Abstract
Hydroxyproline-rich glycoproteins (HRGPs) accumulation and oxidative cross-linking is one of the earliest defense responses in plants against pathogen infection. In the present study HRGP accumulation in three sorghum genotypes i.e. SC146 (resistant), cv. SC326 (intermediately resistant) and BTx623 (susceptible) as a response to Colletotrichum sublineolum isolate CP2126 infection is elucidated. HRGPs were monitored by hydroxyproline (Hyp) estimation. In genotypes SC146 and genotypes SC326 there was a significantly higher amounts of Hyp at 2 days after inoculation (dai) compared to genotype BTx623, indicating an infection induced accumulation of HRGPs. Western blot analysis of acid-ethanol extracted proteins with polyclonal antibody raised against pearl millet purified HRGPs identified four bands with molecular masses of ~65, 45, 17 and 14 kDa as HRGPs. Insolubilization of the 45 kDa protein in genotypes SC146 and SC326 upon infection with C. sublineolum indicates a role of this protein in cell wall cross-linking, coinciding with heavier accumulation of hydrogen peroxide. In addition, tissue print analysis using polyclonal antibody of pearl millet HRGPs recognized these cross-linked proteins to be HRGPs. These findings indicated that HRGPs in sorghum is a component of defense reaction against C. sublineolum infection.
Introduction
Plant cell walls are dynamic structures that vary in their composition depending on their age, type of tissues and taxonomical plant group. Furthermore, they change with physiological fluctuations and after external stimuli from environment. The most abundant structural proteins in plant cell walls are the hydroxyproline rich glycoproteins (HRGPs). They are induced as defense responses, specifically in incompatible plant-pathogen interactions (Davis et al. Citation1997). Functionally, there is evidence that HRGPs act as impenetrable physical barriers against pathogen ingress and that they immobilize the pathogens by binding to their negatively charged surfaces (Leach et al. Citation1982; Mazau et al. Citation1987; Cassab and Varner 1988). HRGPs include extensins, arabinogalactan proteins (AGPs), proline/hydroxyproline-rich proteins (P/HRGPs), and solanaceous lectins (Sommer-Knudsen et al. Citation1998). Among these, the P/HRGPs and extensins are known to be insoluble proteins whereas AGPs are soluble. Most of the earlier studies on infection-induced accumulation of HRGPs were carried out in dicotyledons, e.g. French bean infected with Colletotrichum lindemuthianum (Templeton et al. Citation1990; Millar et al. Citation1992; Bindschedler et al. Citation2006), lettuce infected with Pseudomonas syringae (Bestwick et al. Citation1995), French bean infected with Xanthomonas campestris (Brown et al. Citation1998) and tobacco leaves infected by Erysiphe cichoracearum (Raggi Citation2000). In recent years, reports have emerged pathogens on the accumulation of HRGPs in monocotyledons as a response to pathogens, e.g. in pearl millet with Sclerospora graminicola (Shailasree et al. Citation2004), in wheat infected by Fusarium culmorum (Kang and Buchenauer Citation2003) and in maize treated with a Fusarium monilifore-derived elicitor (García-Muniz et al. Citation1998).
The oxidative cross-linking of cell wall structural proteins is thought to be a rapid defense response to strengthen the cell wall against the invading pathogen prior to the activation of other post-transcription-dependent defence responsed such as accumulation of pathogenesis-related proteins (PR-proteins) and defense genes expression (Bradley et al. Citation1992; Brisson et al. Citation1994). This oxidative cross-linking at the cell surfaces following fungal infection is known to be driven by peroxidases and H2O2, which rapidly accumulates from an oxidative burst (Brisson et al. Citation1994; Ribeiro et al. Citation2006). HRGPs cause cell wall strengthening by the formation of intra- and inter-molecular cross-links resulting in their rapid insolubilization in cell walls. The cross-linking process is reported to involve isodityrosine (IDT) links (Cooper and Varner Citation1984; Epstein and Lamport Citation1984; Fry Citation1986; Smallhood et al. Citation1995). The whole process strongly decreases solubility of HRGPs meaning that they lose their extractability by salts and SDS (Bradley et al. Citation1992). Finally, this leads to the formation of a cross-linked mesh of defined porosity interpenetrated by a cellulose microfibrilar wrap (Epstein and Lamport Citation1984).
