Abstract
The allelopathic effect of some benzoic acid (BA) OH- and OCH3-ring substituents was studied on cucumber root transmembrane potential difference (Vm). Most of the methoxy-BAs induced a rapid Vm depolarization, followed by a Vm hyperpolarization, with the only exception for p-anisic acid (pA). On the other hand, salicylic acid (SA) and 3,4-dimethoxybenzoic acid (DHB) strongly depolarized Vm. A positive correlation was found between Vm hyperpolarization and lipophilicity of methoxylated BAs, whereas a positive correlation was found between lipophilicity and Vm depolarization of hydroxylated BAs. The influence of BAs on K+ was studied by means of specific blocking with Cs+ indicating a possible direct interaction of SA, gallic acid (GA), vanillic acid (VA) and 3,4-dimethoxybenzoic acid (DMB). Interference of BAs with the Vm hyperpolarizing effect of root perfusion with the fungal toxin fusicoccin were also observed.
Introduction
Many phenolic compounds possess allelopathic properties able to produce direct or indirect effects on the growth and development of plants of the same or other species (Siqueira et al. Citation1991, Einhellig Citation1995, Blum Citation1996, Inderjit Citation1996, Seigler Citation1996, Maffei et al. Citation1999, Klejdus & Kuban Citation1999, Reigosa et al. Citation1999, Huang et al. Citation2000, Inderjit et al. Citation2002, Citation2005, Politycka Citation2002, Batish et al. Citation2007). Previous studies have demonstrated that in Cucumis sativus some phenolic compounds cause growth inhibition and decrease crop yield (Lyu & Blum Citation1990, Lyu et al. Citation1990, Gerig & Blum Citation1991, Shafer & Blum Citation1991, Yu & Matsui Citation1997, Politycka Citation2002, Yu et al. Citation2003, Politycka & Bednarski Citation2004). Among these, benzoic acid (BA) -OH ring substituents have been demonstrated to cause an increase in germination percentage, whereas –OCH3 ring substituents decreased the percentage of germination. Moreover, hydroxy substituents increased isocitrate lyase (ICL) activity, one of the main enzymes involved in gluconeogenesis, whereas -OCH3 ring substituents decreased enzyme activity indicating a direct effect on enzyme catalysis (Maffei et al. Citation1999).
Several ways of action are involved in the allelochemical interaction with biochemical and metabolic processes and one of the specific effects of phenolic compounds is the modification of membrane permeability and function (Bergmark et al. Citation1992, Booker et al. Citation1992, Barkosky & Einhellig Citation1993, Lehman et al. Citation1994, Einhellig Citation1995, Blum Citation1996, Seigler Citation1996, Yu & Matsui Citation1997, Sacco & Maffei Citation1997, Sene et al. Citation2000, Barkosky & Einhellig Citation2003, Gniazdowska & Bogatek Citation2005, Pehlivan & Arslan Citation2006). Studies on the influence of phenolic compounds on ion uptake and alteration of transmembrane potential difference (Vm) indicate that some BA derivatives are able to cause a rapid membrane depolarization, owing to their effects on either the diffusion potential or the electrogenic potential of plant root cells (Blake Citation1985). Furthermore, BAs can alter membrane permeability by solubilizing into cellular membranes and/or adsorbing to membranes to produce either a negative surface potential or an increase in the anionic field strength of the membrane (Heipieper et al. Citation1991, Baziramakenga et al. Citation1995, Barron et al. Citation1996). It has been demonstrated that the number and position of BA hydroxy and methoxy ring substituents play an important role in their inhibitory activity towards ion uptake (Yu & Matsui Citation1997, Yu et al. Citation2003, Ye et al. Citation2004) and plant growth (Crisan et al. Citation2007).
The aim of the present work was the evaluation of the effect of some BA hydroxy- and methoxy-ring substituents on membrane potential in cucumber root segments from seedlings germinating either in absence or presence of the various BA derivatives. Since the diterpene glycoside fusicoccin (FC) stimulates proton secretion coupled with cation uptake and enhances the selectivity of the transport system for K+ (Aducci et al. Citation1995, DeMichelis et al. Citation1996, DeBoer Citation1997, van den Wijngaard et al. Citation2005, Bucker et al. Citation2006), FC and and K+ were also studied in relation to BA derivative allelopathic effects.
