PO activity was observed in bacterized plants compared to non-bacterized control plants through out the experimental period. Challenge inoculation in the bacterized plants further increased the PO activity. Maximum induction of PO activity was recorded 72 h after inoculation with M. phaseolina at which period 27% increase over bacterized uninoculated plants was observed ().
Figure 2. Peroxidase activity in black gram against M. phaseolina in response to P. fluorescens isolates Endo2 and Endo35. The enzyme activity was measured calorimetrically. Samples were analysed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time. Bars indicate standard error of the mean and LSD (p = 0.05) = 0.10.
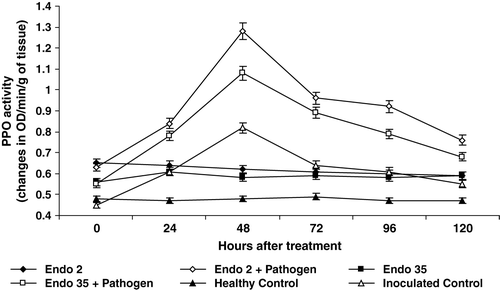
Seed treatment with P. fluorescens significantly induced the activity of PPO in black gram. Among the two strains, P. fluorescens Endo2 induced more PPO compared to Endo35. The PPO activity was significantly higher in bacterized plants compared to non-bacterized control plants through out the experimental period. Challenge inoculation in the bacterized plants further increased the PPO activity. Significant induction in PPO activity was recorded 24 h after challenge inoculation in the bacterized plants. The enzyme activity reached the maximum level 48 h after inoculation with M. phaseolina and then declined. However significantly higher PPO activity was observed in the bacterized and challenge inoculated plants in all sampling days compared to bacterized and uninoculated plants. A 52% increase in PPO activity was recorded 48 h after challenge inoculation with M. phaseolina in the bacterized plants compared to bacterized uninoculated plants ().
Figure 3. Polyphenol oxidase activity in black gram against M. phaseolina in response to P. fluorescens isolates Endo2 and Endo35. The enzyme activity was measured calorimetrically. Samples were analyzed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time. Bars indicate standard error of the mean and LSD (p = 0.05) = 0.05.
Isozyme patterns of PO and PPO
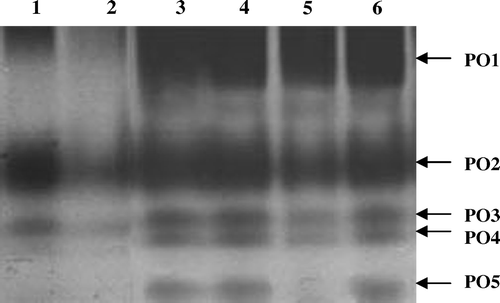
Inoculation with M. phaseolina in black gram induced the activities of PO1, PO2 and PO3. Seed bacterization of black gram with P. fluorescens Endo2 induced the activities of five isozymes (PO1, PO2 PO3, PO4 and PO5), whereas P. fluorescens Endo35 induced the activities of only four isozymes (PO1, PO2, PO3 and PO4). Challenge inoculation with M. Phaseolina in the P. fluorescens Endo35 bacterized plants induced peroxidase isozyme PO5, whereas no significant change in peroxidase isozyme pattern was observed in P. fluorescens Endo2 bacterized plants upon challenge inoculation with M. phaseolina ().
Figure 4. Native PAGE profile of PO induced in response to challenge inoculation of M. phaseolina in black gram. One hundred micrograms equivalent of protein was loaded on each lane. Lane 1, Control challenged with pathogen; Lane 2, Healthy control; Lane 3, Endo2 challenged with pathogen; Lane 4, Endo2 alone treated plants; Lane 5, Endo35 alone treated plants; Lane 6, Endo35 challenged with pathogen.