Colletotrichum sublineolum P. Henn. in Kabat et Bub. infects all aerial parts of the sorghum plant causing the anthracnose. The pathogen is classified as a hemibiotroph with an initial, symptomless biotrophic phase, with thick intracellular primary hyphae. This is followed by development of inter- and intracellular thin necrotrophic secondary hyphae (Wharton and Julian Citation1996; Wharton et al. Citation2001). Recently, we studied the infection biology and initial including induction of peroxidase activity and ROS accumulation in response to C. sublineolum infection in three genotypes of sorghum varying in resistance to the pathogen (P. Basavaraju, NP Shetty, HS Shetty, unpublished results, E de Neergaard and HJL J⊘rgensen). The present study is directed towards elucidation of a role for HRGPs in resistance of sorghum against C. sublineolum infection, leading to cell wall strengthening, against pathogen ingress.
Materials and methods
Host, pathogen, inoculation and sampling
Three sorghum genotypes, i.e. SC146 (=Inbred Stock 12637), SC326 (=Inbred Stock 3758) and BTx623, resistant, intermediately resistant and susceptible, respectively, to Colletotrichum sublineolum isolate CP2126 were used throughout the study. Sorghum plants were grown in the 9′′ earthern plots filled with a mixture of soil:sand:manure (1:1:1) under greenhouse (RH) was maintained approximately at 80 and 60% in day and night, respectively, and temperatures of 27 and 25°C in day and night, respectively. Colletotrichum sublineolum isolate CP2126, originally isolated from cv. TAM 428 (=CP 1943) by C.R. Casela (Embrapa Milho e Sorgo, Sete Lagoas, Brazil), was grown in Potato Dextrose Agar (Hi-Media) at 22±2°C under cycles of 8/16 h near UV light and darkness, respectively.
At the five-leaf stage (24–26 days after sowing), the fourth leaf of each plant was mounted in a horizontal position on bent plastic plates using unbleached cotton strings (Shetty et al. Citation2003). Conidia were harvested in glass-distilled water; the concentration adjusted to 106 spores ml-1 and sprayed onto the mounted leaves until run-off, using a glass hand sprayer. Control plants were sprayed with glass distilled water alone. The treated plants were sealed in plastic bags to provide 100% RH and subsequently placed in darkness in the same growth chamber as before for 24 h. After 24 h, the bags were opened and light applied again. Leaf samples were collected for all experiments at 0, 1, 2, 3, 4 and 5 days after inoculation (dai) along with respective control and stored at −80°C.
Extraction of hydroxyproline (Hyp)
Five gram leaf tissue of distilled water treated and pathogen inoculated plants at each time point were used for cell walls preparation and extraction of Hyp according to the procedures of Shailasree et al. (Citation2004). Hyp content was expressed as µg Hyp mg-1 cell wall (dry weight).
Protein isolation and Western blotting
Cell walls from the leaves of sorghum seedlings were obtained by modifying the procedure of York et al. (Citation1986). Total cell wall proteins were extracted following the procedure described by Leach et al. (Citation1982) and Shailasree et al. (Citation2004). Proteins were subjected to 12% SDS PAGE. Total proteins in the extract were stained with Coonmassie blue. For Western blot analysis the separated proteins were blotted onto polyvinylidene difluoride (PVDF) membrane following Winston et al. (Citation1987) using Multiphor II (Pharmacia, Freiburg, Germany) electrophoretic transfer apparatus according to the manufacturer's protocol. The blots were blocked in 2% fat-free milk in Tris-buffered saline (TBS: 10 mM Tris HCl, pH 8.0 containing 150 mM NaCl). Then were they probed with pAB-P/HRGP (1:1000 dilution), a rabbit polyclonal antibody raised against pearl millet P/HRGPs (Deepak et al. Citation2007a). Subsequently, the blots were incubated with anti-rabbit IgG horseradish peroxidase-conjugate (1:1000 dilution) for 1 h at room temperature. After washing twice with TBST (TBS: 10 mM Tris HCl, pH 8.0 containing 150 mM NaCl, 0.05% Tween 20) for 5 min each, the blots were stained for peroxidase activity with 3,3′-diaminobenzedine (DAB) and H2O2. The developed blots were scanned and the band intensities analyzed using the Bioprofile Image Analysis System (Vilber Lourmat, France).
Detection of H2O2 at host-pathogen interaction sites by DAB staining
Detection of H2O2 accumulation at host-pathogen interaction sites was carried out by staining with 3,3-diaminobenzidine (DAB, D-8001, Sigma) as described by Thordal-Christensen et al. (Citation1997). Leaf samples were harvested from the inoculated plants at 40 h after inoculation (hai) in order to obtain samples after DAB uptake at 48 hai (2 dai, where high HRGP accumulation was seen). Leaf samples of both the resistant genotype SC146 and the susceptible BTx623 were cleared and observed for the dark brown appearance of DAB staining at infection sites by following the method described by Shetty et al. (Citation2003).