Materials and methods
Plant material and seed germination
Cucumber (Cucumis sativus L. var. “Piccolo di Parigi”) seeds were purchased from Franchi Sementi, Bergamo, Italy. Seeds were washed with tap water for 4 h and then placed for 48 h in the dark (28°C isothermal) on 9 cm Petri dishes containing 2 paper filters saturated with 5 ml of 5 mM MES-Na (2-[N'-morpholino] ethanesulfonic acid) buffer pH 6.0, with the addition of 0.5 mM CaSO4. The seeds were also placed in Petri dishes containing 1 mM of each of the BAs listed in . The BAs concentration was chosen after different trials with concentrations ranging from 0.01–2 mM, in accordance with bibliographic data (Maffei et al. Citation1999, Crisan et al. Citation2007). Owing to the strong acidifying effects of some BAs, the pH of the solution was adjusted to pH 6.0 before BAs treatment. For each experiment controls without BAs were also prepared. For treatments and controls, 15 seeds per 10 Petri dishes were incubated in the dark at 28°C. Three days after germination, roots of seedlings were used for membrane potential determination. In all experiments BAs were dissolved in 0.1 M methanol, which was added at the same concentration in all controls. The results are reported as a mean of at least five replications performed the same day on different root segments.
Root segment Vm determination
In the first experiment, cucumber seeds were germinated for three days in the dark and then cut to obtain the root-elongating zone to be directly measured. In the second experiment, two days after germination, seedlings were transferred to an aerated 0.5 mM CaSO4 solution for 24 h in the dark. Membrane potentials were determined in root segments for 100 min. The transmembrane potential difference (Vm) was determined in the root elongating zone with glass micropipettes with a tip resistance of 4–10 MΩ and filled with 1 M KCl. Micropipettes were used as micro-salt bridges to Ag/AgCl electrodes and inserted vertically in the tissue by means of a Narishige micromanipulator (Maffei et al. Citation2004, Maffei & Bossi Citation2006, Maffei et al. Citation2006). Cucumber roots were always equilibrated for 60 min in a 5 mM MES-Na buffer (pH 6.0).
Effect of benzoic acid derivatives on Vm
Vm from root segments of control seedlings germinated for three days in the dark were measured for 30 min, then perfusion was carried on with the addition of 1 mM of the various BAs. This concentration was chosen after several trials ranging from 0.1–5 mM. Vm from root segments of seedlings germinated for three days in the dark in the presence of 1 mM of the various BAs were measured after rinsing the root segments for 30 min in MES–Na buffer. Subsequently, Vm was also measured after the addition of 1 mM of the appropriate BA.
Effect of benzoic acid derivatives on K+ and K+ uptake blocking by Cs+
Vm of root segments obtained from control seedlings aerated for 24 h in a 0.5 mM CaSO4 solution were determined and then root segments were perfused with 1 mM of the various BAs followed by perfusion with 0.25 mM K2SO4. Root segments were also first perfused with 0.25 mM K2SO4 followed by perfusion with 1 mM of the various BAs; 0.25 mM Cs2SO4 was then added and the Vm determined.
Effect of benzoic acid derivatives on Fusicoccin
Vm of root segments obtained from control seedlings aerated for 24 h in a 0.5 mM CaSO4 solution were measured and then root segments were perfused with 10 µM Fusicoccin (FC). Root segments were then perfused with 1 mM of the various BAs. Alternatively FC perfused root segments were perfused with 0.25 mM K2SO4 followed by perfusion with 1 mM of the various BAs.
Statistics
At least five replicates were performed for each experiment. The data obtained were analyzed by standard ANOVA using Systat 10.0 software. Significant differences are intended at p<0.05.
Results
Effect of benzoic acid derivatives on Vm
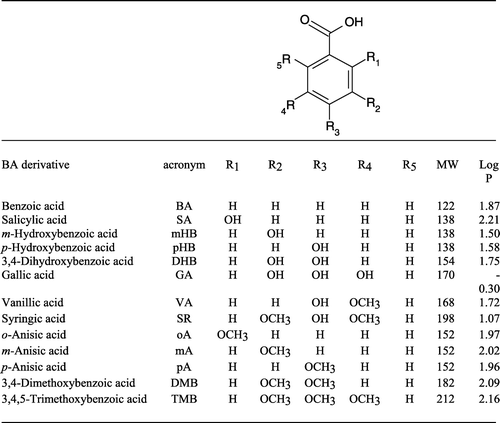
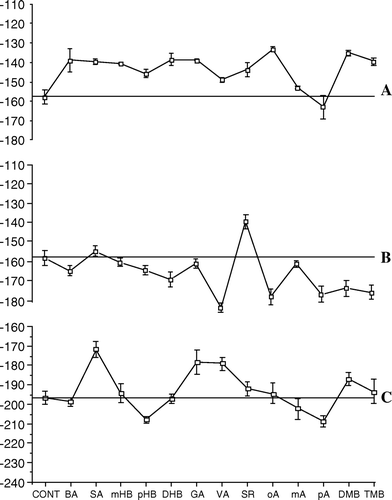
Cucumber root segments from seedlings germinating in Petri dishes with the only presence of MES-Na buffer show different responses to the perfusion with the various benzoic acid (BA) derivatives () when Vm is determined. shows the time-dependent variations in Vm after perfusion with hydroxylated BAs. Almost all hydroxylated BAs, except 3,4-dihydroxybenzoic acid (DHB), induce a rapid and slight depolarization. In particular, after perfusion with benzoic acid (BA) and p-hydroxybenzoic acid (pHB), depolarization is followed by a slow and constant hyperpolarization. The perfusion with salicylic (SA) and gallic acid (GA) induced a slight depolarization followed by a 2 min hyperpolarization with a final stabilization of the Vm towards depolarized values. Perfusion with DHB induced a 2 min slight hyperpolarization followed by depolarization, whereas perfusion with m-hydroxybenzoic acid (mHB) causes a rapid and slight depolarization followed by 1 min hyperpolarization, 13 min depolarization and finally hyperpolarization ().