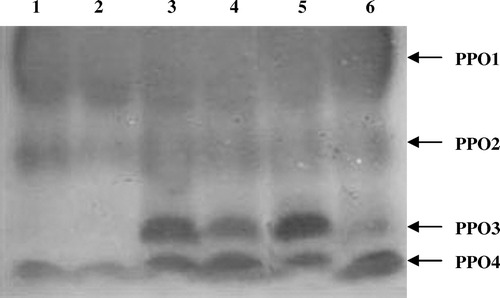
Inoculation with M. phaseolina in black gram induced the activities of PPO1, PPO2 and PPO4. Seed bacterization of black gram with both endophytic P. fluorescens strains induced the activity of another PPO isozyme (PPO3). However, the expression of PPO3 was more in black gram plants bacterized with Endo2 when compared with Endo35 bacterized plants. Challenge inoculation with M. Phaseolina in the P. fluorescens bacterized plants further induced the isozyme PPO3 ().
Figure 5. Native PAGE profile of PPO induced in response to challenge inoculation of M. phaseolina in black gram. One hundred micrograms equivalent of protein was loaded on each lane. Lane 1, Control challenged with pathogen; Lane 2, Healthy control; Lane 3, Endo2 challenged with pathogen; Lane 4, Endo2 alone treated plants; Lane 5, Endo35 challenged with pathogen; Lane 6, Endo35 alone treated plants.
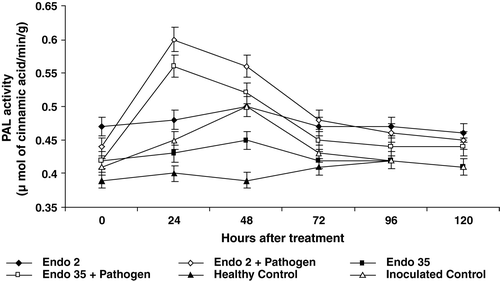
Activity of PAL
Black gram plants bacterized with P. fluorescens strains Endo2 and Endo35 showed elevated levels of PAL activity throughout the experimental period. Changes in inoculation with M. phaseolina in the bacterized plants further increased PAL activity. Among the two strains of P. fluorescens, Endo2 bacterized plants when challange inoculated with M. phaseolina exhibits a higher PAL activity compared to Endo35 bacterized plants. Maximum PAL activity observed 24 h after inoculation, later on enzyme activity decline. A 20 percent increase in PAL activity was recorded 24 h after challenge inoculation in Endo2 bacterized plant compared to uninoculated bacterized plants ().
Figure 6. Phenylalanine ammonia lyase activity in black gram against M. phaseolina in response to P. fluorescens isolates Endo2 and Endo35; (a) without challenge inoculation; (b) after challenge inoculation. The enzyme activity was measured calorimetrically. Samples were analysed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time. Bars indicate standard error of the mean and LSD (p = 0.05) = 0.03.
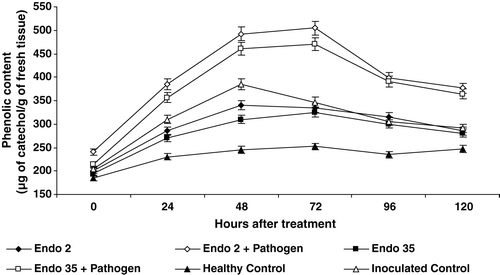
Phenolics content
Black gram plants bacterized with P. fluorescens strains Endo2 and Endo35 showed elevated levels of phenol activity throughout the experimental period. Changes in inoculation with M. phaseolina in the bacterized plants further increased phenol activity. Significant induction in phenol activity was recorded 24 h after challenge inoculation in the bacterized plants. The enzyme activity reached the maximum level 72 h after inoculation with M. phaseolina and then declined. However significantly higher phenol activity was observed in the bacterized and challenge inoculated plants in all sampling days compared to bacterized and uninoculated plants. A 34% increase in phenol activity was recorded 72 h after challenge inoculation with M. phaseolina in the bacterized plants compared to bacterized uninoculated plants ().
Figure 7. Phenol content in black gram against M. phaseolina in response to P. fluorescens isolates Endo2 and Endo35. The enzyme activity was measured calorimetrically. Samples were analysed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time. Bars indicate standard error of the mean and LSD (p = 0.05) = 5.25.
