Abstract
The chemical interaction between plants is known as allelopathy and it is related to the release of substances into the environment. The present study aimed at the evaluation of the allelopathic activity of the leaves of Leonurus sibiricus against the germination and initial growth of Raphanus sativus, Lactuca sativa, and Lepidium sativum. Chemical analyses showed the presence in the leaves of four major flavonoids (quercetin-3-O-α-L-rhamnopyranosyl-(1>6)-β-D-galactopyranoside; rutin; hyperin, and isoquercetrin) and of three minor flavonoidic compounds (genkwanin, 3′-hydroxy genkwanin, and quercetin). Extracts, their chromatographic fractions and pure isolated flavonoids showed different biological activities. A methanol extract of leaves of Leonurus sibiricus caused significant reduction only in the germination of Lactuca sativa, with no effects on the germinative processes of Raphanus sativus and Lepidium sativum. Some chromatographic fractions, containing the flavonoids, showed inhibitory activity on the initial stages of root growth of all tested seeds. The isolated flavonoids, at the higher concentration tested (10−4 M) seemed to be responsible for the inhibition of the germination, as well as the radical elongation. Among pure compounds, 3′-OH-genkwanin and quercetin showed the stronger antigerminative activity at the concentration of 10−4 M, whereas the radical elongation was reduced by rutin, isoquercetrin and 3′-OH-genkwanin. All compounds, tested at concentrations ranging between 10−5 and 10−7 M, showed stimulatory activities.
Introduction
The allelochemicals produced by the secondary metabolism pathways influence the development and the establishment of crops and natural plant communities. Researchers and agriculturists recognize the allelochemicals as a viable alternative to synthetic pesticides, aiming at the environmental pollution decrease and the increase of agricultural productions (Qasem and Foy Citation2001; Duke et al. Citation2002). The chemical richness of the aromatic and medicinal plants constitutes a rich source of biologically active compounds, useful both in medicine and agriculture (Mathela Citation1991; Cutler and Cutler Citation1999).
Leonurus sibiricus L. (Lamiaceae), a plant native of India and now naturalized in South America, contains terpenoids and phenolic substances with demonstrated allelopathic effects (Larcher Citation2000). It was hypothesized that these compounds can explain the invasive behaviour of L. sibiricus in orchards and coffee plantations (Lorenzi Citation2002). This species introduce into the environment radical exudates that increase the germination of rice, wheat and mustard (Mandal Citation2001); the aqueous extract of its leaves inhibits the corn germination and the growth of tomato seedlings (Almeida et al. Citation2003).
Alkaloids (Murakami Citation1943), terpenoids (Savona et al. Citation1982; Boalino et al. Citation2004; Almeida et al. Citation2005) and furanolactones (Satoh et al. Citation2003) have been isolated from the plant, together with rutin and its derivatives and methoxylated flavones (Almeida et al. Citation2006). These latter compounds seem to be important chemotaxonomic markers in the genus Leonurus (Giang et al. Citation2005).
Flavonoids have an important role as defence compounds in plants, as they are signalizing molecules in the reproduction, pathogenesis, and symbiosis. Plants produce flavonoids in large scale and such compounds play relevant roles in plant-plant and plant-microorganisms interactions, when released into the environment (Rice Citation1984; Shirley Citation1996). In the specific literature, several hypotheses have been made on the structure/phytotoxic activity of flavonoids (Macias et al. Citation1997; Bais et al. Citation2003; Parvez et al. Citation2004; Beninger and Hall Citation2005).
The objective of this work was to study the possible allelopathic effect of leaves of Leonurus sibiricus, by an in vitro study of the biological activity of extracts, chromatographic fractions and pure isolated compounds on the germination and the radical growth of Lepidium sativum, Lactuca sativa and Raphanus sativus.
Materials and methods
Plant material
Leaves of Leonurus sibiricus were collected in Botucatu, Sao Paulo State, Brazil, and identified by Clemente José Campos. A voucher specimen of the plant, labelled as 12-706, is deposited at the Herbarium of the Department of Botany, Bioscience Institute at the University of Sao Paulo State, Brazil (BOTU).
