Abstract
Fava d'anta is a tree rich in the flavonoid rutin; it is found mainly in the Brazilian savannah, which is called the cerrado and has a defined drought season. The distribution of rutin in fava d'anta seedlings was studied at different developmental stages and in an adult tree. In addition, the effects of flooding, drought and salinity on the content of this flavonoid in seedlings were investigated. Rutin was found in all of the analyzed fava d'anta plant parts, and its content was always higher than quercetin, a related flavonoid. Young leaves showed the highest rutin content. In general, the stresses caused an increase in both of the flavonoids in the seedling leaves, with some variation depending on the leaf age. Seedlings under stress showed a similar growth to the control seedlings, suggesting that rutin may have a role in protecting the tissues against oxidative damage during drought periods in its natural habitat.
Introduction
Rutin is a flavonoid glycoside (3-O-beta-rhamnoglucoside), which is found in several plant species (Yarnell and Abascal Citation2002). Rutin was isolated and identified in buckwheat by Schunck in the nineteenth century (Couch et al. Citation1946), and currently it is well known for having several pharmacological properties (Wojcicki et al. Citation1995; Abeywardena and Head Citation2001; Holasova et al. Citation2002; Feres et al. Citation2006). An important physiological role of rutin is its strong antioxidant activity (Yokozawa et al. Citation1997).
Onion, wine, grape and buckwheat are food sources containing rutin (Oomah and Mazza Citation1996; Thomson et al. Citation1999), and a small number of species contain sufficient quantities for commercial exploitation, such as Sophora japonica L. and buckwheat (Fagopyrum esculenthum Moench) (Hollman et al. Citation1996; Bruneton Citation1999). The buds and flowers of S. japonica contain between 15–20% of rutin (Harborne Citation1988), while the leaves of buckwheat vary from 2–8% rutin (Oomah and Mazza Citation1996).
Dimorphandra mollis Benth. (Caesalpiniaceae), also known by the common names faveiro or fava d'anta, is a tree found in the Brazilian savannah (cerrado) in the central part of the country (Lorenzi and Matos Citation2002). Fava d'anta is the main source of rutin in Brazil, and the flavonoid is extracted from the immature seedpods of fava d'anta to supply industrial markets with important roles in the cosmetic and pharmaceutical industries (Madeira et al. Citation2002). The pericarp of the fruits may have up to 8% rutin (Silva Citation1986; Chaves and Usberti Citation2003).
Fava d'anta also has an important socio-economic role for the people of the central region of Brazil, because harvesting of the pods helps the income of small family farmers (Lorenzi and Matos Citation2002). The pods are harvested exclusively from plants growing in the wild. Although it is not harmful to the plant growth, depending on the harvest frequency, this socio-economic activity can be a potential hazard to the dissemination of this species and to the natural genetic variability (Gomes and Gomes Citation2000). Therefore, studies have been recommended for the preservation of fava d'anta aiming for sustainable management practices and domestication for agronomical exploitation (Gomes and Gomes Citation2000).
Rutin is formed by addition of a rutinoside (glucose-rhamnose) to position 3 of quercetin (), and isoquercitrin is proposed to be an intermediate in rutin biosynthesis (Suzuki Citation1962; Barber and Behrman Citation1991). Despite several reports on the content of rutin in plants, very little is known about the metabolic control of rutin accumulation or how biotic and abiotic factors affect its content. Rutin is found in the seeds, leaves, flowers and cotyledons of buckwheat, but its synthesis is especially high in young leaves where it seems to play a role as a UV protector (Suzuki et al. Citation2005). Long-term UV-B radiation has a significant effect on buckwheat growth (Gaberscik et al. Citation2002).
As part of an ongoing investigation on the biosynthesis and the metabolic role of rutin in fava d'anta, the objective of this work was to study the variation of the content of rutin and quercetin in different plant parts of fava d'anta seedlings. In addition, the effects of salt and water stresses (flooding and drought) on the rutin and quercetin contents in fava d'anta seedlings and an adult fava d'anta tree were investigated.