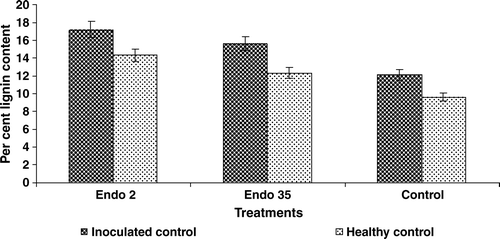
Lignin content
Inoculation with M. phaseolina significantly increased accumulation of lignin in black gram roots in the third day after challenge inoculation. Seed bacterized with P. fluorescens Endo35 also induced accumulation of lignin. A maximum percentage increase in lignin content was observed in black gram plants bacterized with Endo2 compared to the plants bacterized with Endo35. Challenge inoculation with M. phaseolina in the bacterized plants further increased accumulation of lignin ().
Figure 8. Lignin content in black gram against M. phaseolina in response to P. fluorescens isolates Endo2 and Endo35. The enzyme activity was measured calorimetrically. Samples were analysed thrice, and the experiment was performed twice. The treatment values from two independent experiments were averaged and plotted by time. Bars indicate standard error of the mean and LSD (p = 0.05) = 2.71.
Discussion
Plants can acquire enhanced resistance to pathogens after treatment with necrotizing pathogens, non-pathogenic root-colonizing pseudomonads, salicylic acid, β-aminobutyric acid and many other natural or synthetic compounds. The induced resistance is often associated with an enhanced capacity to mobilize infection-induced cellular defence responses (Conratha et al. Citation2002). The results of the present study revealed that treatment of black gram with Endo2 isolate of the P. fluorescens offered protection against dry root rot caused by M. phaseolina. Our results also demonstrated that infection with M. phaseolina strongly induced phenolics and lignin accumulation and the activities of PAL, PO and PPO in black gram tissues. Besides direct antagonistic activity by the production of various bacterial metabolites, induction of systemic resistance by fluorescent pseudomonads against diseases has been established as a mechanism by which the plants defend themselves from pathogen attack (Van Loon et al. Citation1998). Prior application of fluorescent pseudomonads, as seed treatment, induces various defence mechanisms in plants (Chen et al. Citation2000). During root colonization, these bacteria produce antifungal antibiotics, elicit induced systemic resistance in the host plant or interfere specifically with fungal pathogenicity factors (Haas & Defago Citation2005).
Induced resistance by inducing agents in several crops is known to be associated with enhancement of lignification and also increased activities of enzymes involved in phenylpropanoid pathway and PR protein synthesis (Boller & Mauch Citation1988, Hammerschmidt & Kuc Citation1995). Recent studies implies that prior application of fluorescent pseudomonads strengthen host cell wall structures resulting in restriction of pathogen invasion in plant tissue (Chen et al. Citation2000).
Peroxidases represent another component of an early response of plants to pathogen attack and play a key role in the biosynthesis of lignin, which limits the extent of pathogen spread (Bruce & West Citation1989). The products of this enzyme, in the presence of hydrogen donor and hydrogen peroxidase, have antimicrobial activity and even antiviral activity (Van Loon & Callow Citation1983). In bean, rhizosphere colonization of various bacteria induced the peroxidase activity (Zdor & Anderson Citation1992). In the present study, seed bacterization with P. fluorescens significantly induced the activity of PO in black gram. Among the two strains, P. fluorescens Endo2 induced more PO activity compared to Endo35. Significantly higher PO activity was observed in bacterized plants compared to non-bacterized control plants through out the experimental period. Chen et al. (Citation2000) reported higher PO activity in cucumber roots treated with P. corrugata challenged with P. aphanidermatum. In the isozyme study, inoculation with M. phaseolina in black gram induced the activities of PO1, PO2 and PO3. Seed bacterization of black gram with P. fluorescens Endo2 induced the activities of five isozymes (PO1, PO2 PO3, PO4 and PO5). The induced activities of PO in black gram plants upon treatment with P. fluorescens might have involved in lignin biosynthesis which in turn might have contributed for resistance against invasion by M. phaseolina.