Tissue printing
Tissue printing was done as described by Cassab and Varner (Citation1987). Four replicates of four cross-sections each of the inoculated and control SC146, SC326 and leaves of genotypes BTx623 leaves were printed. Leaf samples harvested 2 dai were cross-sectioned, dried on a Kimwipe, and pressed onto a nitrocellulose membrane for 15 sec. The nitrocellulose was previously soaked previously in 0.2 M CaCl2 for 15 min and air-dried. Serial prints were made and stained with Coomassie blue for determination of total protein content.
A parallel set of sections was subjected to immunolabelling with pAB-P/HRGPs. For this the blots were blocked by 3% BSA in TBST for 1 h. They were then treated with a 1:5000 dilution of primary antibody (pAB-P/HRGPs) for 1 h and washed three times with TBST for 10 min each. The blots were later treated with 1:1000 diluted secondary antibody conjugated with horseradish peroxidase and washed three times for 10 min each in TBST. Blots were developed by using DAB and H2O2 and were observed under a stereo microscope (Leica MS5, Germany) at high magnification with light provided from above. Images were recorded with a digital camera (Canon Power Shot S50) attached to the stereomicroscope and processed using Photoshop 8.0.1 (Adobe).
Statistical analysis
Data from studies of Hyp estimation represent continuous variables, which were analyzed by analysis of variance assuming a normal distribution. Hypotheses were rejected at p≤0.05. Data were analyzed by PC-SAS (release 8.2; SAS Institute, Cary, NC). The Hyp estimation experiment was repeated three times with two replicates each, whereas all the other experiments were performed at least twice Only representative data are presented.
Results
Accumulation pattern of HRGPs in sorghum in response to C. sublineolum infection
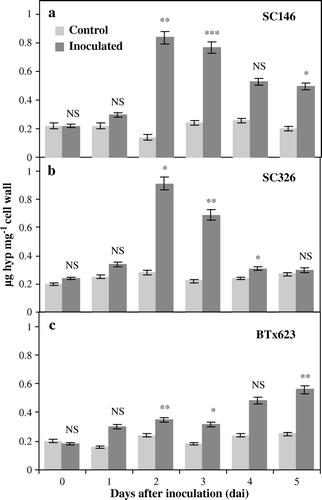
The accumulation pattern of HRGPs, as determined by a time course study of Hyp content in the cell walls of the resistant (SC146), the intermediately resistant (SC326) and the susceptible (BTx623) genotypes of sorghum in response to C. sublineolum infection is seen in . There were no significant differences in Hyp content between the inoculated and control plants of all the three genotypes at 0 and 1 dai. However at 2 and 3 dai, a significantly higher Hyp content was recorded in all the three at 2 and 3 dai inoculated with pathogen to their distilled water controls (a–c). Furthermore compared, SC146 and BTx623 more displayed significantly higher Hyp content until 5 dai (a–c). Whereas genotype SC326 did not accumulate significant amounts of Hyp at the later time points (4 and 5 dai) (Figure b).
Figure 1. Hydroxyproline content in leaves of sorghum as a reaction to anthracnose caused by C. sublineolum. The content of HRGPs, estimated as the content of hydroxyproline (Hyp) in (a) the resistant genotype SC146, (b) the intermediately resistant genotype SC326 and (c) the susceptible genotype BTx623 of sorghum in plants either treated with distilled water (controls) or inoculated with C. sublineolum. Statistical comparisons were made between the inoculated and control samples at each time point in each genotype. Data are the means of two replicates. The number of asterisks indicates the degree of significance. NS: non-significant difference, ***: significant at p≤0.001, **: significant at p≤0.01, *: significant at p≤0.05. Bars represent standard deviations.
Analysis of acid-ethanol extracted proteins and Western blot analysis of HRGPs
Coomassie blue staining of SDS-PAGE separated proteins revealed a number of resolved proteins with molecular weights ranging from ~65 to 7 kDa (data not shown).
Four proteins corresponding to ~65, 45, 17 and 14 kDa were detected on the western blots using pearl millet pAB-P/HRGPs polyclonal antibodies (a). A small induction in the ~17 and 14 kDa proteins was observed after inoculation with the pathogen in leaf samples of the resistant, intermediately resistant and susceptible genotypes. Band intensity analysis also showed a small induction of the ~17 and 14 kDa proteins in infection (b). The ~45 kDa protein was detected in controls of the resistant and intermediately resistant genotypes, but not in C. sublineolum infected samples of these genotypes. However, although this protein band positively detected genotypes in both control and C. sublineolum inoculated plants of the susceptible genotype BTx623.