Material and methods
Plant material
Seeds of fava d'anta were a gift from Dr Geraldo Aclécio de Melo (Universidade Estadual de Montes Claros, Montes Claros, Minas Gerais State, Brazil), and were collected from plants growing in the cerrado of Minas Gerais. They were scarified using sandpaper and germinated in germination boxes with water. The germinated seeds were transferred to 500-ml plastic pots containing vermiculite and received 100 ml of a half-strength nutritive solution once a week (Hoagland and Arnon Citation1950).
Samples were collected from seedlings at different developmental stages: Stage A – seven days after seed germination; in each plant, the root and cotyledons were analyzed together; stage B – 14 days after seed germination; in each plant, the root and shoot + cotyledons were analyzed; stage C – 21 days after seed germination; in each plant, the root, stem, cotyledons and first leaves were analyzed; stage D – 28 days after seed germination; in each plant, the root, stem, cotyledons, and first and second leaves were analyzed; stage E – 35 days after seed germination; in each plant, the root, stem, cotyledons, and first, second and third leaves were analyzed; and stage F – 78 days after seed germination, in each plant, the root, stem, and first leaves, second + third leaves and fourth + fifth leaves were analyzed.
Material from a single fava d'anta tree growing on the campus of our university was also sampled for analysis in February 2007. To our knowledge, there is no record of the age of this tree, but it was estimated to be 20 years old by Dr Jorge Tamashiro from the Department of Botany within our university.
Stressing treatments
Seedlings with five to six leaves growing in 500-ml plastic pots were used for the induction of salt and water stresses. The experiments were carried out in February–March 2007 under greenhouse conditions without any control of light and temperature. The salt stress was induced by watering the plants with 1 l of 75 mM NaCl water solution every second day for 14 days. The seedlings did not receive water in the interval days, and the experiment was interrupted when some leaflets of the treated seedlings fell. Drought stress was induced by supplying the pots with half the water lost in the previous day. At the beginning of the treatment, the pots received an excess of water; after draining the water, they were weighed. On the next day, they were weighed again, and the difference was used to calculate the water to be given to each pot. This watering strategy was prolonged to 25 days of treatment when the pot weight was practically the same for four days and stressed seedlings started to show visible signs of wilting during the hottest period of the day. At this point, the plants were cut in the stem with a razor blade, and the water potential of the whole shoot was determined using a pressure pump (Corvallis, Oregon, USA). The flooding stress was applied by transferring the plastic pots to bigger pots full of water. The water was changed every second day. The treatment was interrupted when some seedlings started to show chlorosis in the basal leaves, which happened at the 18th day of flooding. Control plants were collected for each stress treatment, and they were watered once a day every day. At collection for the analyses, the seedlings were separated into root, stem, and young (1st and 2nd) and old leaves, frozen into liquid N2 and then freeze-dried. Each treatment was made with seven replicates (seedlings).
Measurements of rutin and quercetin content
The samples were finely ground with a mortar and extracted (50–60 mg) in cap-sealed glass tubes with 2 ml of 80% methanol in a water bath at 60°C for 1 h. The extracts were centrifuged in a bench-top centrifuge (14,000 rpm, 10 min), and the pellet was extracted again. The combined supernatants were used for the analysis of rutin and quercetin by high-performance liquid chromatography, which was carried out on a Shimadzu HPLC system. The flavonoids were isocratically separated with an LC18 Supelcosil (Supelco) reversed-phase column using 0.5% acetic acid in acetonitrile as the mobile phase, at a flow rate of 1 ml/min, and detection at 250 and 360 nm using a diode-array-detector. Standard solutions (5 µl of 0.1–1.5 µg/ml) of rutin and quercetin were analyzed by HPLC to establish a calibration curve to determine the quantity of rutin and quercetin in the samples.
