Abstract
Although some moths are known to feed on lichens, few butterflies are reported to feed on them. We report conclusive evidence for the feeding and sequestration of lichen products from the lichen Leproloma sipmanianum by the wild-caught adults and larvae of Talicada nyseus nyseus (Lycaenidae) in Beragala, Uva Province, Sri Lanka. Here, the butterfly lays eggs on Kalanchoe pinnata, growing beside proterozoic rocks on which the lichen grows. HPLC/TLC analysis showed that the lichen compounds enter the adult through its larvae feeding on the lichen. It was also shown that larvae were not viable on the lichen alone without the host plant, but were healthy when the lichen and host plant were present. The presence of lichen compounds in the adult population throughout the season suggests that larvae of T. nyseus nyseus may be feeding on the lichen L. sipmanianum to obtain protection against predators during their life cycle.
Introduction
Lichens are symbiotic organisms of fungi (mycobionts) and algae (phycobionts) comprising about 18,000 species recorded worldwide (Hawksworth et al. Citation1995). In habitats where the amount of available nutrients is limited, lichens may become the dominant flora, thus providing an important potential source of food for herbivores. Lichens accumulate large concentrations of secondary metabolites, in particular aromatic phenolic compounds such as atranorin and usnic acid not found in higher plants (Huneck Citation1999). These compounds comprising up to 25% of the thallus’ dry weight (Romagni et al. Citation2004), originate from the fungal symbiont and, although they were thought to be absent in isolated lichen fungi (Lawrey Citation1989), advances in lichen-mycobiont isolation techniques have led to the production of lichen substances independently of the symbiotic state (Stocker-Wörgötter Citation2001). These compounds have been shown to have an antibiotic, anti-herbivore and plant enzyme inhibitory role in protecting the lichen and its photobiont (Lawrey Citation1986; Huneck and Yoshimura Citation1996; Nanayakkara et al. Citation2005). Lichen compounds such as (−)- and (+)-usnic acid, vulpinic acid and stictic acid have been shown to act as growth retardants on the larvae of a polyphagous insect pest Spodoptera littoralis (Emmerich et al. Citation1993).
Many species of invertebrates live on and among lichens, using them for concealment, shelter and/or food (Gerson and Seaward Citation1977). Other organisms known to consume lichens include orbit mites (Syed and Seaward Citation1984) and terrestrial gastropods (Baur et al. Citation1992). Moths of the family Arctiidae are well known lichen feeders and Hesbacher et al. (Citation1995) have shown that lichen phenolics such as parietin, atranorin and the hydrolytic cleavage product of atranorin (methyl-2,4-dihydroxy-3,6-dimethylbenzoate) were detected in 11 different species. However, Pöykkö and Hyvärinen (Citation2003) showed that members of the Arctiidae preferentially grazed on lichens that did not contain polyphenolic substances, and that these substances affected growth rate and survival of the larvae. Removal of secondary metabolites from the lichen also affects the food choice and survival of lichenivorous moth larvae (Pöykkö et al. Citation2005). Interestingly, females and larvae of the geometrid moth Cleorodes lichenaria prefer a lichen host that assures the shortest larval period (Pöykkö Citation2006). Gowan and Dickson (Citation1971) reported 6 species of Lycaenid butterflies in 4 genera as feeding solely on ‘rock’ or ‘tree’ lichens and this was extended to 14 species of Lycaenid in 7 genera by Pringle et al. (Citation1994). However there is no record of the lichen species or of the chemistry of the lichen.
Recent research in Sri Lanka has shown, for the first time, an association of a widespread butterfly Talicada nyseus nyseus Guerin-Meneville (Red Pierrot) with a leprose lichen Leproloma sipmanianum Kummerling & Leukert at Beragala, (80° 54' 30'' E, 6° 45' 30'' N), growing on rock below Horton Plains, Uva Province, where the natural food plant of the butterfly is Kalanchoe pinnata (Lam) Pers. The larvae have been found feeding on the host plant and on lichen growing on extensive adjacent rock surfaces. Karunaratne et al. (Citation2002) have demonstrated that lichen products found in L. sipmanianum are also present in the wild caught imagines, including zeorin 1, β-sitosterol 2, the fatty acid ester tritetracontylpentanoate 3, atranorin 4 and (+)-usnic acid 5.
In our present study, we conducted a series of experiments to confirm the presence of lichen compounds in a population of the butterfly T. nyseus nyseus, by High Performance Liquid Chromatography (HPLC). In order to determine the stage of entry of lichen compounds into the butterfly, adults, larvae, pupae and larval waste were subjected to chemical analysis.