Figure 2. Western blot analysis showing the temporal accumulation of HRGPs in sorghum using the pAB-P/HRGPs antibody from pearl millet. (a) HRGP accumulation in sorghum leaves of the resistant genotype SC146, the intermediately resistant genotype SC326 and the susceptible BTx623 as a response to C. sublineolum infection. Lane 1: genotype SC146 control; Lane 2: genotype SC146 inoculated; Lane 3: genotype SC326 control; Lane 4: genotype SC326 inoculated; Lane 5: genotype BTx623 control and Lane 6: genotype BTx623 inoculated. (b) Quantification of intensities of the ~45 kDa, 17 kDa and 14 kDa bands using the Bioprofile Image Analysis System. Bars represent standard deviations.
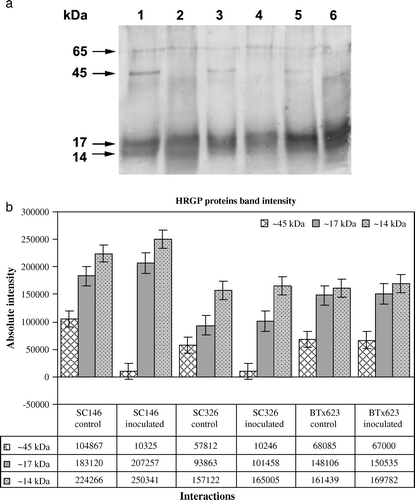
Absence of the ~45 kDa protein in the inoculated resistant and intermediately resistant genotypes but not in the susceptible genotype was also analyzed by the Bioprofile Image Analysis System (b). The ~65 kDa protein was observed with low intensity in the inoculated samples and control samples of all the three content.
H2O2 accumulation at host-pathogen interaction sites
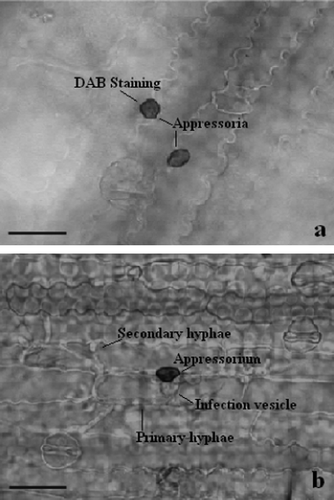
At sorghum-C. sublineolum interaction sites, a dark reddish-brown staining by DAB indicated H2O2 accumulation at 2 dai, mostly at cell walls and in the apoplastic spaces. H2O2 accumulation was seen in one to several cells around the infection sites. Accumulation of H2O2 was predominant in and around the infection structures like appressoria and infection vesicles (a) in the resistant genotype SC 146, whereas, in the susceptible genotype BTx623 lack of accumulation correlated with pathogen growth (b).
Figure 3. Light micrographs of H2O2 accumulation at infection sites of C. sublineolum in leaves of sorghum. H2O2 accumulation is seen by DAB-staining at infection sites in (a) the resistant genotype SC146, whereas in (b) no staining is seen in the susceptible genotype BTx623 where successful development of pathogen took place. Scale bars = 50 µm.
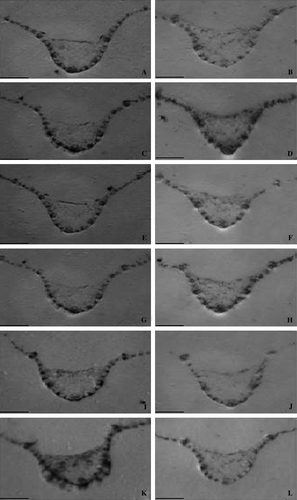
Tissue printing of HRGPs
Tissue print analysis with pAB-P/HRGPs of cross section the three of leaves harvested from genotypes at 2 dai are shown in . The total protein was stained with Coomassie blue in order to show the equal basal level of protein in the resistant genotype SC146 not inoculated (A) and inoculated (C), in the intermediately resistant genotype SC326 not inoculated (E) and inoculated (G) and in the susceptible genotype BTx623 not inoculated (I) and inoculated (K). Both in the resistant genotype SC146 and intermediately resistant genotype SC326, inoculated (D and H) leaves showed a higher level of HRGP in the epidermal region as well as in the vascular tissues when compared to the distilled water treated controls (B and F). In the susceptible genotype BTx623, there was no such HRGP accumulation either in controls (J) or in inoculated samples (L).