Results
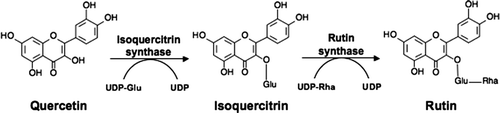
Very low levels of rutin were found in the roots, stems and cotyledons of fava d'anta seedlings in the first two developmental stages (seven and 14 days after germination), and rutin started to appear in appreciable amounts in these organs only after 21 days of germination (A). In the following stages (C to F), rutin always had the highest content in the leaves, especially the older leaves. The rutin content in the cotyledons varied, probably because of the decrease in dry mass as the reserves were consumed to sustain growth. At the final stage, the cotyledons had fallen and they were not analyzed.
Figure 2. Rutin (A) and quercetin (B) contents in the root, stem, cotyledon, youngest leaf (leaf 1), second leaf (leaf 2) and third leaf (leaf 3) of faveiro seedlings at different developmental stages. Bars indicate the standard deviation of the means.
Quercetin was found in much lower amounts than rutin, and was detected in all organs at stage C (B). The quercetin content in the roots of the seedlings at stage F was similar with the content of the leaves; the old leaves had less of this flavonoid. Quercetin and rutin showed similar levels in the roots at stage A.
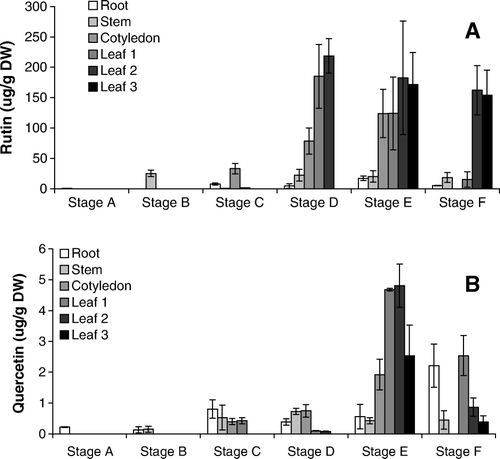
While the literature says that fava d'anta seeds are rich in rutin (Gomes and Gomes Citation2000), its content was low in stage A, where only the root and cotyledon were analyzed. Therefore, rutin and quercetin were analyzed in the testa and cotyledons of dried seeds and seeds imbibed in water for 5 h (). The rutin and quercetin contents were higher in the testa than in the cotyledons and decreased significantly in imbibed seeds. These results explain the low content found in the cotyledons of seedlings at stages A and B () and also suggest that the increased content at later stages was due to biosynthesis.
Figure 3. Rutin (A) and quercetin (B) contents in the cotyledon and testa of dry and imbibed seeds (for 5 h) of faveiro.
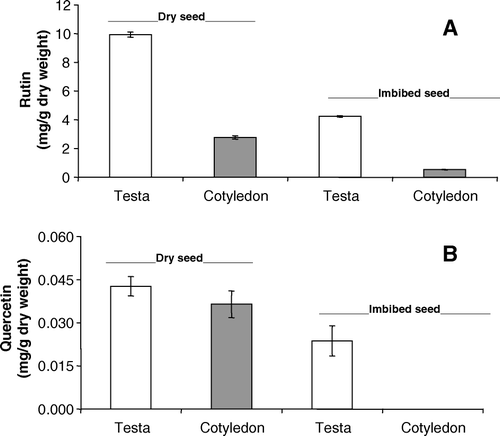
The analysis of an adult fava d'anta plant also showed rutin in higher concentrations than quercetin in all of the tissues and that young tissues have more rutin than old tissues (). On the other hand, with aging, the old leaves become richer in rutin than the young leaves, as was also observed for seedlings. Quercetin also increased in the older leaves.
Figure 4. Rutin (A) and quercetin (B) contents in the leaf bud, bark, and rachis of young (YL) and old leaves (OL) of a faveiro tree. Bars indicate standard deviation of the means.