Extraction and fractionation
Two different extraction procedures have been utilized in order: (i) to obtain a HPLC profile of an aqueous and a methanol extract of fresh leaves of L. sibiricus, and (ii) to isolate the compounds responsible for the biological activity.
HPLC profile – water extract
One g of dry leaves, in three repetitions, was placed in boiling water (50 ml) and filtered. Twenty mg of the residue obtained by liophylization of this extract were diluted in 15 ml of a mixture of methanol and water (1:1) and filtered in m PFE membrane filter and directly analyzed in HPLC.
HPLC profile – methanol extract
Two g of dry leaves were added to 50 ml of chloroform and submitted to ultrasonic extraction for 4 h. The leaves were dried and 1 g was again extracted with 25 ml of methanol in an ultrasonic bath for 4 h. Twenty mg of this extract were filtered by a cartridge (SPE) SEP PAK C18 (360 g) (Waters, MA, USA), activated with 10 ml of methanol and 20 ml of deionized water and eluted with 15 ml of methanol. This extract was filtered in m PFE membrane filter and directly analyzed in HPLC.
Extraction and isolation of flavonoids
A total of 2.3 kg of dry leaves of L. sibiricus were extracted with methanol at room temperature, for five days, resulting, after evaporation under reduced pressure, in 389.6 g of extract. A portion of the methanolic extract (5 g) was fractionated by gel permeation chromatography, using a Sephadex LH-20 column (1.2 m × 6 cm) eluting with methanol. 201 fractions were collected and combined, according their TLC similarity in 75:25 Hexane:Ethyl acetate and 80:18:2 Chloroform:Methanol:Water developed with anisaldeyde and NP-Peg, in 12 main fractions. Major fraction 5, 6, 7, 10, 11 and 12, showing the biological activity, were purified by HPLC.
HPLC analyses
HPLC analyses were performed with a Varian Pro-Star apparatus equipped with a reverse phase silica column (250 mn × 460 mm i.d., 10 m, Phenomenex Luna). The used mobile phase was 28–35% linear gradient with water (30 min), increasing to 35–70% (60 min), 70–100% (65 min) of acetonitrile eluted in 1.0 ml min−1 flow, and monitored using Pro Star 330 ultraviolet detector with photodiode at 254 nm.
Identification of compounds
The isolated flavonoids were identified through 1H and 13C NMR analyses, obtained with a Varian INOVA apparatus operating at 300 and 500 MHz and by comparison with literature data (Agrawal Citation1989).
Biological assays
Aqueous and methanol extracts, at doses of 50, 100, 200, 400 and 800 mg/l, chromatographic fractions at a dose of 1 gl−1, and the isolated flavonoids, at concentrations ranging between 10−4 and 10−7 M, were tested for their effects on the germination and the radical elongation of Lepidium sativum L., Lactuca sativa L. and Raphanus sativus L.
The test seeds were surface-sterilized in 95% ethanol for 15 s and sown in Petri dishes (Ø = 90 mm), containing five layers of Whatman filter paper, impregnated with 7 ml of distilled water (control) or 7 ml of tested solution. The germination conditions were as follow: for radish and cress seeds, 20±1°C, and for lettuce seeds, 24±1°C, with natural photoperiod. Seed germination process was observed directly in Petri dishes, each 24 h. A seed was considered germinated when the protrusion of the radical became evident (De Feo et al. Citation2003). Each determination was repeated three times, using Petri dishes containing 10 seeds each.
Statistical analysis
The obtained data were submitted to the mean variance analysis and polynomial regression for the statistic analysis.
Results and discussion
The results show that both aqueous and methanol extract of Leonurus sibiricus leaves significantly affected only the germination of Lactuca sativa, whereas no activity was registered on the germination of the other two species tested (). On the other hand, the radical elongation of Raphanus sativus (), Lactuca sativa () and Lepidium sativum () was more severely affected by methanol extract, at the highest dose tested. The lower doses of this extract, however, resulted in an increase in radical length of R. sativus (b).