Figure 2. Effect of hydroxylated BAs upon membrane potential in cucumber (Cucumis sativus) root segments.
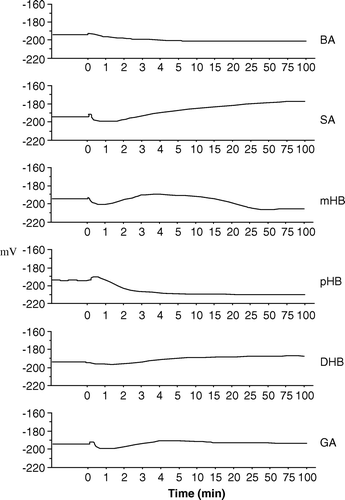
shows the effect of perfusion with methoxylated BAs on Vm. A strong and immediate Vm depolarization was observed after perfusion with vanillic acid (VA), o-anisic acid (oA), m–anisic acid (mA), 3,4,5-trimethoxybenzoic acid (TMB) and 3,4-dimethoxybenzoic acid (DMB). The latter showed a 1 min depolarization followed by two subsequent hyperpolarizations (). A small depolarization followed by hyperpolarization was found after syringic acid (SR) perfusion, whereas perfusion with p-anisic acid (pA) induced a short and slight depolarization followed by a 5 min hyperpolarization and finally by depolarization.
Figure 3. Effect of methoxylated BAs upon membrane potential in cucumber (Cucumis sativus) root segments.
In all treatments, perfusion with MES-Na buffer after BAs perfusion completely recovered the initial Vm, annulling the effect of the various BAs (data not shown).
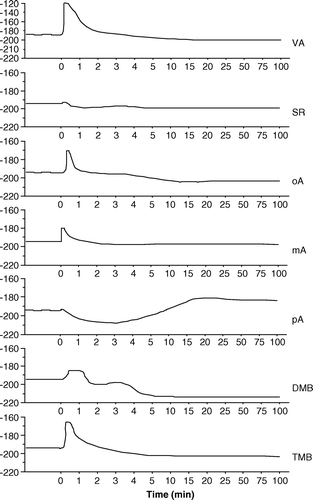
A extends and summarizes the results shown in and by giving the depolarizing or hyperpolarizing Vm after 100 min perfusion with respect to controls along with standard deviation. A consistent and significant Vm was found for SA, DHB and pA. On the contrary, a significant membrane hyperpolarization was found after perfusion with mHB, pHB, VA, SR, oA, DMB and TMB. No significant differences were found after perfusion with the other BAs under study.
Figure 4. Effect of benzoic acid derivatives on cucumber (Cucumis sativus) membrane potentials: depolarization and hyperpolarization with respect to controls. (A) 1 mM benzoic acid derivatives are added to control root segments; (B) Membrane depolarization and hyperpolarization with respect to controls of root segments from seedlings germinated in the presence of 1 mM of the various benzoic acid derivatives; (C) Effect of the addition of 1 mM of the appropriate benzoic acid derivatives on membrane potentials of seedlings germinated in the presence of 1 mM of the various benzoic acid derivatives. Bars indicate standard deviation. Values are expressed as mV.
To better understand the effect of BA derivatives on seed germination, seeds were germinated in the presence of 1 mM of the various BAs and the Vm measured on root segments (B). A significant membrane hyperpolarization was found, with respect to controls (Vm = − 194.84 mV, SD = 2.18), when seeds were grown in the presence of BA, DHB, DMB and TMB, whereas a significant Vm depolarization was found after seed germination in SA, pHB, GA and pA. No significant variations were found for the remaining BAs.
To evaluate the effect of BAs on seedlings grown in the presence of the various BAs, 1 mM of the appropriate BA was perfused into root segments (C). With respect to controls (Vm = − 194.84 mV, SD = 2.18), a significant Vm depolarization was observed after perfusion with SA and pA, whereas a significant Vm hyperpolarization was obtained after perfusion with BA, mHB, DHB, GA, VA, oA, mA, DMB and TMB (C), with no significant variations for the other BAs.