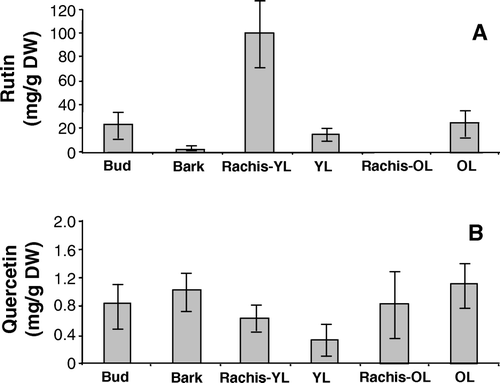
Drought-stressed plants showed shoot water potentials of 2.2–2.7 MPa, while varying from 0.2–0.3 MPa in the unstressed control plants. In general, drought, salt and flooding stresses caused an increase of rutin in all parts of the seedlings (A), except for the old leaves of drought-stressed seedlings, which had a lower rutin content than found in the old leaves of the control plants.
Figure 5. Rutin (A) and quercetin (B) contents and dry mass accumulation (C) in the root, stem and young and adult leaves of faveiro seedlings subjected to drought, salt and flooding stress. *indicates treatments that statistically differed from the control treatment by a 5% Tukey test.
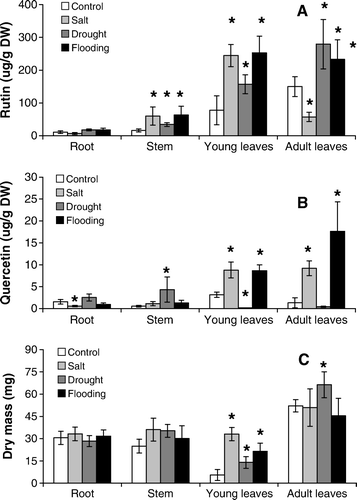
Quercetin was also found in lower amounts than rutin in stressed plants (), and the stress effects were more evident in the leaves, where increases in quercetin were observed with salt and flooding, and drought caused a significant decrease (B).
The dry mass of the root, stem and old leaves practically did not vary among the stressed and control seedlings (C). However, the dry mass was higher in the young leaves of all of the stressed plants.
Discussion
The rutin content was high in the leaves of seedlings and in leaves of a 20-year-old fava d'anta tree, and in both cases, the amount of rutin became higher with leaf aging. The highest concentration of rutin in buckwheat is found in the leaves and flowers (Suzuki et al. Citation2005), but significant amounts are also found in the stem and much less in the roots (Kalinova et al. Citation2006). The same order of magnitude was observed in fava d'anta seedlings.
The analysis of the cotyledons showed an initial low content of rutin but an increase with the seedling growth. This initial low content might be explained by the fast release of rutin from the seeds during germination, which could be an indication that rutin might be an allelopathic compound in fava d'anta. The rutin content in the seed testa was higher than in the cotyledon, however, as the testa forms a thin layer around the cotyledon, its contribution would be less significant in comparison with the amount of rutin released from the cotyledon. The allelopathic effect of rutin and quercetin was previously shown in F. esculentum (Isojima et al. Citation2000; Iqbal et al. Citation2003). However, to the best of our knowledge, there are not any studies on allelopathy with fava d'anta seeds.
Although rutin is released from the cotyledons during the germination of fava d'anta seeds, an increase was observed in this tissue at later stages of the seedling development, suggesting that either it was a consequence of the loss of mass as reserves were drained to support seedling growth, or the cotyledon was able to biosynthesize the flavonoid. Rutin also increased in the tartary buckwheat cotyledon during seed germination in terms of both dry and fresh weight. This was inversely correlated with a decrease of flavonol-3-glucosidase, which promotes the hydrolysis of the rutinoside linked to the heterocyclic C-ring of the flavonol molecule (Suzuki et al. Citation2007). As observed here with fava d'anta seedlings, the quercetin content also increased in the cotyledons of buckwheat (Suzuki et al. Citation2007), reinforcing that this tissue may biosynthesize rutin.