Figure 1. Effects of different doses of aqueous (a) and methanol (b) extracts of Leonurus sibiricus leaves on root elongation of Raphanus sativus, 120 h after sowing.
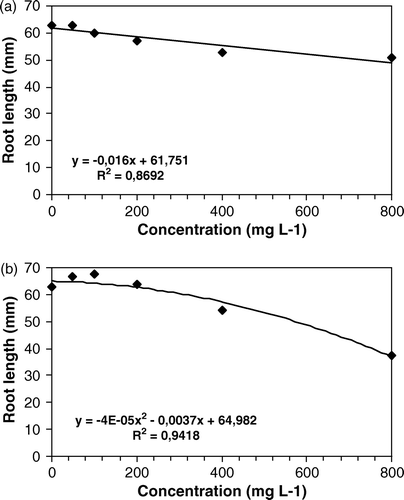
Figure 2. Effects of different doses of aqueous (a) and methanol (b) extracts of Leonurus sibiricus leaves on root elongation of Lactuca sativa, 120 h after sowing.
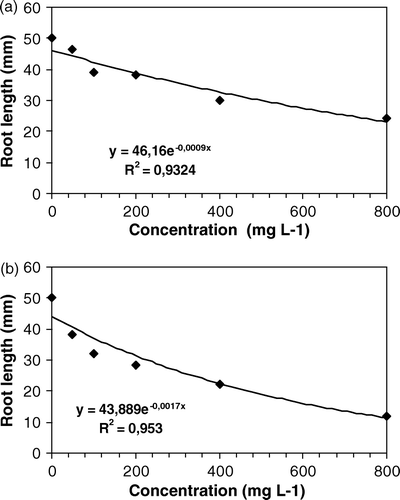
Figure 3. Effects of different doses of aqueous (a) and methanol (b) extracts of Leonurus sibiricus leaves, on root elongation of Lepidium sativum, 120 h after sowing.
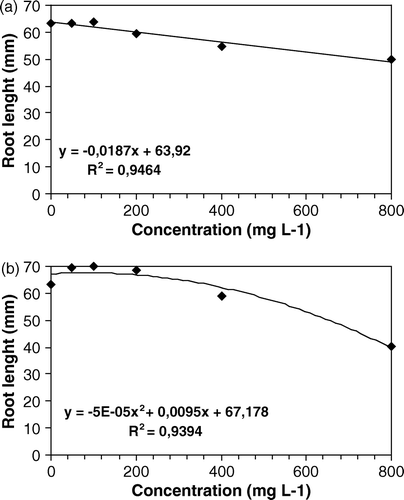
Table 1 Effects of different doses of aqueous and methanol extracts on Leonurus sibiricus on the germination of seeds of Raphanus sativus, Lactuca sativa and Lepidium sativum, 120 h after sowing. Data are the mean of three experiments±SD.
Radical elongation of L. sativa was affected by both aqueous (a) and methanol extract (b), in a dose-dependent manner. Lactuca sativa seed resulted more sensitive to L. sibiricus, as reported in the literature. The germination of L. sativum appeared no sensitive to L. sibiricus extracts (), while radical elongation was affected only at the highest dose tested ().
The statistical analyses with linear arrangement performed on the activity of aqueous extract show a constant inhibition speed, excepting for L. sativa. On the other hand, the second-degree-equations show a maximum point, being the biological activity reduced at different doses. Methanol extract exert a low stimulatory activity on R. sativus and L. sativum at lower doses tested and an inhibitory activity at the higher doses. The analysis of data relative to radical elongation of L. sativa, a species commonly known as sensitive to the allelopathic compounds (Hoagland and Williams Citation2004), have shown an exponential function, with a tendency to reach a plateau in biological activity. Mathematic models demonstrate that in low doses, depending on the species, a stimulation is probable in either germination, development or even in the attraction of polinizers (Stebbing Citation1982; Ferreira and Aquila Citation2000; An and Wagga Citation2005).