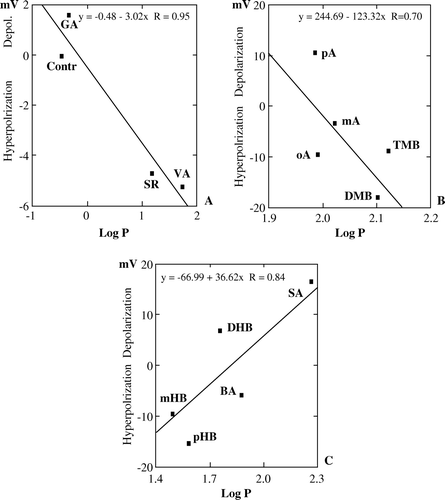
Correlation between benzoic acid derivatives and their partition coefficients
Vm hyperpolarization and depolarization obtained after perfusion of control root segments with the various BA derivatives were plotted against the logarithm of BAs partition coefficients (Log P) between octanol and water, according to Leo et al. (Citation1971). BAs were subdivided into three groups: BAs inducing small Vm hyper-/depolarization; methoxylated-BAs inducing high Vm hyper-/depolarization; hydroxylated-BAs inducing high Vm hyper-/depolarization (). The first group comprised control roots, GA and the two OH-/OCH3- substituted BAs, VA and SR. A similar significant negative correlation was found between Log P and depolarization (A). The same results were obtained with the group containing the methoxylated BAs (B). A significant positive correlation was found between Log P and the ability to depolarize Vm in the remaining group of hydroxylated BAs (C).
Figure 5. Relationship between the potency of BAs to induce hyperpolarization or depolarization and their octanol-water partition coefficient (Log P) in cucumber (Cucumis sativus) root segments. (A) significant positive correlation between Log P of controls and small Vm hyperpolarizing effects of GA and some hydroxymethoxy BAs; (B) Significant positive correlation between Log P and high Vm hyperpolarization induced by some methoxy-BAs; (C) Significant positive correlation between Vm depolarization and Log P of BA and some hydroxy-BAs.
Effect of benzoic acid derivatives in the presence of K+
Root segments of seedlings germinating in Petri dishes with the only MES-Na were perfused for thirty minutes with the various BAs followed by perfusion with 0.25 mM K2SO4. As expected, the latter induced a significant Vm depolarization in control root segments (Vm = − 157.68 mV; SD = 3.65). The addition of almost all BA derivatives, with the exception of pA, caused a significant Vm depolarization with respect to K+ depolarized control roots (A). The experiment was reversed by perfusing root segments first with K2SO4 for 10 min followed by perfusion with the various BAs (B). In this case, with respect to K+ depolarized control root segments (Vm = − 157.68 mV; SD = 3.65), a significant Vm hyperpolarization was found after perfusion with BA, pHB, DHB, VA, oA, pA, DMB and TMB, whereas SR caused a significant Vm depolarization (B). The addition of 0.25 mM Cs2SO4 induced a significant Vm hyperpolarization in K2SO4-depolarized control root segments (Vm = − 196.57 mV; SD = 4.08). However, the addition to K+ depolarized root segments of the various BAs showed a significantly reduced 0.25 mM Cs2SO4-hyperpolarization after perfusion with SA, GA, VA and DMB; whereas hyperpolarization was significantly increased in pHB and pA perfused root segments (C).
Figure 6. Effect of benzoic acid derivatives on K+−dependent membrane depolarization in cucumber (Cucumis sativus) root segments pre-incubated in 0.5 mM CaSO4: membrane depolarization and hyperpolarization with respect to controls. (A) perfusion with 1 mM benzoic acid derivatives to controls root segments for 30 min followed by perfusion with 0.25 mM K2SO4. Controls are root segments perfused with 0.25 mM K2SO4; (B) Addition of 1 mM benzoic acid derivatives after membrane depolarization caused by perfusion of root segments with 0.5 mM K2SO4. Controls are root segments perfused with 0.25 mM K2SO4; C, effect of addition of 0.25 mM Cs2SO4 to root segments perfused as (B) Controls are root segments perfused with 0.25 mM K2SO4 and then with 0.25 mM Cs2SO4. Bars indicate standard deviation. Values are expressed as mV.
Effect of benzoic acid derivatives in the presence of Fusicoccin
The fungal metabolite Fusicoccin (FC) induced a significant Vm hyperpolarization when perfused in control cucumber root segments (Vm = − 241.70 mV; SD = 1.29). The addition of the various BAs to FC hyperpolarized membranes caused a significant Vm depolarization after perfusion with BA, GA and SR, whereas a significant Vm hyperpolarization was obtained after perfusion with oA, mA, pA, DMB and TMB (A).
Figure 7. Effect of benzoic acid derivatives on Fusicoccin (FC) membrane hyperpolarization in cucumber (Cucumis sativus) root segments pre-incubated in 0.5 mM CaSO4: depolarization and hyperpolarization with respect to controls. (A) Addition of 1 mM benzoic acid derivatives to root segments perfused with 10 µM FC; (B) Addition of 1 mM benzoic acid derivatives to root segments perfused with 10 µM FC followed by perfusion with 0.25 mM K2SO4. Controls are root segments perfused with 10 µM FC followed by perfusion with 0.25 mM K2SO4. Bars indicate standard deviation.