In order to determine the effects of lichen compounds on the life cycle of the butterfly and of the progeny, in separate experiments, wild caught adults were caged in pairs in the presence of the host plant and their larvae reared under three different feeding regimes including the host plant K. pinnata and the lichen L. sipmanianum.
Materials and methods
Isolation of lichen substances from L. sipmanianum
Lichen powder (100 g) was ground together with the silica gel (100 g) and subjected to Medium Pressure Liquid Chromatography (eluent: CH2Cl2: hexane (1: 39) to MeOH) to yield, tritetracontylpentanoate 3, atranorin 3, β-sitosterol 2, (+)-usnic acid 5 and zeorin 1 and 3, 6-dimethyl-2-hydroxy-4-methoxybenzoic acid 6 (Kathirgamanathar et al. Citation2006). Compound 6 was not isolated previously from the dichloromethane extract of the lichen (Karunaratne et al. Citation2002). The isolated substances were authenticated using NMR and mass spectrometry.
Extraction and analysis of the T. nyseus nyseus specimens
Wild-caught (two collections made in February 2003 and May 2003) and laboratory-reared specimens (larvae (10), larval waste (15 mg), pupae (5) and the adults (5) which emerged from the larvae from each generation) of T. nyseus nyseus were freeze-dried, ground in a mortar and extracted with CH2Cl2. Solvent was removed under reduced pressure using a rotavapor. The CH2Cl2 extract of each specimen was analysed by HPLC and thin-layer chromatography (TLC).
HPLC analysis
Each CH2Cl2 extract was partially purified by fractionation by gravity column chromatography (eluent: CH2Cl2: hex [1: 4] to CH2Cl2) to obtain the fraction containing the aromatic compounds. They were injected (10 µl of a MeOH solution of 1 mg/ml) on a Nova-Pak C18 reverse phase column (Waters) (3.9×150 mm, 4 µm pore size) in the HPLC system (Waters 2690 Separation Module) equipped with a photodiode array detector (Waters PDA 996). Using linear gradient starting from 100% MeOH to (10% MeOH, 90% H2O adjusted to pH 2 with o-phosphoric acid) in 40 min the aromatic compounds separated. Identification of the lichen compounds was by comparison with retention times of the authentic compounds isolated from L. sipmanianum (Karunaratne et al. Citation2002).
TLC analysis
T. nyseus nyseus CH2Cl2 extracts were compared by co-TLC with authentic samples of zeorin 1 (eluent: MeOH: CH2Cl2 1: 9), β-sitosterol 2 (eluent: CH2Cl2) and tritetracontylpentanoate 3 (eluent: CH2Cl2: hex [3: 2]).
Evaluation of adaptability of wild-caught adults of T. nyseus nyseus collected from Beragala on only K. pinnata in a different environment
Collections of the adults, larvae, pupae and larval waste of T. nyseus nyseus were made in February and May 2003 from Beragala, Sri Lanka from a site where the host plant K. pinnata and the lichen occur in the same location (). A pair of wild-caught imagines was brought to a green house at Horticultural Crop Research and Development Institute (HORDI) reared in 5 separate cages (70×30 cm, each containing 3 potted plants of K. pinnata of approximately the same size) up to the F4 generation. In all cages temperature was 27±4°C and the relative humidity was maintained at 80±4%.
Evaluation of the effects of different nutritional regimes on the development of larvae
Three feeding regimes were set up with 10 larvae in each cage and 2 cages for each feeding regime, one with early instar and another with late instar larvae: (a) with K. pinnata only, (b) with K. pinnata and L. sipmanianum and (c) with L. sipmanianum only. The mean temperature and relative humidity in all cages (70×30 cm) were 27±4°C and 80±4%, respectively during the period of the experiment. Each cage was provided with (Experiments [a] and [c]) three potted plants of K. pinnata of approximately the same size. Lichen containing rocks (Experiments [b] and [c]) were approximate the same size containing comparable lichen patches. Larvae were observed up to the F2 generation.