Figure 4. Tissue print immunoblot localization of HRGPs in sorghum leaves either inoculated with C. sublineolum or left untreated (controls), using pAB-P/HRGPs polyclonal antibodies to localize HRGPs. Tissue prints were made from cross sections of leaves from the resistant genotype SC146 (B: uninoculated, D: inoculated), the intermediately resistant genotype SC326 (F: uninoculated, H: inoculated) and the susceptible genotype BTx623 (J: uninoculated, L: inoculated). The total basal level of protein in the resistant genotype SC146 (A: uninoculated, C: inoculated), the intermediately resistant genotype SC326 (E: uninoculated, G: inoculated) and the susceptible genotype BTx623 (I: uninoculated; K: inoculated) was stained with Coomassie brilliant blue. Scale bars = 3 mm.
Discussion
The present study was undertaken to investigate the possible involvement of HRGPs in the defense response of sorghum against infection by the pathogen C. sublineolum (isolate CP2126) infection. The time-course study on accumulation of HRGPs was determined by monitoring the Hyp content in the cell walls. The colorimetric estimation of Hyp is reported to be a sensitive indicator for the presence of HRGPs (Raggi Citation2000). The analysis of Hyp indicates that the level of wall-bound HRGPs was higher in the leaves of the resistant genotype SC146 and intermediately resistant genotype SC326 than in the susceptible genotype BTx623. A marked increase in the level of these proteins was observed in the genotypes. SC146 and SC326 following inoculation with C. sublineolum. These results confirm and extend several previous studies which have reported more rapid increases in cell wall HRGPs in resistant than in susceptible genotypes in different host-pathogen interactions such as the cucumber – Cladosporium cucumerinum (Hammerschmidt et al. Citation1984), tomato – Fusarium oxysporum f. sp. radicis-lycopersici (Benhamou et al. Citation1991), tobacco – Erysiphe cichoracearum (Raggi Citation2000), wheat – F. culmorum (Kang and Buchenauer Citation2003) and pearl millet – Sclerospora graminicola (Shailasree et al. Citation2004).
HRGPs were extracted from interactions cell walls by a harsher treatment using a combination of acid and ethanol. This procedure denatures most of the other proteins and in the preparations, up to 70% of the proteins may be HRGPs (Mellon and Helgeson Citation1982; Shailasree et al. Citation2004). SDS-PAGE analysis of the acid: ethanol extracted cell wall proteins after Coomassie blue staining showed several proteins with molecular weights of ~65, 45, 17, and 14 kDa (data not shown). Four proteins positively cross-reacted with the polyclonal antibody pAB-P/HRGPs (Deepak et al. Citation2007a). The pAB-P/HRGPs were raised against purified P/HRGPs (Deepak et al. Citation2007b). The reaction of these proteins in the present study with this antibody identifies them as HRGPs. However, a differential pattern of induction of these proteins to C. sublineolum infection in genotypes. SC146, SC326 and BTx623 were recorded. The levels of HRGPs, particularly the ~17 and 14 kDa proteins following inoculation with the pathogen in the leaves of genotype SC146 genotype indicating a role for these HRGPs in the defense of sorghum C. sublineolum infection.
HRGPs are implicated in wall strengthening by formation of intra- and inter-molecular cross-links that involve isodityrosine (IDT) links (Cooper and Varner Citation1984; Epstein and Lamport Citation1984; Fry Citation1986; Smallhood et al. Citation1995). Wojtaszek et al. (Citation1995, Citation1997) suggested that oxidative cross-linking of O-glycosylated HRGPs occurs after pathogen infection and that depends on a pronounced oxidative burst. Although the molecular mechanisms for this process are still not known, the whole process strongly decreases solubility of HRGPs which subsequently against lose their extractability by salts and SDS (Bradley et al. Citation1992). The absence of the ~45 kDa protein in genotypes SC146 and SC326 after inoculation with the pathogen may indicate that it participates in the cross-linking of HRGPs in the presence of H2O2 and peroxidase. Increases in H2O2 accumulation in the resistant genotype SC146 and the intermediately resistant genotype SC326 (P. Basavaraju, N.P. Shetty, H.S. Shetty, unpublished results, E. de Neergaard and H.J.L. J⊘rgensen) and simultaneous absence of the ~45 kDa HRGP protein strongly support its potential involvement in the oxidative cross-linking in response to pathogen ingress. Further evidence for the role of H2O2 in cross-linking of monomeric extensins was provided by Ribeiro et al. (Citation2006). They demonstrated in that cell walls of grapevine (Vitis vinifera cv. Touriga) callus, cell walls that H2O2 stimulated a rapid loss of monomeric GvP1, concomitant with increased accumulation of insoluble GvP1 amino acids and the cell walls showed resistance to digestion by fungal lytic enzymes. Their results hypothesized a cooperative action between the extensin network and the electrostatic interaction with other cell wall proteins in the extracellular matrix. It is important to elucidate the exact role of the ~45 kDa protein in the disease resistance, and therefore, we are further characterizing the ~45 kDa protein in the sorghum-C. sublineolum interaction.