Rutin and quercetin in the leaves showed a similar variation in their contents in the different parts of the seedlings. However, this was not observed in drought-stressed seedlings, where the rutin content increased and quercetin decreased in the leaves. Therefore, this might indicate that rutinoside transference to quercetin is fastest under drought stress.
Crataegus laevigata and Crataegus monogyna subjected to drought, flooding and cold stresses showed different responses in the content of quercetin and rutin (Kirakosyan et al. Citation2004). Whereas drought decreased the quercetin content in C. laevigata, an increase was seen in C. monogyna. By contrast, the amount of rutin increased with drought in both species. Hence, even among related species, variations in quercetin and rutin may not necessarily be correlated. Similar results have also been observed with other plant species subjected to drought and temperature stresses (Abreu and Mazzafera Citation2005).
To cope with biotic and abiotic stresses, plants have evolved antioxidant defense systems, which include enzymes and non-enzymatic compounds, such as amino acids, ascorbate, and thiol compounds (Gratão et al. Citation2005). These defense systems protect plants from the oxidative damage caused by generated reactive oxygen species. Salt and drought stress are well known to generate reactive oxygen species in plant cells (Sakurai et al. Citation2007; Yazici et al. Citation2007; Sakamoto et al. Citation2008). Flavonoids, such as rutin and quercetin, are among the compounds protecting plants from cell damage during biotic and abiotic stresses (Treutter Citation2006; Pourcel et al. Citation2007).
Here, seedlings exposed to three types of stresses showed an increased content of rutin in the leaves and, except for young leaves, the dry mass of roots, stems and old leaves did not differ from the control treatment. One might argue that the lack of difference between the stressed and control seedlings might be because the stresses were not severe; however, as indicated in the Material and methods, the treatments were interrupted when the seedlings showed visible signs of stress, such as wilting, chlorosis and fallen leaflets. Other defence systems, such as enzymes, amino acids and thiol compounds, were not evaluated here. However, the increased content of rutin and quercetin indicate that part of the tolerance of fava d'anta in growing under the cerrado soil and climate conditions may be due to the protective effect of rutin. Young leaves of the stressed seedlings showed a higher mass accumulation than the control seedlings. Although it would appear unexpected, it was previously shown that stressing situations, such as diseases, may induce an increased initial growth (Carneiro et al. Citation1999). However, considering that the seedlings were maintained in the greenhouse during the experimental period and therefore exposed to temperatures higher (~30–34°C) than outside (~28–30°C), the increase of rutin may have caused a protection against the stressful temperatures and even for light.
Most of the Brazilian savannah, named cerrado, may be classified as a seasonal savannah, which is characterized by a defined rainy season and an extended rainless season (Sarmiento Citation1983). Therefore, drought may have played an important role in the development of anatomical and physiological mechanisms by the plants to survive under the cerrado climate conditions (Sarmiento et al. Citation1985; Rundel et al. Citation1998; Marques et al. Citation2000). Although fava d'anta already has appreciable amounts of rutin in its tissues, it was observed here that the content of this flavonoid was increased in the seedlings under three stressing situations. Therefore, these results suggest that a rutin increase may be a response to stress, which enables the growth of fava d'anta in the cerrado. The response observed here will be evaluated next in plants growing in the cerrado during the rainy and dry seasons.
Acknowledgements
N.L. thanks Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP for a student fellowship, and P.M. thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq for a research fellowship.
References
- Abeywardena , MY and Head , RJ. 2001 . Dietary polyunsaturated fatty acid and antioxidant modulation of vascular dysfunction in the spontaneously hypertensive rat . Prostaglan Leuk Essent Fatty Ac. , 65 : 91 – 97 .
- Abreu , IN and Mazzafera , P. 2005 . Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy . Plant Physiol Biochem. , 43 : 241 – 248 .
- Barber , GA and Behrman , EJ. 1991 . The synthesis and characterization of uridine 5′-(beta-L-rhamnopyranosyl diphosphate) and it role in the enzymatic-synthesis of rutin . Arch Biochem Biophys. , 288 : 239 – 242 .