The chromatographic profile of both extracts shows the presence of four main flavonoids: quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)- β-D-galactopyranoside, rutin, hyperin, and isoquercitrin (). The presence of the same main flavonoidic compounds in both extracts suggests similar biological activities; however, methanol extract showed a greater biological activities. It is possible that the different extraction procedure caused an extraction of the same main substances, but in different concentrations, and subsequent different levels of biological activity (Macias et al. Citation1997; Chon et al. Citation2005). This is confirmed in our experiments, where the four compounds were present in methanol extract in quantities about three times that present in aqueous extract (). Methanol appears to be more efficient in extracting flavonoids, especially when these compounds are polar molecules.
Table 2 HPLC quantification of major flavonoids of the aqueous and methanol extracts of Leonurus sibiricus. Data are expressed in mg g−1 of dry leaves±SD.
Also, chromatographic fractions containing flavonoids (5–7 and 10–12) showed biological activity similar to those of methanol extract, in particular against the radical elongation of the three species tested, being the germination little affected ().
Table 3 Biological activtities of Sephadex fractions (1 g l−1) from the methanol extract of Leonurus sibiricus against germination and radical elongation of Raphanus sativus, Lactuca sativa and Lepidium sativum.
These biologically active, flavonoid-rich fractions were further purified by HPLC resulting in seven pure compounds, the diglycosides quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside and rutin from fractions 5 and 6; the two monoglycosides, hyperin and isoquercetrin, from fractions 6 and 7; the two methoxylated flavonoid aglycones, genkwanin and 3′-OH-genkwanin, from fractions 10 and 11, and the flavonoid aglycone quercetin from the fraction 12.
Based on the observation that biological activities of such fractions were most evident only on L. sativa seeds, we decided to utilize this seed in assays with the pure isolated compounds, with the aim to ascertain the possible relationships between the molecular structure of the compounds and the biological activity.
As evident from , it is possible to note that the pure flavonoids exerted their biological activity mainly in the first 48 h. The order of inhibitory potency registered for the isolated flavonoids is as follows: 3′-OH-genkwanin > quercetin > isoquercetrin > rutin > genkwanin > hyperin > quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)- β-D-galactopyranoside.
Table 4 Seed germination of Lactuca sativa under different concentrations of isolated flavonoids.
The biological activity showed by 3′-OH-genkwanin and quercetin may be attributed to presence in the molecules of a catechol group and of a meta oxyl group. Flavonoids with this structural arrangement are reported to alter the permeability of the cellular membranes and to affect the radicular lengthening necessary for the root protrusion (Glass and Dunlop Citation1974; Martínez-Flórez et al. Citation2002; Einhellig Citation2004). On the other hand, the presence of sugar moieties in the flavonoids seems to be uninfluent: in fact, both isoquercetrin and rutin showed inhibitory activity against lettuce germination, whereas hyperin and quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside do not significantly affect the germination.
The inhibitory activity registered on the first day suggests that probably the active flavonoids affected the cellular lengthening mechanism(s) (Hoagland and Williams Citation2004). Moreover, on the fourth day, possible detoxification mechanism(s) might have happened, hindering the effect of inhibitory substances through the activation of the enzymes of the oxidative metabolism, considering that such highly water-soluble flavonoids can be easily transported to the vacuole.
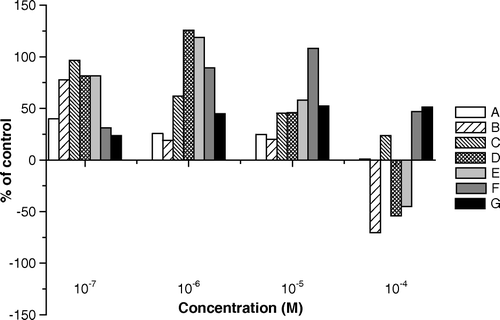
In the evaluation of Lactuca sativa assays, the radical elongation seems to be a good indicator of the allelopathic activity of the tested flavonoids. At concentration of 10−4 M, rutin caused a reduction of the root initial length by 70%, isoquercitrin by 54%, and 3′-OH-genkwanin by 45%. However, at the lowest concentration tested, radical growth was stimulated up to 50% by the aglycones genkwanin and quercetin, and in smaller extents, by hiperine ().