Similar to peroxidase, seed treatment with P. fluorescens significantly induced the activity of PPO in black gram. The PPO activity was significantly higher in bacterized plants compared to non-bacterized control plants through out the experimental period. In case of isozyme studies, inoculation with M. phaseolina in black gram induced the activities of PPO1, PPO2 and PPO4. Seed bacterization of black gram with both endophytic P. fluorescens strains induced the activity of another PPO isozyme (PPO3). Chen et al. (Citation2000) reported that various rhizobacteria and P. aphanidermatum induced PPO activity in cucumber root tissues. The induced PPO might have involved in the resistance of black gram plants to root rot through oxidation of phenolic compounds to fungitoxic quinones.
PAL is the key enzyme of phenylpropanoid metabolism in higher plants which catalyzes the conversion of phenylalanine to trans-cinnamic acid which supplies the precursors for flavonoid pigments, lignins and phytoalexins (Daayf et al. Citation1997). Several studies indicated that the activation of PAL and subsequent increase in phenolic content in plants is a general response associated with disease resistance. Inhibition of PAL affects subsequent pathways of phenolic compound synthesis. Carver et al. (Citation1991) demonstrated that the inhibition of PAL activity in oats by treatment with the PAL inhibitors α-aminooxy-β-phenylpropionic acid and α-aminooxyacetic acid increased the susceptibility of oat tissue to the fungus Erysiphe graminis and significantly reduced the level of the autofluorescence response of the host cell. De Meyer et al. (Citation1999) reported that rhizosphere colonization by P. aeruginosa 7NSK2 activated PAL in bean roots and increased the salicylic acid levels in leaves. In the present study, black gram plants bacterized with P. fluorescens strains Endo2 and Endo35 showed elevated levels of PAL activity throughout the experimental period. Changes in inoculation with M. phaseolina in the bacterized plants further increased PAL activity. Among the two strains of P. fluorescens, Endo2 bacterized plants when inoculated with M. phaseolina exhibits a higher PAL activity compared to Endo35 bacterized plants. The time required to activate the defence mechanisms is important for pathogen suppression. Earlier and higher level of expression of defence enzymes and accumulation of chemicals at the infection site certainly prevent the fungal mycelial colonization. In black gram, the activity of PAL was more in bacterized plants compared to control plants in all sampling days. Upon inoculation with the pathogen, PAL activity rapidly increased and reached the maximum 24 h after inoculation. It has been well documented that P. fluorescens Pf1 induced the PAL activity in rice (Meena et al. Citation1999) and groundnut (Meena et al. Citation2000). Induction of PAL by fluorescent pseudomonads was reported in cucumber against P. aphanidermatum (Chen et al. Citation2000) and in bean against Botrytis cinerea (Zdor & Anderson Citation1992).
Phenolic compounds may also contribute to enhance the mechanical strength of host cell wall and may also inhibit the fungal growth, also because of the fungitoxic in nature of some phenolics. Seed treatment with P. fluorescens 63–28 induced the accumulation of phenolics in tomato root tissues (M'Piga et al. Citation1997). The hyphae of the pathogen surrounded by phenolic substances exhibited considerable morphological changes including cytoplasmic disorganization and loss of protoplasmic content. Accumulation of phenolics by prior application of P. fluorescens in pea has been reported against P. ultimum and F. oxysporum f. sp. pisi (Benhamou et al. Citation1996). The present study also indicates that the highest level accumulation of phenolics was observed in P. fluorescens Endo2 treated black gram plants challenged with the pathogen. Similar findings were reported in rice against R. solani (Meena et al. Citation1999), sugarcane against C. falcatum (Viswanathan & Samiyappan Citation1999) and in groundnut against C. personatum (Meena et al. Citation2000). Hence, the increase in phenolic content in black gram roots following treatment with P. fluorescens might be due to its increased synthesis. Since the production of phenolic compounds depends upon PAL activity (Graham & Graham Citation1991), increase in phenolic content in bacterized roots might be due to increased activity of PAL.