The accumulation of HRGP in response to the C. Sublineolum was investigated by a tissue printing immunoblot technique. The observation of cell wall proteins by this technique was demonstrated at first by Cassab and Varner (Citation1987). It is reported to be a quick and easy method for detecting proteins/HRGPs in different plant tissues (Keller et al. Citation1988; Fritz et al. Citation1991; Hood et al. Citation1991). The results obtained showed accumulation of HRGPs in a tissue specific manner, being highest in the cell walls of epidermal cells and vascular bundles. The differential responses associated with the compatible and incompatible interactions may reflect differences in the ability of individual tissues to respond to a common signal. HRGP localization in vascular tissues is thought to form a part of the defense response designed to prevent spread of the pathogen through the vascular system. Localization of HRGPs in these cells indicates that they may act in facilitating and amplifying defense signals. A similar observation was reported by Narvaez-Vasquez et al. (Narvez-Vásquez et al. Citation2005), who identified three novel hydroxyproline glycopepetide signals (LeHyp sys I II III) present in tomato leaves as components in signal transduction. These signals were synthesized in phloem cells and released after wounding, being transmitted across phloem cells and thereby amplifying defense signals, which in activation of defense genes.
At infection sites the cell wall barriers like formation of papillae and callose deposition were high in the resistant and the intermediately resistant genotypes compared to susceptible genotype BTx623 (P. Basavaraju, unpublished data). The presence of such structural reinforcements at the early stage of infection during the biotrophic stage constitute a strong barrier and also obstructs the formation of further structures like infection vesicles and primary hyphae of C. sublineolum as evidence in SC146 (P. Basavaraju, personal communication). Such barriers could lead to shortage of nutrients or water during the biotrophic phase of hemibiotrophy as these infection vesicles and primary hyphae are known to draw the nutrients across the host plasma membrane (Perfect et al. Citation2001; Oliver and Ipcho Citation2004). Here, we have demonstrated a correlation between pathogen arrest, H2O2 accumulation and HRGPs cross-linking at infection sites at 2 dai.
Our earlier results demostrated that higher accumulation of H2O2 in the resistant than in the susceptible (P. Basavaraju, N.P. Shetty, H.S. Shetty, E. de Neergaard and, H.J.L. J⊘rgensen, unpublished results). H2O2 accumulation started as early as 1 day after inoculation with C. sublineolum, peaking by 2 dai. Furthermore, there was an increase in defense-related, an apoplastic peroxidase activity in the resistant and the intermediately resistant genotypes of sorghum after inoculation with C. sublineolum, with a maximum increase at 2 dai. These observations, together with the results of the present study on accumulation of HRGPs and protein cross-linking of HRGPs in cell walls is an early defense response in sorghum against C. sublineolum infection.
Acknowledgements
We thank the Danish International Development Assistance (DANIDA) for financial support. This work was supported by a grant for Enhancement of Research Capacity (ENRECA), i.e., ‘Systemic Acquired Resistance – an eco-friendly strategy for managing diseases in rice and pearl millet’. The authors thank Professor V. Raggi, Università di Perugia, Italy, for his valuable comments and suggestions during the preparation of the manuscript. The authors thank K. Reilly for cMeHRGP1 cDNA probe. SS acknowledges the research fellowship received from Council of Scientific and Industrial Research, New Delhi, India.
References
- Benhamou , N , Mazau , D , Grenier , M and Esquerre-Tugaye , MT . 1991 . Time-course study of the accumulation of hydroxyproline-rich glycoproteins in root cells of susceptible and resistant tomato plants infected by Fusarium oxysporum f. sp. radicis-lycopersici . Planta , 184 : 196 – 208 .
- Bestwick , CS , Bennett , MH and Mansfield , JW . 1995 . Hrp mutant of Pseudomonas syringae pv. phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce . Plant Physiol. , 108 : 503 – 516 .