Evaluation of survival fitness of T. nyseus nyseus in the presence of supplementary food and on a related Kalanchoe species
A pair male and female wild-caught adults (caught from Royal Botanical Gardens, Peradeniya) were placed in each of 20 cages (70×30 cm) containing three potted plants of K. pinnata and K. laciniata (10 cages for each plant of approximately the same size). Ten cages (5 each containing K. pinnata and K. laciniata) were supplied with 10 cotton receptacles each where 5 were soaked in 10 M sugar solution and the other 5 with bees’ honey solution; the other 10 cages (five each of each plant species) were supplied with 10 cotton receptacles each soaked in only water. The following data were recorded during the experiments: (i) number of eggs laid by a single female; (ii) the time taken for the eggs to hatch; (iii) the time taken from the larval to pupal stages; (iv) the time taken for adults to emerge from pupae and the life span of the adults with and without the artificial food. Data were analyzed using statistical software MINITAB 14 at p=0.05.
Results
The% contribution of compounds in the thallus of the lichen L. sipmanianum () shows that atranorin is a major component and β- sitosterol a minor component of the lichen thallus. The latter substance is a major component (88% of the triterpenoid content) in K. pinnata (Gaind and Gupta Citation1972).
Table 1 Content of compounds in the lichen L. sipmanianum.
The aromatic compound structures are shown in and the HPLC peaks of the wild caught adults, larvae, larval waste, pupae and the authentic compounds found in the lichen (4-6) are shown in . Compound 6, which is presumably related biosynthetically to atranorin 4, was not detected in our previous study. The non-aromatic lichen products 1–3 were detected by TLC and confirmed by co-TLC with authentic samples.
Figure 2. The lichen specific aromatic compounds atranorin 4, (+)-usnic acid 5, 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid 6 common to the butterfly T. nyseus nyseus and the lichen L. sipmanianum.
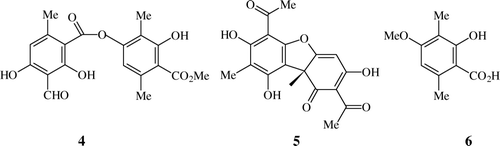
Figure 3. HPLC of:(a)wild-caught adults (b)typical pro .les of larvae,pupae and larval waste collected from Beragala (c)authentic compounds isolated from the lichen.
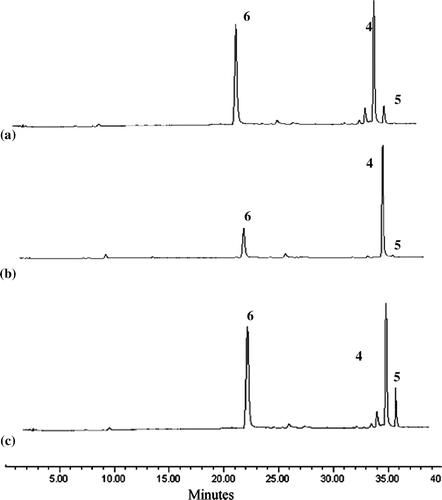
TLC and HPLC profiles of adults, larvae, pupae and larval waste extracts provide unequivocal evidence of the presence of non-aromatic compounds 1–3 and lichen-specific aromatic compounds 4–6 in the butterfly at all stages where compounds 4 and acid 6 were present in larger amounts in all extracts compared to (+)-usnic acid 5 which was observed only in trace amounts. Not surprisingly, the latter was also the least prevalent compound present in the lichen L. sipmanianum (). The presence of lichen substances 4, 5 and 6 and the lichen-specific triterpenoid zeorin 1 in larvae and the larval waste collected (both February and May 2003 collections) from Beragala clearly demonstrate that the larvae had ingested the lichen substances and that these substances are present in the adult butterflies with no apparent adverse effect.
Experiments on larvae and food source
Where the larvae fed only on L. sipmanianum all stages of the life cycle were detrimentally affected. Early instar larvae all died and of the late instar larvae only 3 larger individuals survived to adulthood giving rise to deformed or poorly developed adults of small size. These contained lichen substances 1–6. Adult female butterflies which emerged from the reared larvae did not lay eggs on L. sipmanianum. Where cages contained K. pinnata and L. sipmanianum healthy adults emerged. F1 generation contained the lichen substances. When T. nyseus nyseus specimens were reared up to F2 generations on K. pinnnata or K. laciniata no lichen products were found. However, variations in life cycle and in number of eggs laid are clearly related to additional nutrient supplied in the cages as sugar solution.
Butterflies reared in the presence of the host plant K. pinnata with and without supplementary food (; Experiments [a] and [b]) and in the presence of the related plant species. K. laciniata (; Experiment [c]) showed that T. nyseus nyseus thrived when supplementary food (sugar and honey) was present. When supplementary food was not provided not only was the ovipositing ability of the female impaired but also the adult life span was reduced, indicating that the life cycle of the butterfly was affected by the amount of nutrient available. The foregoing also suggested that T. nyseus nyseus’ host shifts might occur between related plants. In all experiments carried out, the oviposition time ranged between 4–5 days; eggs hatched between 2–8 days; the larval development ranged between 3–16 days; pupae remained dormant for 5–12 days and the life span of the adult ranged between 4–16 days. The average life cycle of T. nyseus nyseus was 21–57 days.