Based on these findings it can be concluded that induction of defence enzymes involved in phenylpropanoid pathway upon treatment with P. fluorescens Endo2 in black gram might lead to induced protection against pathogen infection by synthesizing various defence compounds. Earlier and enhanced activities of PAL, PO and PPO might have involved in the synthesis of phenols and accumulation of lignin which might have suppressed the further colonization of M. phaseolina in black gram. Fluorescent pseudomonads are known to produce lipopolysaccharides (LPS) and salicylic acid which act as local or systemic signal molecules in inducing resistance in plants (Van Peer & Schippers Citation1992, Chen et al. Citation1999). Defence mechanisms in plants can also be induced by factors present in the cell walls of fungi known as “elicitors” (Anderson-Prouty & Albersheim Citation1975, Ebel & Cosio Citation1994, Velazhahan & Vidhyasekaran Citation2000). Such elicitor molecule has been isolated and partially purified from the mycelial walls of M. phaseolina which induced various defence enzymes in suspension-cultured cells of black gram (Ramanathan et al. Citation2000). The elicitor molecules present in M. phaseolina might have induced the expression of defence-related enzymes in black gram. The present studies suggest that the P. fluorescens strains Endo2 and Endo35 might have released a signal and this signal in combination with a signal released by M. phaseolina might have enhanced the defence-related activities in black gram to confer resistance against M. phaseolina.
The senior author is grateful to the Council of Scientific and Industrial Research, New Delhi for the financial assistance provided for the study.
References
- Anderson-Prouty , AJ and Albersheim , P . 1975 . Host-pathogen interactions. VIII. Isolation of a pathogen-synthesized fraction rich in glucan that elicits a defense response in the pathogen's host . Plant Physiol , 56 : 286 – 291 .
- Beckman , CH . 1987 . Nature of wilt diseases. American Phytopathological Society , 174 St Paul, MN : American Phytopathological Society .
- Benhamou , N , Belanger , RR and Paulitz , TC . 1996 . Induction of differential host responses by Pseudomonas fluorescens in Ri T-DNA-transformed pea roots after challenge with Fusarium oxysporum f. sp. pisi and Pythium ultimum . Phytopathology , 86 : 114 – 178 .
- Bhowmik , B , Singh , RP , Jayaraman , J and Verma , JP . 2002 . Population dynamics of cotton endophytic Pseudomonas, their antagonism and productive action against the major pathogen of cotton . Indian Phytopathol , 55 : 124 – 132 .
- Boller , T and Mauch , F . 1988 . Colorimetric assay for chitinase . Meth Enzymol , 161 : 430 – 435 .
- Bradford , HC . 1976 . A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding . Anal Chem , 72 : 248 – 254 .
- Bruce , RJ and West , CA . 1989 . Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean . Plant Physiol , 91 : 889 – 897 .
- Carver , TLW , Robbins , MP and Zeyen , RJ . 1991 . Effects of two PAL inhibitors on the susceptibility and localized autofluorescent host cell responses of oat leaves attacked by Erysiphe graminis DC . Physiol Mol Plant Pathol , 39 : 269 – 287 .
- Chen , C , Bauske , EM , Musson , G , Rodriguezkabana , R and Kloepper , JW . 1995 . Biological control of fusarium wilt in cotton by use of endophytic bacteria . Biol Control , 5 : 83 – 91 .
- Chen , C , Belanger , RR , Benhamou , N and Paulitz , TC . 1999 . Role of salicylic acid in systemic resistance induced by Pseudomonas spp. against Pythium aphanidermatum in cucumber roots . Eur J Plant Pathol , 105 : 477 – 486 .
- Chen , C , Belanger , RR. , Benhamou , N and Paullitz , TC . 2000 . Defense enzymes induced in cucumber roots by treatment with plant-growth promoting rhizobacteria (PGPR) . Physiol Mol Plant Pathol , 56 : 13 – 23 .
- Chesson , A . 1978 . The maceration of lignin in flax under anaerobic conditions . J Appl Bacteriol , 45 : 219 – 230 .
- Conratha , U , Corne , M , Pieterseb , J and Mauch-Mani , BC . 2002 . Priming in plant–pathogen interactions . Trends Plant Sci , 7 : 210 – 216 .
- Daayf , F , Bel-Rhlid , R and Bélanger , RR . 1997 . Methyl ester of p-coumaric acid: A phytoalexin-like compound from long English cucumber leaves . J Chem Ecol , 23 : 1517 – 1526 .
- De Meyer , G , Capieau , K , Audenaert , K , Buchala , A , Metraux , JP and Hofte , M . 1999 . Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean . Mol Plant-Microbe Interact , 12 : 450 – 458 .
- Ebel , J and Cosio , EG . 1994 . Elicitors of plants defense responses . Int Rev Cytol , 148 : 1 – 36 .
- Graham , MY and Graham , TL . 1991 . Rapid accumulation of anionic peroxidase and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan . Plant Physiol , 97 : 1145 – 1155 .
- Haas , D and Defago , G . 2005 . Biological control of soil-borne pathogens by fluorescent pseudomonads . Nat Rev Microbiol , 3 : 307 – 319 .
- Hammerschmidt , R and Kuc , J . 1995 . Induced resistance to disease in plants , 182 Dordrecht, , The Netherlands : Kluwer Academic Publishers .
- Hammerschmidt , R , Nuckles , EM and Kuc , J . 1982 . Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium . Physiol Plant Pathol , 20 : 73 – 82 .
- Hammond-Kosack , KE and Jones , JDG . 1996 . Resistance gene-dependent plant defense responses . Plant Cell , 8 : 1773 – 1791 .
- Jayaraman , KS , Ramanuja , MN , Vijayarahavan , PK and Vaidyanathan , CS . 1987 . Oxidative enzyme in pearl millet . Food Chem , 24 : 203
- Karthikeyan M , Bhaskaran R , Kandan A , Radjacommare R , Ramanathan A , Samiyappan R 2003 . Endophytic bacteria for biological control of dry root rot (Macrophomina phasiolina) in black gram . In: 6th International PGPR workshop 5–10 October 2003 . Calicut , India . Pp 146 – 152 .
- King , EO , Ward , MK and Raney , DE . 1954 . Two simple media for the demonstration of pyocyanin and fluorescein . J Lab Clin Med , 44 : 301 – 307 .
- Krieg NR , Holt JG 1984 . Bergey's manual of systematic bacteriology , 9th ed. Vol. I . Baltimore : The Williams and Wilkins Co .
- Mayer , AM , Harel , E and Shaul , RB . 1965 . Assay of catechol oxidase, a critical comparison of methods . Phytochemistry , 5 : 783 – 789 .
- Meena , B , Radhajeyalakshmi , R , Marimuthu , T , Vidhyasekaran , P , Doraisamy , S and Velazhahan , R . 2000 . Induction of pathogenesis-related proteins, phenolics and phenylalanine ammonia lyase in groundnut by Pseudomonas fluorescens . J. Plant Dis Protect , 107 : 514 – 527 .
- Meena , B , Radhajeyalakshmi , R , Vidhyasekaran , P and Velazhahan , R . 1999 . Effect of foliar application of Pseudomonas fluorescens on phenylalanine ammonia lyase, chitinase and β-1,3-glucanase and accumulation of phenolics in rice . Acta Phytopathol Entomol Hungarica , 34 : 307 – 315 .
- Mittler , R , Simon , L and Lam , E . 1997 . Pathogen-induced programmed cell death in tobacco . J Cell Sci , 110 : 1333 – 1344 .
- M'Piga , P , Belanger , RR , Paulitz , TC and Benhamou , N . 1997 . Increased resistance to Fusarium oxysporum f.sp. radicis-lycopersici in tomato plants treated with endophytic bacterium Pseudomonas fluorescens strain 63–28 . Physiol. Mol Plant Pathol , 50 : 301 – 320 .
- Nejed , P and Johnson , PA . 2000 . Endophytic bacteria induced growth promotion and wilt disease suppression in oilseed rape and tomato . Biol Control , 18 : 208 – 215 .
- Nowak , J , Asiedu , SK , Lazarovits , G , Pillay , V , Stewart , A , Smith , C and Liu , Z . 1995 . “ Enhancement of in vitro growth and transplant stress tolerance of potato and vegetable plantlets co cultured with the plant growth promoting pseudomonas bacterium ” . In Ecophysiology and photosynthetic in vitro cultures , Edited by: Carre , F and Cagvardieff , P . 173 – 179 . France : Comissarat a lenergie atomique .
- Nürnberger , T . 1999 . Signal perception in plant pathogen defense . Cell Mol Life Sci , 55 : 167 – 182 .
- Pleban , S , Ingel , F and Chet , I . 1995 . Control of Rhizoctonia solani and Sclerotium rolfsii in the green house using endophytic bacteria . Eur J Plant Path , 101 : 665 – 672 .
- Rajappan , K and Ramaraj , B . 1999 . Evaluation of fungal and bacterial antagonists against Fusarium moniliformae causing wilt of cauliflower . Ann Plant Prot Sci , 7 : 205 – 207 .
- Ramanathan , A , Vidhasekaran , P and Samiyappan , R . 2000 . Induction of defense mechanisms in greengram leaves and suspension-cultured cells by Macrophomina phaseolina and its elicitors . J Plant Dis. Prot , 107 : 245 – 257 .
- Rangeshwaran , R and Prasad , RD . 2000a . Induction of defense mechanisms in greengram leaves and suspension-cultured cells by Macrophomina phaseolina and its elicitors . J Biol Control , 14 : 9 – 15 .
- Rangeshwaran , R and Prasad , RD . 2000b . Biological control of Sclerotium rots of sunflower . Indian Phytopath , 53 : 444 – 449 .
- Riker , AJ and Riker , RS . 1936 . Introduction to research on plant diseases , 117 New York : John Swift .
- Ross , WW and Sederoff , RR . 1992 . Phenylalanine ammonia lyase from loblolly pine: Purification of the enzyme and isolation of complementary DNA clones . Plant Physiol , 98 : 380 – 386 .
- Saravanakumar D , Lavanya N , Vivekanandan R , Loganathan M , Ramanathan A , Samiyappan R 2003 . PGPR mediated induced systemic resistance (ISR) in mung bean against Macrophomina root rot disease black gram . In 6th International Workshop on PGPR , Calicut , India . pp 146 – 152 .
- Sindhu , JS , Ravi , S and Minocha , JL . 1984 . Peroxidase isozyme patterns in primary trisomics of pearl millet . Theor Appl Genet , 68 : 179 – 182 .
- Sturtz , AV and Christie , BR . 1995 . Endophytic bacterial system governing red clover growth and development . Ann Appl Biol , 126 : 285 – 290 .
- Van Loon , LC , Bakker , PAHM and Pieterse , CMJ . 1998 . Systemic resistance induced by rhizosphere bacteria . Ann Rev Phytopathol , 36 : 453 – 483 .
- Van Loon , LC and Callow , JA . 1983 . “ Transcription and translation in the diseased plant ” . In Biochemical plant pathology , Edited by: Callow , JA . Chichester, , UK : John Wiley and Sons .
- Van Peer , R and Schippers , B . 1992 . Lipopolysaccharides of plant growth–promoting Pseudomonas sp. strain WCS 417r induce resistance in carnation to fusarium wilt . Neth J Plant Pathol , 98 : 129 – 139 .
- Velazhahan , R and Vidhyasekaran , P . 2000 . Isolation of an elicitor from R. solani, the rice sheath blight pathogen which activates phenylpropanoid metabolism in suspension-cultured rice cells . J Pland Dis Prot , 107 : 135 – 144 .
- Viswanathan , R and Samiyappan , R . 1999 . Induction of systemic resistance by plant growth promoting rhizobacteria against red rot disease caused by Colletotrichum falcatum Went in sugarcane . Sugar Tech , 1 : 67 – 76 .
- Zdor , RE and Anderson , AJ . 1992 . Influence of root colonizing bacteria on the defense responses in bean . Plant Soil , 140 : 99 – 107 .
- Zieslin , N and Ben-Zaken , R . 1993 . Peroxidase activity and presence of phenolic substances in peduncles of rose flowers . Plant Physiol Biochem , 31 : 333 – 339 .