- Bindschedler , LV , Whitelegge , JP , Millar , DJ and Bolwell , GP . 2006 . A two component chitin binding protein from French bean association of proline rich protein with a cysteine rich polypeptide . FEBS Lett. , 580 : 1541 – 1546 .
- Bradley , DJ , Kjellborn , P and Lamb , CJ . 1992 . Elicitor and wound induced oxidative cross-linking of a proline-rich plant cell-wall protein: a novel, rapid defence response . Cell , 70 : 21 – 30 .
- Brisson , L , Tenhaken , R and Lamb , CJ . 1994 . Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance . Plant Cell. , 6 : 1703 – 1712 .
- Brown , I , Trethowan , J , Kerry , M , Mansfield , J and Bolwell , GP . 1998 . Localization of components of the oxidative cross-linking of glycoproteins and of callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells . Plant J. , 15 : 333 – 343 .
- Cassab , GI and Varner , JE . 1998 . Cell wall proteins . Ann Rev Plant Physiol. , 39 : 321 – 353 .
- Cassab , GI and Varner , JE . 1987 . Immunocytolocalization of extensin in developing soybean seed coats by immunogold-sliver staining and by tissue printing on nitrocellulose paper . J Cell Biol. , 105 : 2581 – 2588 .
- Cooper , JB and Varner , JE . 1984 . Cross-linking of soluble extensin in isolated cell walls . Plant Physiol. , 76 : 414 – 417 .
- Davis , HA , Daniels , MJ and Dow , JW . 1997 . Induction of extracellular matrix glycoproteins in Brassica petioles by wounding and in response to Xanthomonas campestris . Mol Plant Microbe Interact , 10 : 812 – 820 .
- Deepak , S , Shailasree , S , Sujeeth , N , Ramachandra Kini , K , Shetty , HS and Mithöfer . 2007a . Purification and characterization of proline/hydroxyproline-rich glycoprotein from pearl millet coleoptiles inoculated with downy mildew pathogen Sclerospora graminicola . Phytochemistry , 68 : 298 – 305 .
- Deepak , S , Shailasree , S , Sujeeth , N , Ramachandra Kini , K , Shetty , HS and Mithöfer . 2007b . A role of hydroxyproline-rich glycoproteins in resistance of pearl millet against downy mildew pathogen Sclerospora graminicola . Planta , 226 : 323 – 333 .
- Epstein , L and Lamport , DTA . 1984 . An intramolecular linkage involving isodityrosine in extensin . Phytochemistry , 23 : 1241 – 1246 .
- Fritz , SE , Hood , KR and Hood , EE . 1991 . Localization of soluble and insoluble fractions of Hydroxyproline rich glycoproteins during maize kernel development . J Cell Sci. , 98 : 545 – 550 .
- Fry , SC . 1986 . Cross linking of matrix polymers in the growing cell walls of angiosperms . Ann Rev Plant Physiol. , 37 : 165 – 186 .
- García-Muniz , N , Martínez-Izquierdo , JA and Puigdoménech , P . 1998 . Induction of mRNA accumulation corresponding to a gene encoding a cell wall hydroxyproline-rich glycoprotein by fungal elicitors . Plant Mol Biol. , 38 : 623 – 632 .
- Hammerschmidt , R , Lamport , DTA and Muldoon , EP . 1984 . Cell wall hydroxyproline enhancement and lignin deposition as an early event in the resistance of cucumber to Cladosporium cucumerianum . Physiol Plant Pathol. , 24 : 43 – 47 .
- Hood , KR , Baasiri , RA , Fritz , SE and Hood , EE . 1991 . Biochemical and tissue print analyses of hydroxyproline rich glycoproteins in cell walls of sporophytic maize tissue . Plant Physiol. , 96 : 1214 – 1219 .
- Kang , Z and Buchenauer , H . 2003 . Immunochemical localization of cell wall-bound thionins and hydroxyproline-rich glycoproteins in Fusarium culmorum-infected wheat spikes . J Phytopathol. , 151 : 120 – 129 .
- Keller , B , Tepleton , MD and Lamb , CJ . 1988 . Glycine rich cell wall protein in bean; gene structure and association of the protein with the vascular system . EMBO J. , 3 : 3625 – 3633 .
- Leach , JE , Cantrell , MA and Sequeira , L . 1982 . Hydroxyproline-rich bacterial agglutinin from potato . Plant Physiol. , 70 : 1353 – 1358 .
- Mazau , D , Rumeau , D and Esquerre-Tugaye , MT . 1987 . Molecular approaches to understanding cell surface interactions between plants and fungal pathogens . Plant Physiol Biochem. , 25 : 337 – 343 .
- Mellon , JE and Helgeson , JP . 1982 . Interaction of hydroxyproline-rich glycoproteins from tobacco callus with potential pathogens . Plant Physiol. , 70 : 401 – 405 .
- Millar , DJ , Slabas , AR , Sidebottom , C , Smith , CG , Allen , AK and Bolwell , GP . 1992 . A major stress inducible Mr- 42Kda wall glycoprotien of French bean (Phaseolus Vulgaris L.) . Planta , 187 : 176 – 184 .
- Narvez-Vásquez , J , Pearce , G and Ryan , CA . 2005 . The plant cell wall matrix harbors a precursor of defense signaling peptides . PNAS USA , 102 : 12974 – 12977 .
- Oliver , RP and Ipcho , SVS . 2004 . Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens . Mol Plant Pathol. , 5 : 347 – 352 .
- Perfect , SE , Green , JR and O'Connell , RJ. 2001 . Surface characteristics of necrotrophic secondary hyphae produced by the bean anthracnose fungus, Colletotrichum lindemuthianum . Eur J Plant Pathol. , 107 : 813 – 819 .
- Raggi , V . 2000 . Hydroxyproline-rich glycoprotein accumulation in tobacco leaves protected against Erysiphe cichoracearum by potato virus Y infection . Plant Pathol. , 49 : 179 – 186 .
- Ribeiro , JM , Perira , CS , Soares , NC , Viera , AM , Feijó , JA and Jackson , PA . 2006 . The contribution of extensin network formation to rapid, hydrogen peroxide-mediated increase in grapevine callus wall resistance to fungal lytic enzymes . J Exp Bot. , 57 : 2025 – 2035 .
- Shailasree , S , Kini , KR , Deepak , S , Kumudini , BS and Shetty , HS . 2004 . Accumulation of hydroxyproline-rich glycoproteins in pearl millet seedlings in response to Sclerospora graminicola infection . Plant Sci. , 167 : 1227 – 1234 .
- Shetty , NP , Kristensen , BK , Newman , M-A , M⊘ller , K , Gregersen , PL and J⊘rgensen , HJL . 2003 . Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat . Physiol Mol Plant Pathol. , 62 : 333 – 346 .
- Smallhood , M , Martin , H and Knox , JP . 1995 . An epitope of rice threonine and HRGP is common to cell wall and hydrophobic plasma membrane glycoproteins . Planta , 196 : 510 – 522 .
- Sommer-Knudsen , J , Bacic , A and Clarke , AE . 1998 . Hydroxyproline-rich plant glycoproteins . Phytochemistry , 47 : 483 – 497 .
- Templeton , MD , Dixon , RA , Lamb , CJ and Lawton , MA . 1990 . Hydroxyproline rich glycoprotein transcripts exhibit different spatial patterns of accumulation in compatible and incompatible interactions between Phaseolus vulgaris and Colletotrichum lindemuthianum . Plant Physiol. , 94 : 1265 – 1269 .
- Thordal-Chrsitensen Christensen , H , Zhang , Z , Wei , Y and Collinge , DB . 1997 . Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction . Plant J. , 11 : 1187 – 1194 .
- Wharton , PS and Julian , AM . 1996 . A cytological study of compatible and incompatible interactions between Sorghum bicolor and Colletotrichum sublineolum . New Phytologist , 134 : 25 – 34 .
- Wharton , PS , Julian , AM and O'Connell , RJ . 2001 . Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum . Phytopathology , 91 : 149 – 158 .
- Winston S , Fuller S , Huller J. 1987 . Western blotting In : Ausubel EM . Current protocols in molecular biology New York : John Wiley . p 10.8.1 – 10.8.6 .
- Wojtaszek , P , Trethowan , J and Bolwell , GP . 1995 . Specificity in the immobilization of cell wall proteins in response to different elicitor molecules in suspension-cultured cells of French bean (Phaseolus vulgaris L.) . Plant Mol Biol. , 28 : 1075 – 1087 .
- Wojtaszek , P , Trethowan , J and Bolwell , GP . 1997 . Reconstitution in vitro of the components and conditions required for the oxidative cross linking of extracellular proteins in French bean (Phaseolus vulgaris L.) . FEBS Lett. , 405 : 95 – 98 .
- York , WS , Darvill , AG , McNeil , M , Stevensen , TT and Albersheim , P . 1986 . “ Isolation and characterization of plant cell walls and cell wall components ” . In Methods in enzymology , Edited by: Colowick , SP and Kaplan , NO . Vol. 118 , 3 – 41 . New York : Academic Press .