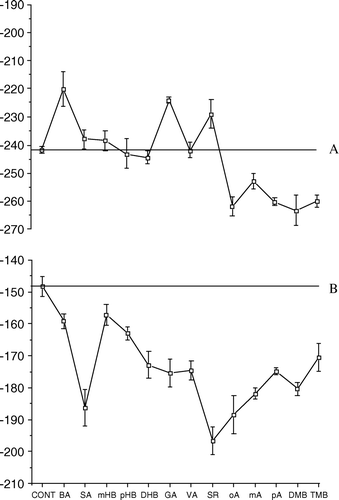
The addition of 0.25 mM K2SO4 to control FC hyperpolarized membranes caused in a few minutes a significant Vm depolarization (Vm = − 148.23 mV; SD 3.16). The subsequent addition of all BAs promoted a significant Vm hyperpolarization by reducing the K2SO4-depolarization (B).
Discussion
Benzoic acid hydroxy- and methoxy-ring substituents alter cucumber root membrane potential and exert several physiological effects, suggesting that membrane interactions are the initial site of action of such molecules (Seigler Citation1996). Rapid Vm changes after perfusion of some BAs confirm the general hypothesis that BAs act at the plasma membrane level (Blake Citation1985, Heipieper et al. Citation1991, Einhellig Citation1995, Baziramakenga et al. Citation1995, Seigler Citation1996, Crisan et al. Citation2007). The results obtained in this study give evidence for a differential effect of BA ring substituents on Vm, with a transient strong Vm depolarization, after perfusion by almost all BA methoxy-ring substituents. A similar differential effect of BA ring substituents was shown in a previous work instead in which a strong inhibitory effect of methoxy-BA and a promoting effect of hydroxy-BA were found on both germination and enzyme activity of isocitrate lyase and catalase (Maffei et al. Citation1999, Crisan et al. Citation2007). The BAs used in this work are likely to be distributed between the lipid components of the cell membrane and the aqueous buffered solution in which they are dissolved (according to their respective partition coefficients) (Leo et al. Citation1971, Yu & Matsui Citation1997). The rapid Vm alterations observed after BAs perfusion may be caused by changes in the permeability of the membrane, resulting in an imbalance in the electroneutral equilibrium. The plot of the difference of Vm after perfusion against Log P, clearly indicates that increasing lipophilicity of hydroxy-ring substituents is correlated to their capability to depolarize the membrane, whereas increased lipophilicity of BA methoxy derivatives parallels their ability to hyperpolarize Vm. In animal cells the relative polarity of either side of the membrane determines the resting, activated (depolarized), or recovering state (hyperpolarized) (Waber Citation1999), in some plants depolarization has been correlated with increased tolerance to abiotic (Olivetti et al. Citation1995) and biotic stress (Maffei et al. Citation2004, Citation2006, Citation2007). The direct comparison between Vm alteration found in the present study and the effect of BA derivatives on germination and enzyme activity and synthesis (Maffei et al. Citation1999, Crisan et al. Citation2007), leads us to suppose the existence of a possible correlation, on the one hand, between depolarizing action of BA OH-ring substituents, and hyperpolarizing activity of BA OCH3-ring substituents and inhibition of some metabolic processes. This would mainly depend on BA's capability to interact with biological membranes (lipophilicity).
The presence of BA and DHB in the incubation medium in which seeds were grown caused a significant Vm hyperpolarization, which was further enhanced by root perfusion with the same BAs. This situation could depend on the higher lipophilicity of DHB with respect to other BA OH-derivatives, with the exception of SA. Another interpretation could be that the increased Vm depolarizing effects of pA might depend on the lower lipophilicity of pA with respect to other methoxy-BAs. These data indicate that the effect of BAs might not be limited to the plasma membrane and that penetration into the cytosol might alter other metabolic processes (Maffei et al. Citation1999).
Rapid and extensive Vm depolarization after SA or DHB perfusion as well as the complete recovery of the Vm after removal of these BAs is in agreement with previous reports (Glass & Dunlop Citation1974).
The reduced Vm depolarization caused by perfusion with most of the BAs following K+ perfusion are consistent and in agreement with previous data reporting the ability of BAs to reduce K+ channel conductance. K+ uptake inhibition has been demonstrated for several BAs, including BA, SA, pHB, DHB, GA and VA (Glass & Dunlop Citation1974, Lyu et al. Citation1990, Yu & Matsui Citation1994, Citation1997, Yu et al. Citation2003). However experiments with the reversal of the treatment, suggest that pretreatment of root segments with BAs increased membrane Vm hyperpolarization [possibly by altering the membrane permeability (Yu & Matsui Citation1997) through action on K+ inward channels (Glass & Dunlop Citation1974)]. However, changes in Vm after perfusion with K+ may also depend on the effects of Ca2 + activity and fluxes (Maffei et al. Citation2007).