- Bruneton , J. 1999 . Pharmacognosy, phytochemistry and medicinal plants , Paris : Lavosier Publications .
- Carneiro , RG , Ferraz , LCCB and Mazzafera , P. 1999 . Carbon partitioning in soybean infected with Meloidogyne incognita and M. javanica . J Nematol. , 31 : 348 – 355 .
- Chaves , MMF and Usberti , R. 2003 . Prediction of Dimorphandra mollis Benth. (“faveiro”) seed longevity . Rev Bras Bot. , 26 : 557 – 564 .
- Couch , JF , Naghski , J and Krewson , CF. 1946 . Buckwheat as a source of rutin . Science. , 103 : 197 – 198 .
- Feres , CAO , Madalosso , RC , Rocha , OA , Leite , JPV , Guimarães , TMDP , Toledo , VPP and Tagliati , CA. 2006 . Acute and chronic toxicological studies of Dimorphandra mollis in experimental animals . J Ethnopharmacol. , 108 : 450 – 456 .
- Gaberscik A , Voncina M , Trost T , Germ M , Biorn . 2002 . Growth and production of buckwgeat (Fagopyrum esculentum) treated with reduced, ambient, and enhanced UV-B radiation . J Photochem Photobiol B: Biol . 66 : 30 – 36 .
- Gomes , LJ and Gomes , MAO. 2000 . O extrativismo e biodiversidade: o caso da fava-d′anta . Ciência Hoje. , 5 : 66 – 69 .
- Gratão , PL , Polle , A , Lea , PJ and Azevedo , RA. 2005 . Making the life of heavy-stressed plants a little easier . Func Plant Biol. , 32 : 481 – 494 .
- Harborne , JB. 1988 . The flavonoids: advances in research since 1980 , New York : Chapman and Hall .
- Hoagland DR , Arnon DI. 1950 . The water culture method for growing plants without soil . Circular, 347 Berkley: California Agriculture Experimental Station . 32 .
- Holasova , M , Fiedlerova , V , Smrcinova , H , Orsak , M , Lachman , J and Vavreinova , S. 2002 . Buckwheat – the source of antioxidant activity in functional foods . Food Res Intern. , 35 : 207 – 211 .
- Hollman , PCH , Hertog , MGL and Katan , MB. 1996 . Analysis and health effects of flavonoids . Food Chem. , 57 : 43 – 46 .
- Iqbal , Z , Hiradate , S , Noda , A , Isojima , SI and Fujii , Y. 2003 . Allelopathic activity of buckwheat: isolation and characterization of phenolics . Weed Sci. , 51 : 657 – 662 .
- Isojima , S , Iqbal , Z , Koizumi , A and Fujii , Y. 2000 . Allelopathy of Fagopyrum esculentum: analysis of allelochemicals . J Weed Sci Technol. , 45 : 92 – 93 .
- Kalinova , J , Triska , J and Vrchotova , N. 2006 . Distribution of vitamin E, squalene, epicatechin, and rutin in common buckwheat plants (Fagopyrum esculentum Moench) . J Agric Food Chem. , 54 : 5330 – 5335 .
- Kirakosyan , A , Kaufman , P , Warber , S , Zick , S , Aaronson , K , Bolling , S and Chang , SC. 2004 . Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants . Physiol Plant. , 121 : 182 – 186 .
- Lorenzi , H and Matos , FJA. 2002 . Plantas medicinais do Brasil: nativas e exóticas , Nova Odessa, , Brazil : Instituto Plantarum .
- Madeira FA , Ferreira GDC , Leite JPV , Machado A , Abreu WM. 2002 . Inventário de espécimes de Dimorphandra do cerrado mineiro utilizados para a obtenção de rutina e otimização do seu processo produtivo . In : XVII Simpósio de Plantas Medicinais do Brasil . Cuiabá, MT, Brazil .