Figure 4. Chromatographic profile of aqueous (I) and methanol (II) extracts of leaves of Leonurus sibiricus showing the main flavonoidic compounds.
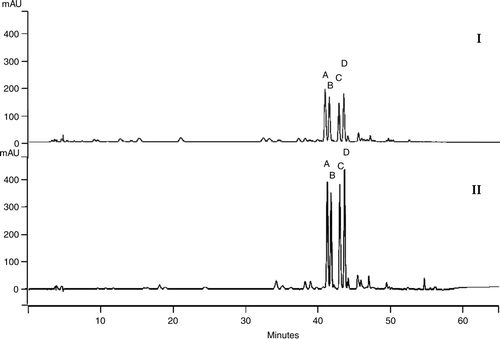
At concentration of 10−5 M, all the tested substances stimulated the radical elongation with this decreasing order of potency: genkwanin > 3′-OH-genkwanin > quercetin > hyperin = isoquercitrin > quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside > rutin. At this concentration, the aglycone flavonoids seem to promote the radical elongation more effectively than the glycosylated flavonoids.
A similar activity was registered when the compounds were tested at 10−6 M, with the following decreasing order of potency: isoquercitrin > 3′-OH-genkwanin > genkwanin > hyperin > quercetin > quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside > rutin. At this concentration, it is evident that the diglycosides tested presented low stimulating activity if compared to other flavonoids.
The flavonoid aglycones showed lower stimulatory activity at concentration of 10−7 M (). At this concentration, the order of stimulatory potency is as follows: hyperin > isoquercitrin = 3′-OH-genkwanin > rutin > quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside > genkwanin > quercetin.
Figure 5. Effects of different concentrations of the flavonoids isolated from L. sibiricus leaves on root elongation of Lactuca sativa, 120 h after sowing. A = quercetin-3-O-α-L-rhamnopyranosyl-(1 > 6)-β-D-galactopyranoside; B = rutin; C = hyperin; D = isoquercetrin; E = 3′-OH-genkwanin; F = genkwanin; G = quercetin.
The different biological activity among chromatographic fractions containing flavonoids and the pure isolated compounds can be attributed to possible synergistic effects.
Our results relative to the biological activities on seeds of rutin and isoquecitrin agree with some literature reports (Parvez et al. Citation2004). Recently, the flavonoid-3-glycosides isorhamnetin-3-O-rutinoside and isorhamnetin-3-O-robinobioside, isolated from the cactaceous Hylocereus undatus, have been reported for their allelopathic properties (Parvez et al. Citation2004).
Among the flavonoid aglycones tested, the methoxylated compounds showed a stronger biological activity, probably depending on the presence of a catechol group and of a metha-oxyl on the ring A. These data agree with those reported in the literature. (Macias et al. Citation1997; Rice-Evans and Packer Citation1998; Einhellig Citation2004; Parvez et al. Citation2004). It is further possible that the activity of the isolated flavonoid aglycones on radical elongation, as shown in , might be due to the presence of a radical in the ring B, possibly responsible for the allelopathic activity (Macias et al. Citation1997; Bais et al. Citation2003; Parvez et al. Citation2004; Beninger and Hall Citation2005).
In literature, some hypotheses have been made about the structural characteristics necessary for anti-oxidant and allelopathic activities: (i) the presence of a catechol group in the ring B, which enable the molecule to interact with free oxygen molecules; (ii) the presence of a double bond at the carbons 2/3, along with an oxygen at the position 4 of the ring C; and (iii) the presence of a hydroxyl or methoxyl group at the positions 3, 5, and 7 of the ring A. These structural characteristics favour the enhancement of the electronic stability of the free radical and, therefore, confer anti-oxidant properties of the flavonoids (Bors et al. Citation1990; Martínez-Flórez et al. Citation2002). Thus, the activity on the radical elongation might be probably due to an anti-oxidant activity of the flavonoids, able to capture free radicals, principally in the intracellular medium, where such molecules assume a negative charge at neuter pH (Martínez-Flórez et al. Citation2002). As a consequence, in low concentrations, such compounds can cause the promotion of growth (Worsham Citation1987; Macias et al. Citation1997; Parvez et al. Citation2004), perhaps due to a more effective utilization of cellular enzymes, proteins and electron carriers. High concentrations of flavonoids, on the other hand, could act as membrane hyperpolarizers, altering the ATP pump, making the flavonoids toxic for the cells, and therefore reducing their growth.