Cesium acts as a high affinity, strongly voltage-dependent blocker of inward K+ channels, and submillimolar concentrations are sufficient to block 50% of K+ uptake in the several systems tested (Sheahan et al. Citation1993, Draber & Hansen Citation1994, Hedrich et al. Citation1995, Zimmermann & Sentenac Citation1999, White & Broadley Citation2000). Salicylic acid, GA, VA and DMB significantly lowered the inhibitory effect of Cs+, whereas pHB and pA increased Cs+ inhibition. Since Cs+ transport is not relevant to the overall ion movement (Bellando et al. Citation1995) it seems reasonable to suppose that the above BAs might have an effect on the mechanism of Cs+ inhibitory action on K+ inward channels, even though the effects of Cs+ may also be indirect. Alternatively, these BAs might act on H+ pump operation, by affecting ATPase and/or ATP production, as suggested by some authors (Einhellig Citation1995, Yu & Matsui Citation1997). However, a preliminary result on H+ extrusion following pA incubation in cucumber root segments does not support the hypothesis of an increased H+-ATPase activity (Camusso et al. unpublished). Another possibility would be the action of BAs on anion channels; opening anion channel would usually depolarize Vm (Olivetti et al. Citation1995).
The fungal toxin FC exerts several physiological effects on plants, including acidification of the incubation medium through stimulation of H+ extrusion, stimulation of K+ uptake and hyperpolarization of the Vm. FC is an ideal tool for studying interference of phytochemicals with membranes since FC actually interferes with a general transduction pathway of the plant cell (Aducci et al. Citation1995 and references therein). BA, and the trisubstituted GA and SR reduced the FC-hyperpolarizing effect on root Vm, whereas the methoxylated BAs oA, mA, pA, DMB and TMB increased Vm hyperpolarization by increasing the FC effect on Vm. Since methoxylated BAs do not induce H+ extrusion when tested in root segments (Camusso et al. unpublished), a possible interaction of methoxylated-BAs with FC might be in binding to its receptor, because of their interaction with membrane fluidity. In fact, hyperpolarization prompted by oA, mA, pA, DMB and TMB, positively correlates with their Log P, whereas hydroxylated BAs (particularly SA) show a positive correlation between depolarization and their Log P. An interpretation could be that FC effect on cucumber root membranes is sensitive to alteration in membrane fluidity caused by hyperpolarizing or depolarizing effects of BAs, which correlate to their Log P. However, the effect of BAs on other cell compartments cannot be excluded. Whether BAs affect the binding of FC to the FC binding protein is at present unknown (DeMichelis et al. Citation1996). The strong depolarization of FC hyperpolarized membranes following addition of K+ has been explained as the increase of net K+ uptake with consequent increase of pH in both the cytosol and the vacuole (Bellando et al. Citation1995). The general reduction of the Vm depolarizing effect caused by the addition of BAs suggests both their influences on gating properties of K+ channels and a direct effect on FC activity.
In conclusion, the results of this investigation confirm the hypothesis that BAs may exert a significant allelopathic action on cucumber by interfering with several physiological and metabolic processes. Their action is linked to the nature of the ring substituents, with promoting and inhibiting effects associated to the presence of hydroxy- and methoxy-ring substituents, respectively. By inducing changes in Vm, BAs appear to interfere with K+ and FC, with consequent alteration of the cell osmolarity and whole cell and tissue physiology.
Acknowledgements
This work was supported by a M.U.R.S.T grant (ex quota 60%).
References
- Aducci , P , Marra , M , Fogliano , V and Fullone , MR . 1995 . Fusicoccin receptors – perception and transduction of the fusicoccin signal . J Experim Botany , 46 : 1463 – 1478 .
- Barkosky , RR and Einhellig , FA . 1993 . Effects of salicylic acid on plant water relationships . J Chem Ecol , 19 : 237 – 247 .
- Barkosky , RR and Einhellig , FA . 2003 . Allelopathic interference of plant-water relationships by parahydroxybenzoic acid . Botan Bull Academia Sinica , 44 : 53 – 58 .
- Barron , D , Balland , C , Possety , F , Ravanel , P and Desfougeres , A . 1996 . Prenyl flavonoids and membrane permeability . Acta Botanica Gallica , 143 : 509 – 520 .
- Batish , DR , Lavanya , K , Singh , HP and Kohli , RK . 2007 . Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea . Plant Growth Regul , 51 : 119 – 128 .
- Baziramakenga , R , Leroux , GD and Simard , RR . 1995 . Effects of benzoic and cinnamic acids on membrane permeability of soybean roots . J Chem Ecol , 21 : 1271 – 1285 .
- Bellando , M , Marré , MT , Sacco , S , Talarico , A , Venegoni , A and Marré , E . 1995 . Transmembrane potential-mediated coupling between H+ pump operation and K+ fluxes in Elodea densa leaves hyperpolarized by fusicoccin, light or acid load . Plant Cell Environ , 18 : 963 – 976 .
- Bergmark , CL , Jackson , WA , Volk , RJ and Blum , U . 1992 . Differential inhibition by ferulic acid of nitrate and ammonium uptake in Zea mays l . Plant Physiol , 98 : 639 – 645 .
- Blake , NE . 1985 . “ Effects of allelochemicals on mineral uptake and associated physiological processes ” . In The chemistry of allelopathy, biochemical interactions among plants , Edited by: Thompson , AC . 161 – 178 . Washington, DC : ACS, Symposium Series .
- Blum , U . 1996 . Allelopathic interactions involving phenolic acids . J Nematol , 28 : 259 – 267 .
- Booker , FL , Blum , U and Fiscus , EL . 1992 . Short-term effects of ferulic acid on ion uptake and water relations in cucumber seedlings . J Experim Botany , 43 : 649 – 655 .
- Bucker , CA , de Souza , SR and Fernandes , MS . 2006 . Effects of fusicoccin and vanadate on proton extrusion and potassium uptake by rice . J Plant Nutrit , 29 : 485 – 496 .
- Crisan , M , Grozav , M , Kurunczi , L , Ilia , G and Bertea , C . 2007 . Inhibitory effects of some synthetic monoethanolamine salts of para-substituted benzoic acids and corresponding benzoic acids on cucumber seed germination . J Plant Interact , 2 : 53 – 61 .
- DeBoer , B . 1997 . Fusicoccin – a key to multiple 14-3-3 locks? . Trends Plant Sci , 2 : 60 – 66 .
- DeMichelis , MI , Rasi Caldogno , F , Pugliarello , MC and Olivari , C . 1996 . Fusicoccin binding to its plasma membrane receptor and the activation of the plasma membrane H+-ATPase.III. Is there a direct interaction between the fusicoccin receptor and the plasma membrane H+-ATPase? . Plant Physiol , 110 : 957 – 964 .
- Draber , S and Hansen , UP . 1994 . Fast single-channel measurements resolve the blocking effect of Cs+ on the K+ channel . Biophys J , 67 : 120 – 129 .
- Einhellig FA . 1995 . Allelopathy – current status and future goals . In : Allelopathy . Acs symposium series . pp 1 – 24 .
- Gerig , TM and Blum , U . 1991 . Effects of mixtures of 4 phenolic acids on leaf-area expansion of cucumber seedlings grown in Portsmouth B1 soil materials . J Chem Ecol , 17 : 29 – 40 .
- Glass , ADM and Dunlop , J . 1974 . Influence of phenolic acids on ion uptake . Plant Physiol , 54 : 855 – 858 .
- Gniazdowska , A and Bogatek , R . 2005 . Allelopathic interactions between plants. Multi site action of allelochemicals . Acta Physiol Plant , 27 : 395 – 407 .
- Hedrich , R , Moran , O , Conti , F , Busch , H , Becker , D , Gambale , F , Dreyer , I , Kuch , A , Neuwinger , K and Palme , K . 1995 . Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators . Eur Biophys J , 24 : 107 – 115 .
- Heipieper , HJ , Keweloh , H and Rehm , HJ . 1991 . Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli . Appl Environ Microbiol , 57 : 1213 – 1217 .
- Huang , ZQ , Liao , LP and Wang , SL . 2000 . Allelopathy of phenolics from decomposing stump-roots in replant Chinese fir woodland . J Chem Ecol , 26 : 2211 – 2219 .
- Inderjit . 1996 . Plant phenolics in allelopathy . Botanical Rev 62 : 186 – 202 .
- Inderjit , Streibig JC , Olofsdotter M . 2002 . Joint action of phenolic acid mixtures and its significance in allelopathy research . Physiol Plant 114 : 422 – 428 .
- Inderjit , Weston LA , Duke SO . 2005 . Challenges, achievements and opportunities in allelopathy research . J Plant Interact 1 : 69 – 81 .
- Klejdus , B and Kuban , V . 1999 . Plant phenolic compounds in allelopathy . Chemicke Listy , 93 : 243 – 248 .
- Lehman , ME , Blum , U and Gerig , TM . 1994 . Simultaneous effects of ferulic and p-coumaric acids on cucumber leaf expansion in split-root experiments . J Chem Ecol , 20 : 1773 – 1782 .
- Leo , A , Hansch , C and Elkins , D . 1971 . Partition coefficients and their uses . Chem Rev , 71 : 525 – 616 .
- Lyu , SW and Blum , U . 1990 . Effects of ferulic acid, an allelopathic compound, on net P, K, and water uptake by cucumber seedlings in a split-root system . J Chem Ecol , 16 : 2429 – 2439 .
- Lyu , SW , Blum , U , Gerig , TM and Obrien , TE . 1990 . Effects of mixtures of phenolic acids on phosphorus uptake by cucumber seedlings . J Chem Ecol , 16 : 2559 – 2567 .
- Maffei , M , Bertea , CM , Garneri , F and Scannerini , S . 1999 . Effect of benzoic acid hydroxy and methoxy ring substituents during cucumber (Cucumis sativus L) germination. I. Isocitrate lyase and catalase activity . Plant Sci , 141 : 139 – 147 .
- Maffei M , Bossi S . 2006 . Electrophysiology and plant responses to biotic stress . In : Volkov , Alexaner, Plant electrophysiology – theory & methods . Berlin, Springer-Verlag . pp 461 – 481 .
- Maffei , M , Bossi , S , Spiteller , D , Mithöfer , A and Boland , W . 2004 . Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components . Plant Physiol , 134 : 1752 – 1762 .
- Maffei , M , Mithöfer , A and Boland , W . 2007 . Before gene expression: Early events in plant-insect interaction . Trends Plant Sci , 12 : 310 – 316 .
- Maffei , ME , Mithöfer , A , Arimura , GI , Uchtenhagen , H , Bossi , S , Bertea , CM , Starvaggi Cucuzza , L , Novero , M , Volpe , V , Quadro , S and Boland , W . 2006 . Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide . Plant Physiol , 140 : 1022 – 1035 .
- Olivetti , GP , Cumming , JR and Etherton , B . 1995 . Membrane potential depolarization of root cap cells precedes aluminum tolerance in snapbean . Plant Physiol , 109 : 123 – 129 .
- Pehlivan , E and Arslan , G . 2006 . Uptake of metal ions on humic acids . Energy sources part A: Recovery utilization and environmental effects , 28 : 1099 – 1112 .
- Politycka , B . 2002 . Physiological responses of cucumber to allelochemicals of phenolic compounds . Allelopathy J , 10 : 85 – 103 .
- Politycka , B and Bednarski , W . 2004 . Oxidative burst and lipoxygenase activity induced by hydroxycinnamic acids in cucumber roots . Allelopathy J , 14 : 187 – 196 .
- Reigosa , MJ , Souto , XC and Gonzalez , L . 1999 . Effect of phenolic compounds on the germination of six weeds species . Plant Growth Regul , 28 : 83 – 88 .
- Sacco , S and Maffei , M . 1997 . The effect of isosakuranetin (5,7-dihydroxy 4′-methoxy flavanone) on potassium uptake in wheat root segments . Phytochemistry , 46 : 245 – 248 .
- Seigler , DS . 1996 . Chemistry and mechanisms of allelopathic interactions . Agronomy J , 88 : 876 – 885 .
- Sene , M , Dore , T and Pellissier , F . 2000 . Effect of phenolic acids in soil under and between rows of a prior sorghum (Sorghum bicolor) crop on germination, emergence, and seedling growth of peanut (Arachis hypogea) . J Chem Ecol , 26 : 625 – 637 .
- Shafer , SR and Blum , U . 1991 . Influence of phenolic acids on microbial populations in the rhizosphere of cucumber . J Chem Ecol , 17 : 369 – 389 .
- Sheahan , JJ , Ribeironeto , L and Sussman , MR . 1993 . Cesium-insensitive mutants of Arabidopsis thaliana . Plant J , 3 : 647 – 656 .
- Siqueira , JO , Nair , MG , Hammerschmidt , R and Safir , GR . 1991 . Significance of phenolic-compounds in plant-soil-microbial systems . Crit Rev Plant Sci , 10 : 63 – 121 .
- van den Wijngaard , PWJ , Sinnige , MP , Roobeek , I , Reumer , A , Schoonheim , PJ , Mol , JNM , Wang , M and De Boer , AH . 2005 . Abscisic acid and 14-3-3 proteins control K+ channel activity in barley embryonic root . Plant J , 41 : 43 – 55 .
- Waber , S . 1999 . “ Modes of action at the target site ” . In Natural products from plants , Edited by: Kaufman , PB , Cseke , LJ , Waber , S , Duke , JA and Brielmann , HL . 157 – 182 . Boca Raton : CRC Press .
- White , PJ and Broadley , MR . 2000 . Mechanisms of cesium uptake by plants . New Phytol , 147 : 241 – 256 .
- Ye , SF , Yu , JQ , Peng , YH , Zheng , JH and Zou , LY . 2004 . Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates . Plant Soil , 263 : 143 – 150 .
- Yu , JQ and Matsui , Y . 1994 . Phytotoxic substances in root exudates of cucumber (Cucumis sativus L) . J Chem Ecol , 20 : 21 – 31 .
- Yu , JQ and Matsui , Y . 1997 . Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings . J Chem Ecol , 23 : 817 – 827 .
- Yu , JQ , Ye , SF , Zhang , MF and Hu , WH . 2003 . Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber . Biochem Syst Ecol , 31 : 129 – 139 .
- Zimmermann , S and Sentenac , H . 1999 . Plant ion channels: from molecular structures to physiological functions . Curr Opin Plant Biol , 2 : 477 – 482 .