- Marques , AR , Garcia , QS , Rezende , JLP and Fernandes , GW. 2000 . Variations in leaf characteristics of two species of Miconia in the Brazilian cerrado under different light intensities . Trop Ecol. , 41 : 47 – 60 .
- Oomah , BD and Mazza , G. 1996 . Flavonoids and antioxidative activities in buckwheat . J Agric Food Chem. , 44 : 1746 – 1750 .
- Pourcel , L , Routaboul , J-M , Cheynier , V , Lepiniec , L and Debeaujon , I. 2007 . Flavonoid oxidation in plants: from biochemical properties to physiological functions . Trends Plant Sci. , 12 : 29 – 36 .
- Rundel , PW , Sharifi , MR , Gibson , AC and Esler , KJ. 1998 . Structural and physiological adaptation to light environments in neotropical Heliconia (Heliconiaceae) . J Trop Ecol. , 14 : 789 – 801 .
- Sakamoto , H , Matsuda , O and Iba , K. 2008 . ITN1, a novel gene encoding an ankyrin-repeat protein that affects the ABA-mediated production of reactive oxygen species and is involved in salt-stress tolerance in Arabidopsis thaliana . Plant J. , 56 : 411 – 422 .
- Sakurai T , Plata G , Rodriguez-Zapata F , Seki M , Salcedo A , Toyoda A , Ishiwata A , Tohme J , Sakaki Y , Shinozaki K , Ishitani M . 2007 . Sequencing analysis of 20,000 full-length cDNA clones from cassava reveals lineage specific expansions in gene families related to stress response . BMC Plant Biol. 7:66. Available online at: http://www.biomedcentral.com/content/pdf/1471-2229-7-66.pdf .
- Sarmiento , G. 1983 . Ecosystems of the world – tropical savannas. The savannas of tropical America , Amsterdam : Elsevier .
- Sarmiento , G , Goldstein , G and Meinzer , F. 1985 . Adaptative strategies of woody species in neotropical savannas . Biol Rev Camb Philos Soc. , 60 : 315 – 355 .
- Silva , MF. 1986 . Dimorphandra (Caesalpiniaceae). Flora Neotropica , New York : The New York Botanical Garden .
- Suzuki , H. 1962 . Hydrolysis of flavonoid glycosides by enzymes (rhamno diastase) from Rhamnus and other sources . Arch Biochem Biophys. , 99 : 476 – 483 .
- Suzuki , T , Honda , Y and Mukasa , Y. 2005 . Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves . Plant Sci. , 168 : 1303 – 1307 .
- Suzuki , T , Kim , SJ , Takigawa , S , Mukasa , Y , Hashimoto , N , Saito , K , Noda , T , Matsuura-Endo , C , Zaidul , ISM and Yamauchi , H. 2007 . Changes in rutin concentration and flavonol-3-glucosidase activity during seedling growth in tartary buckwheat (Fagopyrum tataricum Gaertn.) . Can J Plant Sci. , 87 : 83 – 87 .
- Thomson , C , Bloch , A and Hasler , CM. 1999 . Position of the American Dietetic Society: functional foods . J Am Diet Assoc. , 99 : 1280 – 1281 .
- Treutter , D. 2006 . Significance of flavonoids in plant resistance: a review . Environ Chem Lett. , 4 : 147 – 157 .
- Wojcicki , J , Barcewwiszniewska , B , Samochowiec , L and Rozewicka , L. 1995 . Extractum-Fagopyri reduces atherosclerosis in high-fat diet fed rabbits . Pharmazie. , 50 : 560 – 562 .
- Yarnell , E and Abascal , K. 2002 . Overview of drug herb interactions . Altern Complem Therap. , 8 : 87 – 96 .
- Yazici , I , Tuerkan , I , Sekmen , AH and Demiral , T. 2007 . Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation . Environ Exp Bot. , 61 : 49 – 57 .
- Yokozawa , T , Dong , E , Liu , ZW and Shimizu , M. 1997 . Antioxidative activity of flavones and flavonols in vitro . Phytother Res. , 11 : 446 – 449 .