The available literature suggests another possible explanation for the changes registered in seed germination and radical elongation. The presence of flavonoids in the solution could cause a decrease of the osmotic potential and therefore, causing difficulties in absorption of solutes by radical hairs and consequently reduced radical growth (Ferreira and Aquila Citation2000). This appears to be improbable in our experiments, since the Refractive Index of the initial and final tested solutions resulted unaltered (data not given).
Acknowledgements
We are very grateful to Dr Daniel Rinaldi and to Dr Clenilson M. Rodrigues at the Institute of Chemistry of the Sao Paulo State University (UNESP) Campus of Araraquara, Brazil, for their help in HPLC and NMR analyses.
References
- Agrawal PK 1989 . Carbon-13 NMR of flavonoids . Amsterdam : Elsevier .
- Almeida , LFR , Delachiave , MEA and Marques , MOM. 2005 . Composição química do óleo essencial de Leonurus sibiricus . Braz J Medicinal Plants. , 8 : 31 – 35 .
- Almeida LFR , Delachiave MEA , Braga JF , Pinho SZ . 2003 . Ação alelopática de extratos aquosos de rubim (Leonurus sibiricus) na germinação e desenvolvimento inicial de alface, tomate e milho . IX Congresso Brasileiro de Fisiologia Vegetal, Atibaia-SP, Brasil, 12–20 September, p. 56 .
- Almeida LFR , Rinaldo D , Martins CR , Sannomiya M , Santos LC , Delachiave MEA , Vilegas W . 2006 . Constituintes químicos de Leonurus sibiricus (Lamiaceae) . Congresso da Sociedade Brasileira de Química; 23–29 Maio; Águas de Lindóia-SP, Brazil, p 94 .
- An , M and Wagga , W. 2005 . Mathematical modeling of dose-response relationship (Hormesis) in allelopathy and its application. Nonlinearity Biol . Toxicol Med. , 3 : 153 – 172 .
- Bais , HP , Vepachedu , R , Gilroy , S , Callawa , RM and Vivanco , JM. 2003 . Allelopathy and exotic plants: from genes to invasion . Science. , 301 : 1377 – 1380 .
- Beninger , CW and Hall , JC. 2005 . Allelopathic activity of luteolin 7-O-(-glucuronide isolated from Chrysanthemum morifolium L . Biochem Systemat Ecol. , 33 : 103 – 111 .
- Boalino , DM , McLean , S , Reynolds , WF and Tinto , WF. 2004 . Labdane Diterpenes of Leonurus sibiricus . J Nat Prod. , 67 : 714 – 717 .
- Bors , W , Heller , W , Michel , C and Saran , M. 1990 . Flavonoids as antioxidants: determination of radical scavenging efficiencies . Meth Enzymol. , 186 : 343 – 355 .
- Chon , SUK , Jang , HG , Kim , DK , Kim , YM , Boo , HO and Kim , YJ. 2005 . Allelopathic potential in lettuce (Lactuca sativa L.) plants . Scientia Horticult. , 106 : 309 – 317 .
- Cutler HG , Cutler SJ . 1999 . Agrochemicals and pharmaceuticals: The connection . In: Cutler HG , Cutler SJ Biologically active natural products: Agrochemicals . Boca Raton, FL : CRC Press . p. 1 – 14
- De Feo , V , De Martino , L , Quaranta , E and Pizza , C. 2003 . Isolation of phytotoxic compounds from Tree-of-Heaven (Ailanthus altissima Swingle) . J Agric Food Chem. , 51 : 1177 – 1180 .
- Duke , SO , Rimando , AM , Baerson , SR , Scheffler , BE , Ota , E and Belz , RG. 2002 . Strategies for the use of natural products for weed management . J Pesticide Sci. , 27 : 298 – 306 .
