Abstract
A total of 73 different varietal groups and cultivars of Vietnamese rice (Oryza sativa L.) were evaluated for the allelopathic potential on barnyardgrass (Echinochloa crus-galli) in laboratory, greenhouse and field screenings. In a bioassay, Y1, U17, Nep Thom and Lua Huong, cultivars showed the highest weed-suppressing ability against the length of shoot and root of barnyardgrass. Y-1, Nhi Uu and Khau Van exerted significant inhibitory effects in greenhouse screening. In the field study, Phuc Tien obtained the highest weed-suppressing potential. The allelopathic activity of Vietnamese rice showed cultivar-dependence and varietal group-dependence, of which hybrid group cultivars (H) showed the highest inhibition, followed by the local non-sticky cultivars (LNS), foreign (F), traditional sticky (TS), and traditional upland sticky (TUS), while the least were the local upland non-sticky (LUNS) cultivars. The inhibition exerted in both bioassay and greenhouse was lower than the observed weed suppression in the field by 15–20%; however, rice cultivars against the growth of barnyardgrass in greenhouse screening showed more correlation to the results obtained in the field than that of the laboratory, but both laboratory and greenhouse screenings may arduously reflect the actual weed-suppressing ability of rice exhibited in the field. The obtained data might be useful for the enhancement of the weed-suppressing ability of rice in this country.
Introduction
Rice (Oryza sativa L.) is the major food crop in Vietnam and the stable food of Asian countries and many regions in the world. The cultivated area of rice accounted for > 7.7 million ha in 2004, which provided 35.5 million tons of rice, of which 4.2 million tons were exported (Khanh et al. Citation2006). Rice is grown in both upland and lowland areas and can be planted all year round, with two or three crops per year. However, rice crops in Vietnam have currently encountered abiotic and biotic factors, causing low rice yield compared to other rice-producing countries. Among the adverse factors, weed infestation is a major biotic constraint and one of the biggest challenges to rice production in Vietnam, which increases severe economic losses (about 46% rice yield reduction in Mekong Delta) (Chin Citation2001). Herbicide application can minimize the time spent on weed control and stabilize the rice yield. However, the overuse of synthetic chemicals for weed control is a serious problem in Vietnam, leading to environmental pollution, unsafe agricultural products, weed-resistant herbicides, and human health concerns. Therefore, improvement of crop quality and yield as well as reducing the dependency on synthetic herbicides and agrochemicals in agricultural production are important tasks. Biological weed management by using allelopathy may affect a yield improvement without environmental cost, which is one of the most important considerations for scientists working to secure the world's food supply for future generations. However, in Vietnam, biological control of weeds in crops is somewhat less known and experienced than breeding, cultivating, and protecting crops from insects and diseases. Nonetheless, studies on allelopathy have just been carried out sporadically in Vietnam: Chau et al. (Citation2008) recently examined the 19 Vietnamese improved indica rice cultivars in a laboratory bioassay and reported that eight cultivars obtained high allelopathic properties on lettuce (Lactuca sativa), kale (Brassica oleracea) and weedy rice (Oryza sativa).
Barnyardgrass (Echinochloa crus-galli) is one of the greatest yield-limiting weeds in both the irrigated rice and in direct-sowing rice areas in Vietnam. It is difficult to control because the phenotype and morphology of this weed are most similar with rice, and easy to adapt for growth under dry and wet conditions therefore causing a greater problem in upland rice and direct-sowing rice. Hence, the most environmentally acceptable and sustainable approach would be utilization of allelopathy for barnyardgrass. The subject of allelopathy in rice has been receiving much attention from scientists with the positive aspects of allelopathy as an ecological control by selecting rice cultivars with greater allelopathic potential (Fujii Citation1992; Garrity et al. Citation1992; Navarez and Olofsdotter Citation1996; Hassan et al. Citation1998; Chou Citation1999; Dilday et al. Citation2000; Lee et al. Citation2003; Berendji et al. Citation2008). It has been known that the allelopathic characteristics in rice depend on varietal difference (Dilday et al. Citation1989, Citation1994, Citation2000; Olofsdotter et al. Citation1997; Lee et al. Citation2004). Dilday et al. (Citation1994) examined about 10,000 accessions from rice germplasm collections for allelopathic effects on ducksalad [Heteranthera limosa (Sw.) Willd]. Olofsdotter et al. (Citation1997) reported that 45 out of 1000 screened rice cultivars contained promising allelopathic activity against one or more weeds. About 20–40% of 1000 rice cultivars in Egypt have shown strong allelopathic activity against indicator plants (Hassan et al. Citation1998). Also, Garrity et al. (Citation1992) investigated the differential weed suppression ability of upland rice cultivars and reported rice with strong allelopathic property often obtains high yield, and sufficient plant characters (plant height and leaf area). These studies imply that it might be possible to develop and use an allelopathic component to control barnyardgrass via plant breeding.