Table 2 Content of lichen compounds in wild-caught specimens (imagines, larvae, larval waste and pupae) and the laboratory reared specimens of T. nyseus nyseus.
Table 3 Average life-time (days) of different stages of T. nyseus nyseus reared on K. pinnata and K. laciniata.
Discussion
The butterfly T. nyseus nyseus is widespread throughout Sri Lanka, being equally abundant in gardens and in the wild (Woodhouse Citation1952) where the food plant K. pinnata is also widely available and frequently planted. The adults of T. nyseus nyseus feed on nectar from the bushes of Lantana camara (Singh Citation2005) growing freely in the same location. In contrast, L. sipmanianum has only been recorded at altitudes above 1000 m in Uva Province (Orange et al. Citation2001), so is not available to the butterfly over most of its range. As such, when reared at lower altitudes on Kalanchoe species the larvae and butterfly do not contain lichen products. However the experiments on feeding regimes and supplementary diet show that the life cycle and growth rate of T. nyseus nyseus is strongly affected by the nutritional value of the diet, as demonstrated for moths by Pöykkö and Hyvärinen (Citation2003) where growth rate and survival are adversely affected by the presence of lichen compounds. Pöykkö and Hyvärinen also showed that the effect is proportional to the amount of lichen compound ingested. During the period of population sampling at Beragala the butterflies sampled all contained the specific lichen products atranorin 4, (+)-usnic acid 5, 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid 6 and zeorin 1, suggesting that this population of T. nyseus nyseus were regularly feeding on L. sipmanianum in combination with Kalanchoe pinnata. Both atranorin 4 and usnic acid 5 have been shown to have toxic and adverse effects on the growth and development of generalist herbivores (Lawrey Citation1986; Emmerich et al. Citation1993; Romagni et al. Citation2004). While this paper has shown that lichen compounds in L. sipmannianum had deleterious effects on larvae fed only on the lichen, larvae fed on L. sipmanianum together with the food plant were healthy. Lichen products pass through the larvae of T. nyseus nyseus and enter the adult butterflies with no apparent adverse effect on their life cycle, lending credence to the beneficial effects of a host plant/lichen diet. Regular sampling of the population throughout the butterfly season at Beragala showed that lichen compounds were present in all butterflies sampled at this site, suggesting that the larvae are regularly feeding on L. sipmannianum together with Kalanchoe pinnata. It is well known that Monarch butterflies (Nymphalidae) accumulate toxic cardiac glycosides in milkweeds, which are used by the adults to deter predators (Reichstein et al. Citation1968), and we suggest that the presence of toxic products in the larvae and adult butterfly of T. nysseus nyseus may deter predators. Further observations on predators of this population of Talicada nysseus nyseus in upland Sri Lanka are required to elucidate this hypothesis.
Acknowledgements
Authors thank the NSF and NRC Sri Lanka and IFS Sweden and the OPCW for a research grant to Dr Veranja Karunaratne. Pat Haynes and anonymous reviewers are thanked for helpful comments and further information.
References
- Baur , A , Baur , B and Froberg , L . 1992 . The effect of lichen diet on the growth rate of rock-dwelling land snails Chondrina clienta (Westerlund) and Balea perversa (Linnarus) . J Moll Stud , 58 : 345 – 347 .
- Emmerich , R , Giez , I , Lange , OL and Proksch , P . 1993 . Toxicity and antifeedant activity of lichen compounds against the polyphagous herbivorous insect Spodopera littoralis . Phytochemistry , 33 : 1389 – 1394 .
- Gaind , KN and Gupta , RL . 1972 . Alkanes, alkanols, triterpenes and sterols of Kalanchoe pinnata . Phytochemistry , 11 : 1500 – 1502 .
- Gerson , U and Seaward , MRD . 1977 . “ Lichen-invertebrate associations ” . In Lichen ecology , Edited by: Seaward , MRD . 69 – 119 . New York : Academic Press .
- Gowan , C and Dickson , CGC . 1971 . Life histories of the South African Lycaenid butterflies , Cape Town : Purnell .
- Hawksworth , DL , Kirk , PM , Sutton , BC and Pegler , DN . 1995 . Dictionary of fungi , Wallingford, , UK : CAB International .