- Einhellig FA 2004 . Mode of allelochemical action of phenolic compounds . In: Macias FA Galindo JCG Molinillo JMG Cutler HG Allelopathy: Chemistry and mode of action of allelochemicals . Boca Raton, FL : CRC Press . p. 217 – 238 .
- Ferreira , AG and Aquila , MEA. 2000 . Alelopatia: Uma área emergente da ecofisiologia . Revista Brasileira de Fisiologia Vegetal. , 12 : 175 – 204 .
- Giang , PM , Son , PT , Matsunami , K and Otsuka , H. 2005 . New Labdane-Type Diterpenoids from Leonurus heterophyllus SW . Chem Pharmaceut Bull. , 53 : 938 – 941 .
- Glass , ADM and Dunlop , J. 1974 . Influence of phenolic acids on ion uptake: 4. Depolarization of membrane potentials . Plant Physiol. , 54 : 855 – 858 .
- Hoagland , RE and Williams , RD. 2004 . “ Bioassays-useful tolls of the study of allelopathy ” . In Allelopathy: Chemistry and mode of action of allelochemicals , Edited by: Macias , FA , Galindo , JCG , Molinillo , JMG and Cutler , HG . 315 – 341 . Boca Raton, FL : CRC Press .
- Larcher , W. 2000 . Ecofisiologia vegetal , Stuttgard : Eugen Ulmer .
- Lorenzi , H. 2002 . Plantas daninhas do Brasil: terrestres, aquáticas, parasitas, tóxicas e medicinais , 278 São Paulo : Nova Odessa .
- Macias , FA , Molinillo , JMG , Torres , A , Varela , RM and Castellano , D. 1997 . Bioactive flavonoids from Helianthus annuus cultivars . Phytochemistry. , 45 : 683 – 687 .
- Mandal , S. 2001 . Allelopathic activity of root exudates from Leonurus sibiricus L. (Raktodrone) . Weed Biol Manage. , 1 : 170 – 175 .
- Martínez-Flórez , SJ , González-Gallego , J , Culebras , M and Tuñón , MJ. 2002 . Los flavonoides: propiedades y acciones antioxidantes . Nutricion Hospitalaria. , 6 : 271 – 278 .
- Mathela CS 1991 . Allelochemicals in Medicinal and Aromatic Plants . In: Narwal SS Tauro P Allelopathy in agriculture and forestry . Jodhper, , India : Scientific Publishers . p. 213 – 228 .
- Murakami , S. 1943 . Stachydrim in Leonurus sibiricus L . Acta Phytochem , 13 : 161 – 184 .
- Parvez , MM , Tomita-Yokotani , K , Fujii , Y , Konishi , T and Iwashina , T. 2004 . Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa . Biochem Systemat Ecol. , 32 : 631 – 635 .
- Qasem , JR and Foy , CL. 2001 . Weed allelopathy, its ecological impacts and future prospects: A review . J Crop Product. , 4 : 43 – 119 .
- Rice EL 1984 . Allelopathy . 2nd ed . New York : Academic Press .
- Rice-Evans , CA and Packer , L. 1998 . Flavonoids in health and disease , 447 – 467 . New York : Marcel Dekker .
- Satoh , M , Satoh , Y , Isobe , K and Fujimoto , Y. 2003 . Studies of the constituents of Leonurus sibiricus . Chem Pharmaceut Bull. , 51 : 341 – 342 .
- Savona , G , Piozzi , F , Bruno , M and Rodriguez , B. 1982 . Diterpenoids from Leonurus sibiricus . Phytochemistry. , 21 : 2699 – 2701 .
- Shirley , BW. 1996 . Flavonoid biosynthesis: new functions for an old pathway . Trends Plant Sci. , 31 : 377 – 382 .
- Stebbing , ARD. 1982 . Hormesis – the stimulation of growth by low levels of inhibitors . Sci Total Environ. , 22 : 213 – 234 .
- Worsham , AD. 1987 . “ Germination of witch weed seeds ” . In Parasitic weeds in agriculture , Edited by: Musselman , L . 45 – 61 . Boca Raton, FL : CRC Press .