In the trend of exploring the allelopathic potential of rice, many screening methods have been developed; several were simple and reliable in evaluating the allelopathic activity of rice (Fujii and Shibuya Citation1992; Navarez and Olofsdotter Citation1996; Chung et al. Citation2004). Laboratory screening is useful and necessary for initial allelopathic investigation in rice cultivars, but the results need to be further validated in greenhouse and field screening before any conclusion can be made. Therefore, the main objective of this study was to assess the allelopathic potential of 73 Vietnamese rice cultivars against the growth of barnyardgrass in laboratory, greenhouse and field screenings. The variation of weed suppressing ability among rice cultivars and varietal groups and the correlation coefficient between laboratory-field and greenhouse-field screenings were also evaluated.
Materials and methods
Preparation of rice cultivars and barnyardgrass seeds
The selected 73 rice cultivars – widely cultivated in Vietnam – were kindly provided by Hanoi National Seedbank in Vietnam in 2004. Rice cultivars were classified into six types based on the different varietal groups of their origins and habitats: (i) foreign cultivars (F) (16); (ii) hybrid cultivars (H) (26); (iii) traditional sticky cultivars (TS) (9); (iv) traditional upland sticky cultivars (TUS) (6); (v) local non-sticky cultivars (LNS) (8); and (vi) local upland non-sticky cultivars (LUNS) (8). All of these rice cultivars were Indica type.
Barnyardgrass seeds were collected at Yeoju Experimental Farm of Konkuk University, South Korea in 2004. Empty and undeveloped seeds were discarded by floating in tap water. The remaining seeds were air-dried and then hermetically stored at −20°C. Before conducting the experiment, for breaking of dormancy, both rice and barnyardgrass seeds were incubated at 40°C for five days. Before use in the bioassay, barnyardgrass seeds were sterilized with 1% sodium hypochlorite for 30 sec and rinsed several times with distilled water. In the germination test, the germination ratio of seeds was randomly checked and showed to be more than 90%.
Laboratory screening
A total of 73 Vietnamese rice cultivars were screened in the laboratory for their allelopathic potential against barnyardgrass. Twenty healthy seeds of each rice cultivar were evenly sown in a Petri dish (9 cm diameter) lined with filter paper and added to 10 ml of distilled water. Simultaneously, 20 seeds of barnyardgrass were evenly inter-planted between the rice seeds. The Petri dishes were transferred into a growth chamber (25°C, 4000 lux, lighted time: 9:00–17:00 h, humidity: 75%). After seven days, the numbers of germinating barnyardgrass seeds were counted, and the length of shoots and roots was measured. Entire seedlings of barnyarngrass were dried in an oven at 60°C for five days, to determine the dry weight.
Greenhouse screening
All cultivars examined in the laboratory were continuously screened in a greenhouse. Briefly, 10 seeds were planted in a Petri dish (9 cm diameter) lined with the double water-wetted filter paper (Whatman No. 42). The Petri dishes were transferred into a growth chamber (28°C, 4000 lux, lighted time: 9:00–17:00 h, humidity: 75%) for three days of germination. A set of plastic trays formed with small pots (38 ml, 5 cm in diameter) and larger pots (70 ml, 7 cm in diameter) (Modern 50 PS and Green PS) were used. The holes in the bottom of the pots were covered by a plastic label to deter water leaking out. For easier removable purpose, the small pot was discretely cut from the set of trays, and inserted in a large pot, then filled with a wet soil media (Gongryongsanto: pH 4.5–5.8, EC 1.0±0.2, N 1100±100 mg/kg, P2O5 400±100 mg/kg). One healthy seedling amongst 10 germinated rice seedlings in three-day-old rice cultivars was selected and grown at the center of the inserted small pot-tray. The trays of planted rice seedlings were transferred to a greenhouse in the summer season of 2005. Greenhouse temperature was maintained around 25–30°C and tap water was provided every two days to all pots. After 28 days of growing, 12 healthy seeds of barnyardgrass were evenly transplanted around the rice plant. The pots planted with barnyardgrass seeds only were used as the controls. After five days, barnyardgass seedlings were thinned to the remaining six healthiest plants. Fourteen days after barnyardgrass seeds transplanting, all of the six barnyardgrass plants were cut at soil surface. Plant height of the weed was measured and the dried weight was calculated following the method used in the laboratory bioassay.
Field screening
Field screening was conducted at the Experimental Farm of Konkuk University in the summer season of 2006. Three rice cultivars from each rice varietal group (F, LNS, H, TS, TUS, and LUNS) (total 18 rice cultivars) were selected in random for the field screening. Rice seeds were soaked in tap water for two days and treated with a fungicidal chemical (Kyung Nong Co. Ltd., Seoul, