Abstract
Legume plants enter two important endosymbioses – with soil fungi, forming phosphorus acquiring arbuscular mycorrhiza (AM), and with nitrogen-fixing bacteria, leading to the formation of nitrogen-fixing root nodules. Both symbioses have been studied extensively because these symbioses have great potential for agricultural applications. Although 80% of all living land plants form AM, the nitrogen-fixing root nodule symbiosis with rhizobia is almost exclusively restricted to legumes. Despite varying degree of differences in the morphological responses induced by both endosymbionts in the host plants, significant similarities in the development of both fungal and bacterial symbioses have been reported. The signal perception and signal transduction cascades that initiate nodulation and mycorrhization in legumes partially overlap. Legume genes have been identified that are required for the establishment of both AM and root nodule symbiosis and are referred to as the common SYM genes. Genetic dissection of the common SYM signal transduction pathway required for bacterial and fungal root endosymbiosis has not only unraveled the players involved but also provided a first glimpse at conservation and specialization of signaling cascades essential for nodulation and mycorrhiza development. Based on the observation of common signaling cascades, it is tempting to speculate that the root nodule symbiosis, where fossil records date back to the late Cretaceaous, adopted and subsequently modified more ancient signal transduction pathways leading to AM formation, having already been in place 400 million years ago. This review discusses the common aspects of recognition of mycorrhizal fungi and Rhizobium by the host, and further signal transduction that leads to an effective symbiosis.
Keywords:
Introduction
Plant roots are exposed to a range of soil microorganisms with which they form a variety of interactions. Some interactions are beneficial whereas some are detrimental for the plant. The microorganisms grow on plants as a food resource or habitat niche. In one such symbiotic interaction, the roots of many plants are infected by certain fungi (mycorrhizal association) that help them acquire phosphate from the soil (Smith & Read Citation1997, Smith et al. Citation2003). In return for its services, the fungus is provided with a carbon source, derived from plant photosynthesis, for biosynthesis and energy metabolism. In arbuscular mycorrhizae, arbuscules are the major site of nutrient exchange and mycorrhiza-specific plant phosphate transporters are exclusively localized at the arbuscular interface (Harrison et al. Citation2002). Arbuscules are branched, microscopic haustorial structures of the fungal symbiont that form within living cortical cells of the root. In the other key agricultural symbiosis, rhizobial bacteria set up house-keeping in the roots of legumes, such as pea, soybeans, and alfalfa, where the plant provides its beneficial endosymbiont with photosynthate, together with other nutrients, in exchange for valuable fixed nitrogen in the form of ammonium and amino acids (Udvardi & Day Citation1997). Mycorrhiza and Rhizobia are natural mini-fertilizer factories that are an economical and safer source of plant nutrition than synthetic chemical fertilizers, which contribute substantially to environmental pollution. These associations can increase agricultural production and improve soil fertility and therefore have great potential as a supplementary, renewable, and environment friendly source of plant nutrients. By providing essential nutrients, these microbes help both their host plants and the world's agricultural systems (Marx Citation2004). Newman and Reddell (Citation1987) have reported that from 202 species of legumes, 90% had been found to form mycorrhizal symbiosis. Arbuscular mycorrhizal (AM) association enhances legume growth and nitrogen fixation by improved phosphorus (P) nutrition. Nitrogen and phosphorus concentration and nodule formation increase with the mycorrhizal inoculum in the soil (Azcon & El-Atrash Citation1997). Both interactions are extreme in terms of host specificity. Whereas nodulation is almost exclusively restricted to legumes and requires the organogenesis of a root nodule (Schultze & Kondorosi Citation1998, Vessey et al. Citation2005, Küster et al. Citation2007), in AM formation there is very little host specificity and more than 80% of terrestrial plants enter into symbiosis with fungi of the phylum Glomeromycota (Schussler et al. Citation2001, Garg et al. Citation2006). Organogenesis does not occur in AM, but the root system can be heavily affected in its development, structure and differentiation (Berta et al. Citation1990, Schellenbaum et al. Citation1991, Hooker & Atkinson Citation1992, Berta et al. Citation1995, Forbes et al. Citation1996, Norman et al. Citation1996). Changes frequently include a more extensive and branched root system, with a larger proportion of smaller diameter, higher order roots (Berta et al. Citation2002, Gamalero et al. Citation2002).
Although symbiotic microbes colonize root tissues intracellularly during nodule and AM endosymbioses, they stay separated from the plant cytoplasm by the highly specialized perisymbiotic membranes (Provorov et al. Citation2002). Comparable to root nodules, where nutrient exchange takes place across the perisymbiotic membrane surrounding the nitrogen-fixing bacteroids (Day et al. Citation2001), solute exchange during AM occurs at the perisymbiotic membranes that surround the arbuscules (Gianinazzi-Pearson Citation1996, Harrison Citation1999a, Parniske Citation2000).
In both interactions, the two partners engage in a complex molecular conversation that allows the microbes to infect the plant cells and then entice the cells to undergo the developmental changes necessary for establishing the symbioses. In addition to clarifying the molecular underpinnings of these symbioses, the findings are shedding light on plant evolution. Detection of fungi in fossilized plants indicates that the association with mycorrhizal fungi was important for the evolution of land plants in mid-Paleozoic (480–360 mya) (Simon et al. Citation1993, Remy et al. Citation1994, Read et al. Citation2000, Lum & Hirsch Citation2003). By contrast, the N2-fixing symbiosis in legumes is much more recent, with nodulation presumably originating in the early Tertiary period during the Cretaceous (60–70 mya) (Lum & Hirsch Citation2003). This has long led plant researchers to suspect that rhizobia might have made use of some of the same plant machinery as used by the fungi to establish their symbioses – a hypothesis supported by the recent molecular findings showing that some of the same plant genes involved in rhizobial invasions are needed for the establishment of fungal infections (Kistner & Parniske Citation2002, Marx Citation2004).
Successful establishment of symbiosis is accompanied by various biological processes: signal transduction, organogenesis and maintenance of symbiotic structure, metabolite exchange between host and symbiont, senescence and turnover of symbiotic structures. Host development during both AM and root nodule symbiosis is triggered by the exchange of different signaling molecules but several signal transduction components are shared (Parniske Citation2004, Harrison Citation2005, Oldroyd et al. Citation2005, Takeda et al. Citation2007). This is evident from genetic screening in several legume species, where mutants have been identified, which are defective in root nodule symbiosis, and also exhibit defects in arbuscular mycorrhizal symbiosis (Harrison Citation2005). Common symbiosis mutants have been identified in Medicago sativa, Pisum sativum, Lotus japonicus, Medicago truncatula, Phaseolus vulgaris, Vicia faba, and Melilotus alba (Duc et al. Citation1989, Bradbury et al. Citation1991, Sagan et al. Citation1995, Shirtliffe & Vessey Citation1996, Schauser et al. Citation1998, Szczyglowski et al. Citation1998, Wegel et al. Citation1998, Catoira et al. Citation2000, Senoo et al. Citation2000, Kawaguchi et al. Citation2002, Lum et al. Citation2002). This implies that the evolutionary more recent root nodule symbiosis evolved by recruiting a subset of genes involved in the older AM symbiosis (LaRue & Weeden Citation1994). The genes, required for both AM and root nodule symbioses, are referred to as common symbiosis (sym) genes (Stougaard Citation2001, Kistner & Parniske Citation2002) and at least seven common sym genes exist in legumes (Kistner et al. Citation2005). Several common sym genes have been cloned. Among them, receptor kinases, putative channel proteins and nucleoporins contribute to a common symbiosis pathway (Endre et al. Citation2002, Stracke et al. Citation2002, Ané et al. Citation2004, Lévy et al. Citation2004, Imaizumi-Anraku et al. Citation2005, Kanamori et al. Citation2006).
Early signaling and microbial entrance into the plant
The development of both the fungal and bacterial symbioses can be divided into an analogous sequence of events: (i) Attraction, (ii) recognition, (iii) contact, (iv) entrance, and (v) development and differentiation ().
Figure 1. Schematic representation of the analogous sequence of events in the progression of AM fungal and Rhizobium-legume symbioses. Rhizobia get entrapped within a curled root hair and AM fungal hyphae develop appressoria on the surface of the root, both the microbes penetrate the host cells and the final step involves the differentiation of fungal hyphae into arbuscules and that of Rhizobium into nitrogen-fixing bacteroids housed in the cells of the nodule.
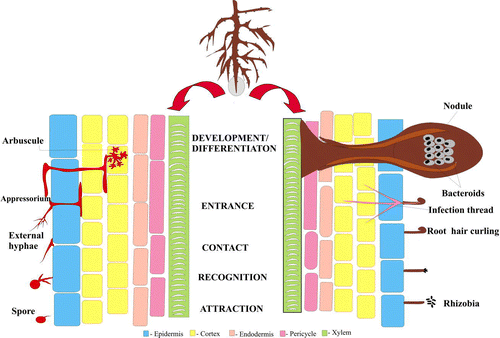
There are clear similarities between the symbioses at each of these steps. Flavonoids, the molecules secreted by plant roots and seeds induce rhizobial nod genes as well as AM fungal spore germination and hyphal colonization. In both bacterial and fungal symbioses, an increase in flavonoid levels is seen within the plant root in response to colonization. In the Rhizobium symbiosis, this is referred to as the Ini (increase in nod gene inducing flavonoids) response because there is an increase in the flavonoids, which in a positive feedback loop induce bacterial nod genes (Recourt et al. Citation1992). Increased flavonoid biosynthesis is also detected in mycorrhizal roots. α-phenylalanine ammonia-lyase (PAL) and chalcone synthase transcripts were upregulated in Medicago truncatula in response to colonization by Glomus versiforme, localizing in cells containing arbuscules (Harrison & Dixon Citation1993, Citation1994). Some flavonoids and phenolic compounds have been shown to stimulate the spore germination and hyphal growth of AM fungi (Vierheilig et al. Citation1998, Akiyama et al. Citation2002). Furthermore, it was found that an enhancement of mycorrhizal colonization seen in response to Nod factor was correlated with an enhancement of specific flavonoids known to stimulate fungal colonization (Xie et al. Citation1995). However, recently, strigolactones (a group of sesquiterpenes) exuded from host roots have been identified as an inducer of hyphal branching in AM fungi. These signaling molecules called ‘branching factors’ are released by host roots and induce extensive hyphal branching in AM fungi (Akiyama et al. Citation2005, Akiyama & Hayashi Citation2006, Paszkowski Citation2006a). Upon induction of their nod genes, rhizobia synthesize nod factor, a lipooligosaccharide signal molecule that is more typical of fungal cell wall than of Gram-negative bacteria (Lum et al. Citation2002). Ca2 + signaling is an important step during both symbioses (Oldroyd & Downie Citation2004, Navazio et al. Citation2006).
Attachment and penetration of AM fungi or rhizobia is a multi-step procedure that involves varying degrees of host participation. Physiologically, the details of the internalization of the fungus or bacteria show the greatest similarity (Lum & Hirsch Citation2003). The plant does not appear to assist in the penetration of the fungus through the plant cell wall and therefore, it is thought that as with the pathogenic fungi, AM fungi exert mechanical pressure in order to enter. The appressorium may serve that function. In addition, degradative enzymes such as exo- and endoglucanases, cellulases, xyloglucanases, and pectolytic enzymes are produced, all of which could assist in the degradation and penetration of the cell wall (Harrison Citation1999b). Rhizobium bacteria also release hydrolytic enzymes that act on the plant cell wall (Martinez-Molina et al. Citation1979). Specialized interfaces develop between the plant and fungal hyphae or bacteroids. The periarbuscular interface carries xyloglucans, nonesterified polygalaturonans, arabinogalactans and hydroxyproline-rich glycoproteins, which are characteristics of the plant cortical cell walls and similar to components in pea bacteroid compartments (Perotto et al. Citation1990, Lum & Hirsch Citation2003). Furthermore, microsymbionts are surrounded by a plant-derived membrane within the plant cell, the perihaustorial membrane around arbuscules or the peribacteroid membrane around the bacteroids in the root nodules. Wyss et al. (Citation1990) reported that polypeptides, immunologically cross-reactive with nodules-specific plant proteins (‘nodulins’), are also specifically induced in mycorrhizal plant roots, i.e., are ‘mycorrhizins’. Genre et al. (Citation2005) targeted roots with Gigaspora hyphae and reported that, before infection, the epidermal cell assembles a transient intracellular structure with a novel cytoskeletal organization- pre-penetration apparatus (PPA). First, the nucleus rapidly migrates to a position just below the appressorium, then it moves away, leaving behind it an aggregation of microtubules, actin microfilaments, and ER cisternae. This aggregation becomes organized into a finger-shaped structure, the pre-penetration apparatus, which projects into the cell lumen. The PPA defines a trajectory through the cell, which presages the path of the invading fungal hypha (Reinhardt Citation2007). It plays a central role in the elaboration of the apoplastic interface compartment through which the fungus grows when it penetrates the cell lumen. Genre and Bonfante (Citation2005) have suggested that PPA formation in the epidermis can be correlated with DMI-dependent transcriptional activation of the Medicago early nodulin gene ENOD11. Pre-penetration apparatus formed during AM resembles the cortical pre-infection threads formed during nodulation. In the case of nitrogen-fixing bacteria, the host synthesizes a unique membrane-matrix tube, called the infection thread, which initiates within the curled root hair as a membrane invagination. Rhizobia enter the infection thread and progress down the root hair by repeated cell division. The infection thread tip grows continuously ahead of the bacteria, following some distance behind the downward-migrating root hair nucleus. In underlying cortical cell layers, transcellular cytoplasmic bridges (also called preinfection threads) are formed in advance of the progressing infection thread (Genre & Bonfante Citation2005).
As legumes can enter into both types of endosymbioses, i.e., with soil fungi, forming phosphorus-acquiring arbuscular mycorrhiza, and with nitrogen-fixing bacteria, leading to nitrogen-fixing root nodules, they are ideal plants to study the signaling events that control these two endosymbiotic systems. Most progress in understanding the molecular basis of the signaling cascade that leads to symbiotic interactions has come from studies on the nodulation process in the two model legumes Lotus japonicus (Lotus) (Stougaard Citation2001) and Medicago truncatula (Medicago) (Cook Citation1999).
Conservation of signal-transduction pathways
Considering the apparent analogies in the infection process, including the only recently discovered establishment of an epidermal pre-penetration apparatus during AM that resembles the cortical pre-infection threads formed during nodulation (Genre & Bonfante Citation2005), an overlap in the activation of gene expression can be expected. This overlap is particularly evident for the signal perception and signal transduction cascades that initiate nodulation and mycorrhization (Parniske Citation2004). Molecular and genetic studies have shown that the infection processes in both the symbioses are strikingly similar and that both microsymbionts induce a common signaling cascade during initiation of root nodules and AM (Cullimore & Denarie Citation2003). Studies on legume mutants defective in early stages of both endosymbioses suggest the existence of a so-called ‘Myc factor’, that, similar to the action of Nod-factors triggering nodulation, initiates AM formation (Cullimore & Denarie Citation2003). This fungal signal molecule has not yet been characterized, but recent studies have demonstrated that AM fungi do indeed produce a diffusible signal molecule that can induce responses in the legume host (Kosuta et al. Citation2003, Limpens & Bisseling Citation2003). The identification of additional components within the symbiotic nitrogen fixation signaling cascade indicates that proteins with related functions might be involved in mycorrhizal signaling. For example, a functional equivalent of the putative Nod factor receptors LjNFR1 and LjNFR5 (Oldroyd et al. Citation2005, Oldroyd & Downie Citation2006, Paszkowski Citation2006b) could exist for ‘Myc factor’ perception. Smit et al. (Citation2005) have reported the identification of the Medicago GARS- type protein Nodulation Signaling Pathway 1 (NSP1), which is essential for all known Nod factor-induced changes in gene expression. Kaló et al. (Citation2005) have shown that nodulation signaling pathway genes (NSP2) from Medicago truncatula encode a GARS protein essential for Nod-factor signaling. NSP2 functions downstream of Nod-factor-induced calcium spiking and a calcium/calmodulin-dependent protein kinase. It is also likely that components corresponding to the GARS transcription factors MtNSP1 and MtNSP2 (Kaló et al. Citation2005, Smit et al. Citation2005) are present to modulate gene expression in response to calcium oscillation during mycorrhizal signaling. Bonfante et al. (Citation2000) have proposed that LjSym4 is required for the initiation or coordinated expression of the host plant cell's accommodation program, allowing the passage of both microsymbionts through the epidermis layer.
The availability of marker genes which are activated during both symbiotic interactions as well as host mutants, blocked in the infection by either microsymbiont, have provided the means to study whether the induction of the infection – related genes involve common mechanisms. The analysis of legume nodulation mutants showed that a number of these mutants are also impaired in their ability to form the AM-symbiosis. Hence, although the microsymbionts of these symbioses are different types of organisms, they both establish endosymbiosis with plant roots and a wide range of molecular data confirm the existence of a genetic program that regulates the accommodation of microsymbiont inside plant cells (Parniske Citation2000). In contrast to studies dedicated to nodulation, targeted approaches addressing AM have so far revealed a markedly smaller number of genes activated in arbuscules (Franken & Requena Citation2001), e.g., the phosphate transporter MtPt4 (Harrison et al. Citation2002), the plasma-membrane H1-ATPase Mtha1 (Krajinski et al. Citation2002), the germin-like protein MtGlp1 (Doll et al. Citation2003), the glutathione S-transferase MtGst1 (Wulf et al. Citation2003), and the Ser carboxypeptidase MtScp1 (Liu et al. Citation2003). Experiments making use of suppression subtractive hybridization (SSH) cDNA libraries, cDNA-array hybridizations, and real-time reverse transcription (RT)-PCR experiments identified several dozens of mycorrhiza-related M. truncatula genes (Liu et al. Citation2003, Wulf et al. Citation2003, Brechenmacher et al. Citation2004, Küster et al. Citation2004, Manthey et al. Citation2004, Weidmann et al. Citation2004). Regardless of the progress made and largely due to the obligate biotrophy of AM fungi (Franken & Requena Citation2001) as well as the presence of different stages of AM formation in mycorrhizal roots (Gianinazzi-Pearson & Brechenmacher Citation2004), knowledge on genes activated during AM is still limited in relation to more than a thousand genes identified as up-regulated during different steps of root nodule initiation and function using different cDNA-based macro- and microarrays (Colebatch et al. Citation2002, Citation2004, Fedorova et al. Citation2002, El Yahyaoui et al. Citation2004, Kouchi et al. Citation2004, Lee et al. Citation2004).
Küster et al. (Citation2007) applied in silico and microarray-based transcriptome profiling approaches to uncover the transcriptome of developing root nodules and AM roots of the model legume Medicago truncatula and found several hundred genes to be activated in different stages of either symbiosis, with almost 100 genes being co-induced during nodulation and in arbuscular mycorrhiza. These co-induced genes can be associated with different cellular functions required for symbiotic efficiency, such as the facilitation of transport processes across the perisymbiotic membranes that surround the endosymbiotic bacteroids in root nodules and the arbuscules in AM roots. The first AM-activated genes with relevance to arbuscule physiology were two plasma membrane H+-ATPases from tobacco, where the corresponding protein and enzyme activities were localized to the periarbuscular membrane (Gianinazzi-Pearson et al. Citation2000). In M. truncatula, the corresponding H+-ATPase MtHa1 has been localized to the periarbuscular membrane recently (Valot et al. Citation2006). In addition to a specific expression in AM roots (compared to non-mycorrhizal roots, Krajinski et al. Citation2002), MtHa1 was also found to be induced during nodulation, illustrating the common requirement for an acidification of the perisymbiotic space in either root endosymbiosis (Manthey et al. Citation2004), most probably to facilitate energy-dependent transport across the perisymbiotic membranes. Amongst the symbiotically induced genes encoding membrane proteins, also the multifunctional aquaporin MtNip1, identified to be mycorrhiza-induced by Brechenmacher et al. (Citation2004) and a membrane nodulin of unknown function originally identified in soybean symbiosome membranes (Winzer et al. Citation1999) deserve attention. Together, the up-regulation of different genes encoding symbiosome membrane nodulins indicates that the peribacteroid and periarbuscular membranes share common structural properties.
With respect to membrane formation, the symbiotically induced annexin MtAnn2 might play a role in calcium dependent membrane reorganization during the colonization of plant tissues, as proposed for other plant annexins (Manthey et al. Citation2004). Since the invading microsymbionts are surrounded by membranes of plant origin, such processes are obviously relevant in both the root nodule and the AM symbiosis. The in situ localization of MtAnn2 promoter activity showed an induction of the gene in the nodule primordium and in arbuscule-containing cells (Manthey et al. Citation2004).
A polygalacturonase and an endo- 1,3-1,4-beta-D-glucanase gene are co-induced in nodules as well as AM, indicating the recruitment of similar cell wall modifying enzymes for modification of extracellular matrices surrounding symbiotic structures. Serine carboxypeptidase gene MtScp1 and a cysteine protease (genes encoding enzymes involved in protein processing) were also shown to be activated in both symbioses (Liu et al. Citation2003). Targeted studies demonstrated that genes expressed during root nodule initiation and infection, e.g., the early nodulin genes Enod2, Enod5, Enod11, Enod12 and Enod40 are also transcriptionally activated during AM (van Rhijn et al. Citation1997, Albrecht et al. Citation1998, Albrecht et al. Citation1999, Journet et al. Citation2001, Chabaud et al. Citation2002, Fehlberg et al. Citation2005). Whereas Enod2, Enod11 and Enod12 encode proline-rich putative cell wall proteins, Enod5 specifies an arabinogalactan protein and Enod40 encodes a small regulatory peptide (Scheres et al. Citation1990, Schultze & Kondorosi Citation1998, Journet et al. Citation2001). In Rhizobium interaction, Enod40 is first expressed in pericycle cells opposite the protoxylem poles within a few hours of Nod-factor application and markedly before the first cell divisions occur in the root cotex. It has been postulated that in AM, interacting cytokinin induces ENOD40 (van Rhijn et al. Citation1997).
Using a genetic approach, plant mutants unable to nodulate and affected in early responses to Nod factors have been identified in several legumes including Medicago truncatula (Catoira et al. Citation2000, Ben Amor et al. Citation2003), Pisum sativum (Walker et al. Citation2000), and Lotus japonicus (Madsen et al. Citation2003, Radutoiu et al. Citation2003). Some of these mutants are unable to establish a symbiosis with both their rhizobial partner and also with arbuscular endomycorrhizal (AM) fungi, and these have been called does not make infections (dmi) or common symbiotic (sym) mutants. In M. truncatula one mycorrhizal phenotype, represented by three mutants, has been described so far. In the symbiosis mutants dmi1, dmi2, and dmi3, the fungal growth is arrested at the early epidermal cell layer; they only very rarely have infections of root cortex cells (Sagan et al. Citation1995). In M. truncatula, dmi1 and dmi2 mutants are blocked in most Nod factor responses but still exhibit rapid calcium influx and root-hair deformation after Nod factor addition, whereas a dmi3 mutant shows, in addition, the calcium-spiking response (Shaw & Long Citation2003). dmi2 and its ortholog in L. japonicus encode receptor-like kinases (Endre et al. Citation2002, Stracke et al. Citation2002). dmi1 and the L. japonicus homologs have been shown to encode membrane spanning ion channel-like proteins (Ané et al. Citation2004, Imaizumi-Anraku et al. Citation2005), and dmi3 encodes a putative calcium/calmodulin dependent protein kinase and could play a role in interpreting calcium signatures elicited in response to rhizobia and possibly AM fungi (Lévy et al. Citation2004, Mitra et al. Citation2004). All of the genes encode proteins involved in signal transduction, but how these components are incorporated in the global mycorrhiza signaling pathway is still unknown (Krajinski & Frenzel Citation2007).
The mutant Nod factor perception (nfp) of M. truncatula (Ben Amor et al. Citation2003), like sym10 mutants of P. sativum (Walker et al. Citation2000) and nfr5 and nfr1 mutants of L. japonicus (Radutoiu et al. Citation2003), are completely unresponsive to Nod factors but are still capable of establishing a symbiotic interaction with AM fungi. Based on these observations, it was proposed that dmi1, dmi2 and dmi3 are involved in a common signaling pathway (called the common SYM pathway) implicated in the establishment of both the mycorrhizal and bacterial endosymbioses (Catoira et al. Citation2000), whereas SYM10 (in P. sativum), NFR5, NFR1 (in L. japonicus), and NFP (in M. truncatula), acting upstream of the common SYM pathway, would be specifically involved in rhizobial Nod factor perception (Walker et al. Citation2000, Ben Amor et al. Citation2003, Radutoiu et al. Citation2003). Kistner et al. (Citation2005) tested the AM phenotype of nodulation-impaired mutants and by complementation analysis, defined seven L. japonicus common symbiosis genes (SYMRK, CASTOR, POLLUX, SYM3, SYM6, SYM15, and SYM24) that are required for both fungal and bacterial entry into root epidermal or cortical cells.
MtSucS1, one of the five sucrose synthase genes of M. truncatula was found to be up-regulated not only in root nodules, but also in AM roots (Hohnjec et al. Citation1999). Based on the expression of reporter gene fusions in transgenic roots, Hohnjec et al. (Citation2003) demonstrated its expression both in the central nodule tissues and in the arbuscule-containing cells of mycorrhizal roots, which was in accordance with in situ localizations of sucrose synthase transcripts in arbuscule-containing cells of different plants (Blee & Anderson Citation2002).
Leghemoglobins (Lb), which are the most abundant and best-characterized nodule-specific proteins, are expressed in the infected cells just prior to the onset of nitrogen fixation. Three genes encoding the leghemoglobin VfLb29 as well as the truncated hemoglobin genes MtTrHb1 and MtTrHb2 were up-regulated during nodulation and mycorrhization. Whereas the VfLb29 gene was identified in the course of the reverse Northern blot screening for symbiotically induced Vicia faba genes mentioned above (Fruhling et al. Citation1997), the two genes encoding truncated hemoglobins were found by in silico analyses (Vieweg et al. Citation2005). An 85 bp sequence of the VfLb29 promoter is necessary for the expression in arbuscules, whereas this element is not involved in the expression in nodules. Within this 85 bp sequence, a palindromic sequence, also present in the promoter of the AM-specific phosphate transporter StPt3, was identified (Fehlberg et al. Citation2005). The arbuscule-specific expression was observed not only in Vicia faba, but also in other legumes including the model legume M. truncatula and in the non-legume tobacco. This indicates a general AM-specific trigger for VfLb29 gene expression under arbuscular mycorrhizal conditions (Vieweg et al. Citation2004). A similar highly specific activity in arbuscule-containing cells, as shown for the VfLb29 promoter (Vieweg et al. Citation2004), has also been demonstrated for the promoters of two phosphate transporter genes, MtPt4 of M. truncatula (Harrison et al. Citation2002) and Stpt3 of potato (Rausch et al. Citation2001).
Conclusions and future prospects
The roots of most higher plants are able to establish an endosymbiotic association with soil fungi to form arbuscular mycorrhiza, whereas only legumes are able to engage in the evolutionary younger nitrogen-fixing root-nodule symbiosis with bacteria. The recognition of mycorrhiza and rhizobia, and further signal transduction that leads to an effective symbiosis is complex and is controlled by multiple receptor and signaling pathways. Little is known about the crucial stage of the interaction that follows the initial fungal–plant contact and precedes infection and in particular the nature of the molecular/cellular dialog that is required for recognition of the fungal partner and successful infection. However, genetic studies performed with several legume genera, such as Pisum, Medicago, and Lotus, have revealed that a small group of plant genes are essential for successful root penetration. These genes were originally identified by virtue of their role in early steps of Rhizobium-elicited nodulation, and, in particular, in transducing the specific rhizobial symbiotic signal (Nod factor) perceived by root hairs, essential for bacterial infection. In the case of the model legume Medicago truncatula, mutations in three distinct genes (Doesn’t make infections1 [DMI1], DMI2, and DMI3) result in a block of root infection by either Sinorhizobium meliloti or AM fungi. The role of the DMI genes in Nod factor signaling has led to the proposition that AM fungi generate analogous Myc signals, whose perception is required to initiate infection. In addition to these genetic data, molecular studies in a variety of legumes have revealed that a number of host genes expressed early during nodulation, including ENOD2, ENOD5, ENOD11, ENOD12, and ENOD40, are also transcribed during root colonization by AM fungi. These findings have an important implication, since in contrast to Rhizobium, AM fungi have the ability to interact with a wide range of higher plants. Assuming that the mechanisms by which AM fungi infect their various hosts are similar, it implies that SYM and ENOD genes, required for the interaction of legumes with both micro-symbionts, are most probably widespread in the plant kingdom. Although, with the exception of Parasponia andersonni, non-leguminous plants are unable to establish a symbiosis with Rhizobium, they seem to harbour a perception mechanism by which Nod factors can be recognized. Obviously this perception mechanism is not maintained by non-legumes to recognize rhizobial Nod factors. However, since its activation leads to ENOD12 transcription, it is worthwhile to study whether molecules of AM fungi are natural ligands. Although the function of the non-leguminous perception mechanism is not clear, it seem probable that it has a widespread occurrence, and that the Nod factor perception mechanism of legumes has evolved from it. Despite the recent progress, researchers have a long way to go to get a complete picture of the genetic interplay involved in rhizobial and mycorrhizal symbiosis. A clearer view of the molecular evolution of endosymbiosis will emerge from genetic/genomic analyses of M. truncatula and L. japonicus. Consequently, the often asked questions of what genes allow legumes to form root nodules and how this pathway evolved from the older endomycorrhizal symbiosis will come closer to being answered. With a more complete understanding of early signaling pathways, it should become apparent which symbiosis genes are missing from crop plants that do not form endosymbiosis with nitrogen-fixing bacteria such as wheat and rice.
Acknowledgements
The financial support provided by CSIR, New Delhi, India is thankfully acknowledged.
References
- Akiyama , K and Hayashi , H . 2006 . Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots . Ann Bot , 97 : 925 – 931 .
- Akiyama , K , Matsuoka , H and Hayashi , H . 2002 . Isolation and identification of a phosphate deficiency-induced c-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots . Mol Plant-Microbe Interact , 15 : 334 – 340 .
- Akiyama , K , Matsuoka , H and Hayashi , H . 2005 . Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi . Nature , 435 : 750 – 751 .
- Albrecht , C , Geurts , R and Bisseling , T . 1999 . Legume nodulation and mycorrhizae formation; two extremes in host specificity meet . EMBO J , 18 : 281 – 288 .
- Albrecht , C , Geurts , R , Lapeyrie , F and Bisseling , T . 1998 . Endomycorrhizae and rhizobial Nod-factors both require SYM8 to induce the expression of the early nodulin genes PsENOD5 and PsENOD12A . Plant J , 15 : 605 – 614 .
- Ané , JM , Kiss , GB , Riely , BK , Penmetsa , RV , Oldroyd , GED , Ayax , C , Lévy , J , Debellé , F , Baek , J-M , Kaló , P , Rosenberg , C , Roe , BA , Long , SR , Dénarié , J , Cook , DR and Ane , JM . 2004 . Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes . Science , 303 : 1364 – 1367 .
- Azcon , R and El-Atrash , F . 1997 . Influence of arbuscular mycorrhizae and phosphorus fertilization on growth, nodulation and N2 fixation (15N) in Medicago sativa at four salinity levels . Biol Fertil Soils , 24 : 81 – 86 .
- Ben Amor , B , Shaw , SL , Oldroyd , GE , Maillet , F , Penmetsa , RV , Cook , D , Long , SR , Dénarié , J and Gough , C . 2003 . The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation . Plant J , 34 : 495 – 506 .
- Berta , G , Fusconi , A and Hooker , JE . 2002 . “ Arbuscular mycorrhizal modifications to plant root systems ” . In Mycorrhizal technology: from genes to bioproducts — achievement and hurdles in arbuscular mycorrhizal research , Edited by: Gianinazzi , S and Schuepp , H . 71 – 101 . Basel, , Switzerland : Birkhauser Verlag Publisher .
- Berta , G , Fusconi , A , Trotta , A and Scannerini , S . 1990 . Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root systems of Allium porrum L . New Phytol , 114 : 207 – 215 .
- Berta , G , Trotta , A , Fusconi , A , Hooker , JE , Munro , M , Atkinson , D , Giovannetti , M , Morini , S , Fortuna , P , Tisserant , B , Gianinazzi-Pearson , V and Gianinazzi , S . 1995 . Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera . Tree Physiol , 15 : 281 – 294 .
- Blee , KA and Anderson , AJ . 2002 . Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules . Plant Mol Biol , 50 : 197 – 211 .
- Bonfante P , Genre A , Faccio A , Martini I , Schauser L , Stougaard J , Webb J , Parniske M. . 2000 . The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells . Mol Plant-Microbe Interact 13 (10) : 1109 – 1120 .
- Bradbury , SM , Peterson , RL and Bowley , SR . 1991 . Interaction between three alfalfa nodulation genotypes and two Glomus species . New Phytol , 119 : 115 – 120 .
- Brechenmacher , L , Weidmann , S , van Tuinen , D , Chatagnier , O , Gianinazzi , S , Franken , P and Gianinazzi-Pearson , V . 2004 . Expression profiling of up-regulated plant and fungal genes in early and late stages of Medicago truncatula-Glomus mosseae interactions . Mycorrhiza , 14 : 253 – 262 .
- Catoira , R , Galera , C , De Billy , F , Penmetsa , RV , Journet , EP , Maillet , F , Rosenberg , C , Cook , D , Gough , C and Denarie , J . 2000 . Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway . Plant Cell , 12 : 1647 – 1666 .
- Chabaud , M , Venard , C , Defaux-Petras , A , Becard , G and Barker , DG . 2002 . Targeted inoculation of Medicago truncatula in vitro root cultures reveals MtENOD11 expression during early stages of infection by arbuscular mycorrhizal fungi . New Phytol , 156 : 265 – 273 .
- Colebatch , G , Desbrosses , G , Ott , T , Krusell , L , Montanari , O , Kloska , S , Kopka , J and Udvardi , MK . 2004 . Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus . Plant J , 39 : 487 – 512 .
- Colebatch , G , Kloska , S , Trevaskis , B , Freund , S , Altmann , T and Udvardi , MK . 2002 . Novel aspects of symbiotic nitrogen fixation uncovered by transcript profiling with cDNA arrays . Mol Plant Microbe Interact , 15 : 411 – 420 .
- Cook , DR . 1999 . Medicago truncatula – a model in the making! . Curr Opin Plant Biol , 2 : 301 – 304 .
- Cullimore , JV and Denarie , J . 2003 . How legumes select their sweet talking symbionts . Science , 302 : 630 – 633 .
- Day , DA , Kaiser , BN , Thomson , R , Udvardi , MK , Moreau , S and Puppo , A . 2001 . Nutrient transport across symbiotic membranes from legume nodules . Austr J Plant Physiol , 28 : 667 – 674 .
- Doll , J , Hause , B , Demchenko , K , Pawlowski , K and Krajinski , F . 2003 . A member of the germin-like protein family is a highly conserved mycorrhiza-specific induced gene . Plant Cell Physiol , 44 : 1208 – 1214 .
- Duc , G , Trouvelot , A , Gianinazzi-Pearson , V and Gianinazzi , S. 1989 . First report of non-mycorrhizal plant mutants (Myc−) obtained in pea (Pisum sativum L.) and fababean (Vicia faba L.) . Plant Sci , 60 : 215 – 222 .
- El Yahyaoui , F , Küster , H , Ben Amor , B , Hohnjec , N , Pühler , A , Becker , A , Gouzy , J , Vernié , T , Gough , C and Niebel , A . 2004 . Expression profiling in Medicago truncatula identifies more than 750 genes differentially expressed during nodulation, including many potential regulators of the symbiotic program . Plant Physiol , 136 : 3159 – 3176 .
- Endre , G , Kereszt , A , Kevei , Z , Mihacea , S , Kaló , P and Kiss , GB . 2002 . A receptor kinase gene regulating symbiotic nodule development . Nature , 417 : 962 – 966 .
- Fedorova , M , van de Mortel , J , Matsumoto , PA , Cho , J , Town , CD , VandenBosch , KA , Gantt , JS and Vance , CP . 2002 . Genome-wide identification of nodule-specific transcripts in the model legume Medicago truncatula . Plant Physiol , 130 : 519 – 537 .
- Fehlberg , V , Vieweg , MF , Dohmann , EMN , Hohnjec , N , Puhler , A , Perlick , AM and Küster , H . 2005 . The promoter of the leghaemoglobin gene VfLb29: Functional analysis and identification of modules necessary for its activation in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots . J Exp Bot , 56 : 799 – 806 .
- Forbes , PJ , Ellison , CH and Hooker , JE . 1996 . The impact of arbuscular mycorrhizal fungi and temperature on root system development . Agronomie , 16 : 617 – 620 .
- Franken , P and Requena , N . 2001 . Analysis of gene expression in arbuscular mycorrhiza: new approaches and challenges . New Phytol , 150 : 431 – 439 .
- Fruhling , M , Roussel , H , Gianinazzi-Pearson , V , Puhler , A and Perlick , AM . 1997 . The Vicia faba leghemoglobin gene VfLb29 is induced in root nodules and in roots colonized by the arbuscular mycorrhizal fungus Glomus fasciculatum . Mol Plant-Microbe Interact , 10 : 124 – 131 .
- Gamalero , E , Martinotti , M G , Trotta , A , Lemanceau , P and Berta , G . 2002 . Morphogenetic modifications induced by Pseudomonas fluorescens A6RI and Glomus mosseae BEG12 in the root system of tomato differ according to plant growth conditions . New Phytol , 155 ( 2 ) : 293 – 300 .
- Garg , N and Geetanjali , Kaur A . 2006 . Arbuscular mycorrhiza: nutritional aspects . Arch Agron Soil Sci , 52 : 593 – 606 .
- Genre , A and Bonfante , P . 2005 . Building a mycorrhizal cell: How to reach compatibility between plants and arbuscular mycorrhizal fungi . J Plant Interact , 1 : 3 – 13 .
- Genre , A , Chabaud , M , Timmers , T , Bonfante , P and Barkerb , DG . 2005 . Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago Truncatula root epidermal cells before infection . Plant Cell , 17 ( 12 ) : 3489 – 3499 .
- Gianinazzi-Pearson , V and Brechenmacher , L . 2004 . Functional genomics of arbuscular mycorrhiza: decoding the symbiotic cell programme . Can J Bot , 82 : 1228 – 1234 .
- Gianinazzi-Pearson , V . 1996 . Plant cell responses to arbuscular mycorrhiza fungi: Getting to the roots of the symbiosis . Plant Cell , 8 : 1871 – 1883 .
- Gianinazzi-Pearson , V , Arnould , C , Oufattole , M , Arango , M and Gianinazzi , S . 2000 . Differential activation of H + -ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco . Planta , 211 : 609 – 613 .
- Harrison , MJ , Dewbre , GR and Liu , J . 2002 . A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi . Plant Cell , 14 : 2413 – 2429 .
- Harrison , MJ . 1999a . Biotrophic interfaces and nutrient transport in plant/fungal symbioses . J Exp Bot , 50 : 1013 – 1022 .
- Harrison , MJ . 1999b . Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis . Ann Rev Plant Physiol Plant Mol Biol , 50 : 361 – 389 .
- Harrison , MJ and Dixon , RA . 1993 . Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular-arbuscular mycorrhizal associations in roots of Medicago truncatula . Mol Plant-Microbe Interact , 6 : 643 – 654 .
- Harrison , MJ and Dixon , RA . 1994 . Spatial patterns of expression of flavonoid/isoflavonoid pathway genes during interactions between roots of Medicago truncatula and the mycorrhizal fungus Glomus versiforme . Plant J , 6 : 9 – 20 .
- Harrison , MJ . 2005 . Signaling in the arbuscular mycorrhizal symbiosis . Ann Rev Microbiol , 59 : 19 – 42 .
- Hohnjec , N , Becker , JD , Puhler , A , Perlick , AM and Küster , H . 1999 . Genomic organization and expression properties of the MtSucS1 gene, which encodes a nodule-enhanced sucrose synthase in the model legume Medicago truncatula . Mol Gen Genet , 261 : 514 – 522 .
- Hohnjec , N , Perlick , AM , Puhler , A and Küster , H . 2003 . The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi . Mol Plant-Microbe Interact , 16 : 903 – 915 .
- Hooker , JE and Atkinson , D . 1992 . Vesicular-arbuscular fungi induced alteration in poplar root system morphology . Plant Soil , 145 : 207 – 214 .
- Imaizumi-Anraku , H , Takeda , N , Charpentier , M , Perry , J , Miwa , H , Umehara , Y , Kouchi , H , Murakami , Y , Mulder , L and Vickers , K . 2005 . Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots . Nature , 433 : 527 – 531 .
- Journet , EP , El-Gachtouli , N , Vernoud , V , de Billy , F , Pichon , M , Dedieu , A , Arnould , C , Morandi , D , Barker , DG and Gianinazzi-Pearson , V . 2001 . Medicago truncatula ENOD11: A novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells . Mol Plant-Microbe Interact , 14 : 737 – 748 .
- Kaló , P , Gleason , C , Edwards , A , Marsh , J , Mitra , RM , Hirsch , S , Jakab , J , Sims , S , Long , SR , Rogers , J , Kiss , GB , Downie , JA and Oldroyd , GED . 2005 . Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators . Science , 308 : 1786 – 1789 .
- Kanamori , N , Madsen , LH , Radutoiu , S , Frantescu , M , Quistgaard , EM , Miwa , H , Downie , JA , James , EK , Felle , HH , Haaning , LL , Jensen , TH , Sato , S , Nakamura , Y , Tabata , S , Sandal , N and Stougaard , J . 2006 . A nucleoporin is required for induction of Ca2 + spiking in legume nodule development and essential for rhizobial and fungal symbiosis . Proc Natl Acad Sci USA , 103 : 359 – 364 .
- Kawaguchi , M , Imaizumi-Anraku , H , Koiwa , H , Niwa , S , Ikuta , A , Syono , K and Akao , S . 2002 . Root, root hair, and symbiotic mutants of the model legume Lotus japonicus . Mol Plant Microbe Interact , 15 : 17 – 26 .
- Kistner , C and Parniske , M . 2002 . Evolution of signal transduction in intracellular symbiosis . Trends Plant Sci , 7 : 511 – 518 .
- Kistner , C , Winzer , T , Pitzschke , A , Mulder , L , Sato , S , Kaneko , T , Tabata , S , Sandal , N , Stougaard , J , Webb , KJ , Szczyglowski , K and Parniske , M . 2005 . Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis . Plant Cell , 17 : 2217 – 2229 .
- Kosuta , S , Chabaud , M , Lougnon , G , Gough , C , Denarie , J , Barker , DG and Becard , G . 2003 . A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula . Plant Physiol , 131 : 952 – 962 .
- Kouchi , H , Shimomura , K , Hata , S , Hirota , A , Wu , G-J , Kumagai , H , Tajima , S , Suganuma , N , Suzuki , A and Aiko , T . 2004 . Large-scale analysis of gene expression profiles during early stages of root nodule formation in a model legume, Lotus japonicus . DNA Res , 11 : 263 – 274 .
- Krajinski , F and Frenzel , A . 2007 . Towards the elucidation of AM-specific transcription in Medicago truncatula . Phytochemistry , 68 : 75 – 81 .
- Krajinski , F , Hause , B , Gianinazzi-Pearson , V and Franken , P . 2002 . Mtha1 a plasma membrane H+-ATPase gene from Medicago truncatula, shows arbuscule-induced expression . Plant Biol , 4 : 754 – 761 .
- Küster , H , Hohnjec , N , Krajinski , F , El Yahyaoui , F , Manthey , K , Gouzy , J , Dondrup , M , Meyer , F , Kalinowski , J and Brechenmacher , L . 2004 . Construction and validation of cDNA-based Mt6k-RIT macro- and microarrays to explore root endosymbioses in the model legume Medicago truncatula . J Biotechnol , 108 : 95 – 113 .
- Küster , H , Vieweg , MF , Manthey , K , Baier , MC , Hohnjec , N and Perlick , AM . 2007 . Identification and expression regulation of symbiotically activated legume genes . Phytochemistry , 68 : 8 – 18 .
- LaRue , TA and Weeden , NF . 1994 . “ The symbiosis genes of the host ” . In Proceedings of the 1st European Nitrogen Fixation Conference , Edited by: Kiss , GB and Endre , G . 147 – 151 . Szeged : Officina Press .
- Lee , H , Hur , C-G , Oh , CJ , Kim , HB , Park , S-Y and An , CS . 2004 . Analysis of the root nodule-enhanced transcriptome in soybean . Mol Cells , 18 : 53 – 62 .
- Lévy , J , Bres , C , Geurts , R , Chalhoub , B , Kulikova , O , Duc , G , Journet , EP , Ane , JM , Lauber , E and Bisseling , T . 2004 . A putative Ca2 + and calmodulin-dependent protein kinase required for bacterial and fungal symbioses . Science , 303 : 1361 – 1364 .
- Limpens , E and Bisseling , T . 2003 . Signaling in symbiosis . Curr Opin Plant Biol , 6 : 343 – 350 .
- Liu , J , Blaylock , LA , Endre , G , Cho , J , Town , CD , VandenBosch , KA and Harrison , MJ . 2003 . Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis . Plant Cell , 15 : 2106 – 2123 .
- Lum , MR and Hirsch , AM . 2003 . Roots and their symbiotic microbes: Strategies to obtain nitrogen and phosphorus in a nutrient-limiting environment . J Plant Growth Regul , 21 : 368 – 382 .
- Lum , MR , Li , Y , LaRue , TA , David-Schwartz , R , Kapulnik , Y and Hirsch , AM . 2002 . Investigation of four classes of non-nodulating white sweetclover (Melilotus alba annua Desr.) mutants and their responses to arbuscular-mycorrhizal fungi . Integ Comp Biol , 42 : 295 – 303 .
- Madsen , EB , Madsen , LH , Radutoiu , S , Olbryt , M , Rakwalska , M , Szczyglowski , K , Sato , S , Kaneko , T , Tabata , S and Sandal , N . 2003 . A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals . Nature , 425 : 637 – 640 .
- Manthey , M , Krajinski , F , Hohnjec , N , Firnhaber , C , Pühler , A , Perlick , AM and Küster , H . 2004 . Transcriptome profiling in root nodules and arbuscular mycorrhiza identifies a collection of novel genes induced during Medicago truncatula root endosymbioses . Mol Plant Microbe Interact , 17 : 1063 – 1077 .
- Martinez-Molina , E , Morales , VM and Hubbell , DH . 1979 . Hydrolytic enzyme production by Rhizobium . Appl Environ Microbiol , 38 : 1186 – 1188 .
- Marx , J . 2004 . The roots of plant-microbe collaborations . Science , 304 : 234 – 236 .
- Mitra , RM , Gleason , CA , Edwards , A , Hadfield , J , Downie , JA , Oldroyd , GE and Long , SR . 2004 . A Ca2 + /calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning . Proc Natl Acad Sci USA , 101 : 4701 – 4705 .
- Navazio , L , Moscatiello , R , Genre , A , Novero , M , Baldan , B , Bonfante , P and Mariani , P . 2006 . A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells . Plant Physiol , 144 : 673 – 681 .
- Newman , EI and Reddell , P . 1987 . The distribution of mycorrhizas among families of vascular plants . New Phytol , 106 : 745 – 751 .
- Norman , JR , Atkinson , D and Hooker , JE . 1996 . Arbuscular mycorrhizal fungal-induced alteration to root architecture in strawberry and induced resistance to the root pathogen Phytophthora fragariae . Plant Soil , 185 : 191 – 198 .
- Oldroyd , GE and Downie , JA . 2004 . Calcium, kinases and nodulation signaling in legumes . Nat Rev Mol Cell Biol , 131 : 566 – 576 .
- Oldroyd , GE and Downie , JA . 2006 . Nuclear calcium changes at the core of symbiosis signalling . Curr Opin Plant Biol , 9 ( 4 ) : 351 – 357 .
- Oldroyd , GE , Harrison , MJ and Udvardi , M . 2005 . Peace talks and trade deals. Keys to long-term harmony in legume-microbe symbioses . Plant Physiol , 137 : 1205 – 1210 .
- Parniske , M . 2000 . Intracellular accommodation of microbes by plants: A common developmental program for symbiosis and disease? . Curr Opin Plant Biol , 3 : 320 – 328 .
- Parniske , M . 2004 . Molecular genetics of the arbuscular mycorrhizal symbiosis . Curr Opin Plant Biol , 7 : 414 – 421 .
- Paszkowski , U . 2006a . A journey through signaling in arbuscular mycorrhizal symbioses . New Phytol , 172 : 35 – 46 .
- Paszkowski , U . 2006b . Mutualism and parasitism: The yin and yang of plant symbioses . Curr Opin Plant Biol , 9 : 364 – 370 .
- Perotto , S , Vandenbosch , KA , Brewin , NJ , Faccio , A , Knox , JP and Bonfante-Fasolo , P . 1990 . “ Modifications of the host cell wall during root colonization by Rhizobium and VAM fungi ” . In Endocytobiology IV , Edited by: Nardon , P , Gianinazzi-Pearson , V , Grenier , AM , Margulis , M and Smith , DC . 114 – 117 . Paris : INRA Press .
- Provorov , NA , Borisov , AY and Tikhonovich , IA . 2002 . Developmental genetics and evolution of symbiotic structures in nitrogen- fixing nodules and arbuscular mycorrhiza . J Theor Biol , 214 : 215 – 232 .
- Radutoiu , S , Madsen , LH , Madsen , EB , Felle , HH , Umehara , Y , Gronlund , M , Sato , S , Nakamura , Y , Tabata , S and Sandal , N . 2003 . Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases . Nature , 425 : 585 – 592 .
- Rausch , C , Daram , P , Brunner , S , Jansa , J , Laloi , M , Leggewie , G , Amrhein , N and Bucher , MA . 2001 . A phosphate transporter expressed in arbuscule-containing cells in potato . Nature , 414 : 462 – 466 .
- Read , DJ , Duckett , JG , Francis , R , Ligrone , R and Russel , A . 2000 . Symbiotic fungal associations in “lower” land plants . Philos Trans Roy Soc Biol Sci , 355 : 815 – 831 .
- Recourt , K , van Tunen , AJ , Mur , LA , van Brussel , AAN , Lugtenberg , JW and Kijne , JW . 1992 . Activation of flavonoid biosynthesis in roots of Vicia sativa subsp. nigra plants by inoculation with Rhizobium leguminosarum biovar viciae . Plant Mol Biol , 19 : 411 – 420 .
- Reinhardt , D . 2007 . Programming good relations – development of the arbuscular mycorrhizal symbiosis . Curr Opin Plant Biol , 10 : 98 – 105 .
- Remy , W , Taylor , TN , Hass , H and Kerp , H . 1994 . Four hundred-million-year-old vesicular arbuscular mycorrhizae . Proc Natl Acad Sci USA , 91 : 11841 – 11843 .
- Sagan , M , Morandi , D , Tarenghi , E and Duc , G . 1995 . Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis . Plant Sci , 111 : 63 – 71 .
- Schauser , L , Handberg , K , Sandal , N , Stiller , J , Thykjaer , T , Pajuelo , E , Nielsen , A and Stougaard , J . 1998 . Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus . Mol Gen Genet , 259 : 414 – 423 .
- Schellenbaum , L , Berta , G , Ravolanirina , F , Tisserant , B , Gianinazzi , S and Fitter , AH . 1991 . Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.) . Ann Bot , 67 : 135 – 141 .
- Scheres , B , van Engelen , F , van der Knaap , E , van de Wiel , C , van Kammen , A and Bisseling , T . 1990 . Sequential induction of nodulin gene expression in the developing pea nodule . Plant Cell , 2 : 687 – 700 .
- Schultze , M and Kondorosi , A . 1998 . Regulation of symbiotic root nodule development . Ann Rev Genet , 32 : 33 – 57 .
- Schussler , A , Schwarzott , D and Walker , C . 2001 . A new fungal phylum, the glomeromycota: Phylogeny and evolution . Mycol Res , 105 : 1413 – 1421 .
- Senoo , K , Solaiman , MZ , Kawaguchi , M , Imaizumi-Anraku , H , Akao , S , Tanaka , A and Obata , H . 2000 . Isolation of two different phenotypes of mycorrhizal mutants in the model legume plant Lotus japonicus after EMS-treatment . Plant Cell Physiol , 41 : 726 – 732 .
- Shaw , SL and Long , SR . 2003 . Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells . Plant Physiol , 131 : 976 – 984 .
- Shirtliffe , SJ and Vessey , JK . 1996 . A nodulation (Nod+/Fix−) mutant of Phaseolus vulgaris L. has nodule-like structures lacking peripheral vascular bundles (Pvb−) and is resistant to mycorrhizal infection (Myc−) . Plant Sci , 118 : 209 – 220 .
- Simon , L , Bousquet , J , Lovesque , RC and Lalonde , M . 1993 . Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants . Nature , 363 : 67 – 69 .
- Smit , P , Raedts , J , Portyanko , V , Debellé , F , Gough , C , Bisseling , T and Geurts , R . 2005 . NSP1 of the GRAS protein family is essential for rhizobial nod factor-induced transcription . Science , 308 : 1789 – 1791 .
- Smith , SE , Smith , AF and Jakobsen , I . 2003 . Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses . Plant Physiol , 133 : 16 – 20 .
- Smith , S and Read , D . 1997 . Mycorrhizal symbiosis , 453 – 469 . London : Academic Press .
- Stougaard , J . 2001 . Genetics and genomics of root symbiosis . Curr Opin Plant Biol , 4 : 328 – 335 .
- Stracke , S , Kistner , C , Yoshida , S , Mulder , L , Sato , S , Kaneko , T , Tabata , S , Sandal , N , Stougaard , J and Szczyglowski , K . 2002 . A plant receptor-like kinase required for both bacterial and fungal symbiosis . Nature , 417 : 959 – 962 .
- Szczyglowski , K , Shaw , RS , Wopereis , J , Copeland , S , Hamburger , D , Kasiborski , B , Dazzo , FB and de Bruijn , FJ . 1998 . Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus . Mol Plant Microbe Interact , 11 : 684 – 697 .
- Takeda , N , Kistner , C , Kosuta , S , Winzer , T , Pitzschke , A , Groth , M , Sato , S , Kaneko , T , Tabata , S and Parniske , M . 2007 . Proteases in plant root symbiosis . Phytochemistry , 68 : 111 – 121 .
- Udvardi , MK and Day , DA . 1997 . Metabolite transport across symbiotic membranes of legume nodules . Annu Rev Plant Physiol Plant Mol Biol , 48 : 493 – 523 .
- Valot , B , Negroni , L , Zivy , M , Gianinazzi , S and Dumas-Gaudot , E . 2006 . A mass spectrometric approach to identify arbuscular mycorrhiza-related proteins in root plasma membrane fractions . Proteomics , 6 : S145 – 155 .
- Van Rhijn , P , Fang , Y , Galili , S , Shaul , O , Atzmon , N , Wininger , S , Eshed , Y , Lum , M , Li , Y and To , V . 1997 . Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction pathways used in forming arbuscular mycorrhizae and Rhizobium-induced nodules may be conserved . Proc Natl Acad Sci USA , 94 : 5467 – 5472 .
- Vessey , KJ , Pawlowski , K and Bergman , B . 2005 . Root-based N2-fixing symbioses: Legumes, actinorhizal plants, Parasponia sp. and cycads . Plant Soil , 274 : 51 – 78 .
- Vierheilig , H , Bago , B , Albrecht , C , Poulin , M J and Piche , Y . 1998 . Flavonoids and arbuscular-mycorrhizal fungi . Adv Exp Med Biol , 439 : 9 – 33 .
- Vieweg , MF , Fruhling , M , Quandt , H-J , Heim , U , Baumlein , H , Puhler , A , Küster , H and Perlick , AM . 2004 . The promoter of the Vicia faba L. leghemoglobin gene VfLb29 is specifically activated in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots from different legume and non-legume plants . Mol Plant-Microbe Interact , 17 : 62 – 69 .
- Vieweg , MF , Hohnjec , N and Küster , H . 2005 . Two genes encoding different truncated hemoglobins are regulated during root nodule and arbuscular mycorrhiza symbioses of Medicago truncatula . Planta , 220 : 757 – 766 .
- Walker , SA , Viprey , V and Downie , JA . 2000 . Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by nod factors and chitin oligomers . Proc Natl Acad Sci USA , 97 : 13413 – 13418 .
- Wegel , E , Schauser , L , Sandal , N , Stougaard , J and Parniske , M . 1998 . Mycorrhiza mutants of Lotus japonicus define genetically independent steps during symbiotic infection . Mol Plant Microbe Interact , 11 : 933 – 936 .
- Weidmann , S , Sanchez , L , Descombin , J , Chatagnier , O , Gianinazzi , S and Gianinazzi-Pearson , V . 2004 . Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula . Mol Plant Microbe Interact , 17 : 1385 – 1393 .
- Winzer , T , Bairl , A , Linder , M , Linder , D , Werner , D and Müller , P . 1999 . A novel 53-kDa nodulin of the symbiosome membrane of soybean nodules, controlled by Bradyrhizobium japonicum . Mol Plant Microbe Interact , 12 : 218 – 226 .
- Wulf , A , Manthey , K , Doll , J , Perlick , AM , Linke , B , Bekel , T , Meyer , F , Franken , P , Küster , H and Krajinski , F . 2003 . Transcriptional changes in response to arbuscular mycorrhiza development in the model plant Medicago truncatula . Mol Plant Microbe Interact , 16 : 306 – 314 .
- Wyss , P , Mellor , RB and Wiemken , A . 1990 . Vesicular-arbuscular mycorrhizas of wild-type soybean and non-nodulating mutants with Glomus mosseae contain symbiosis-specific polypeptides (mycorrhizins), immunologically cross-reactive with nodulins . Planta , 182 : 22 – 26 .
- Xie , ZP , Staehelin , C and Vierheilig , H . 1995 . Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans . Plant Physiol , 108 : 1519 – 1525 .