- Hesbacher , S , Giez , I , Embacher , G , Fiedler , K , Max , W , Trawoger , A , Türk , R , Lange , OL and Proksch , P . 1995 . Sequestration of lichen compounds by lichen-feeding members of the Arctiidae (Lepidoptera) . J Chem Ecol , 12 : 2079 – 2089 .
- Huneck , S . 1999 . The significance of lichens and their metabolites . Naturwissenschaften , 86 : 559 – 570 .
- Huneck , S and Yoshimura , I . 1996 . Identification of lichen substances , 493 New York : Springer .
- Karunaratne , V , Bombuwela , K , Kathirgamanathar , S , Kumar , V , Karuaratne , DN , Ranawana , KB , Wijesundara , DSA , Weerasooriya , A and De Silva , ED . 2002 . An association between the butterfly Talicada nyseus and the lichen Leproloma sipmanianum as evidenced from chemical studies . Current Sci , 83 : 741 – 745 .
- Kathirgamanathar S , Wickramasinghe A , Karunananda Bombuwela K , Wolseley , Karunaratne , V . 2006 . Chemistry of two new Leprarioid lichens from Sri Lanka . J Nat Sci Foundation Sri Lanka 34 : 85 – 90 .
- Lawrey , JD . 1986 . Biological role of lichen substances . Bryologist , 89 : 111 – 122 .
- Lawrey , JD . 1989 . Lichen secondary compounds: Evidence for a correspondence between antiherbivore and antimicrobial function . Bryologist , 92 : 326 – 328 .
- Nanayakkara , C , Bombuwela , K , Kathirgamanathar , S , Adikaram , NKB , Wijesundara , DSA , Hariharan , GN , Wolseley , P and Karunaratne , V . 2005 . Effect of some lichen extracts from Sri Lanka on larvae of Aedes aegypti and the fungus Cladosporium cladosporioides . J Nat Sci Foundation Sri Lanka , 33 : 147 – 149 .
- Orange A Wolseley P Karunaratne V Bombuwela K 2001 . Two Leprarioid lichens new to Sri Lanka . In : McCarthy PM Kantvilas G Louwhoff SHJJ . Lichenological contributions in honour of Jack Elix. Bibliotheca Lichenologica , J. Cramer . Berlin, Stuttgart : Bibliotheca Lichenologica 78 : 327 – 333 .
- Pöykkö , H , Hyvärinen , M and Backor , M . 2005 . Removal of lichen secondary metabolites affects food choice and survival of lichenivorous moth larvae . Ecology , 86 : 2623 – 2632 .
- Pöykkö , H . 2006 . Females and larvae of a geometrid moth, Cleorodes lichenaria, prefer a lichen host that assures shortest larval period . Experim Entomol , 35 : 1669 – 1676 .
- Pöykkö , H and Hyvärinen , M . 2003 . Host preference and performance of Licheniverous Eilema spp. larvae in relation to lichen secondary metabolites . J Animal Ecol , 72 : 383 – 390 .
- Pringle E , Henning G , Ball J editors. 1994 Pennington's butterflies of Southern Africa . Cape Town : Struick .
- Reichstein , T , Euw , JV , Parsons , JA and Rothschilde , M . 1968 . Heart poisons in the Monarch butterfly . Science NY , 161 : 861 – 866 .
- Romagni , JG , Rosell , RC , Nanayakkara , NPD and Dayan , FE . 2004 . “ Ecophysiology and potential modes of action for selected lichen secondary metabolites ” . In Allelopathy , Edited by: Macias , FA , Galindo , JCG , Molinillo , JM and Cutler , HG . 13 – 33 . New York : CRC Press .
- Singh , AP . 2005 . Initial colonisation of Red Pierrot butterfly, Talicada nyseus nyseus Guerin (Lycaenidae) in the lower western Himalaya: An indicator of the changing environment . Current Sci , 89 : 41 – 42 .
- Stocker-Wörgötter , E. 2001 . Experimental lichenology and microbiology of lichens: Culture experiments, secondary chemistry of cultured mycobionts, resynthesis, and thallus morphogenesis . Bryologist , 104 : 576 – 581 .
- Syed , EL and Seaward , MRD . 1984 . The association of orbit mites with lichens . Zool J Linn Soc , 80 : 369 – 420 .
- Woodhouse , LGO. 1952 . The butterfly fauna of Ceylon , 2nd ed , Colombo : Ceylon Gov. Press .
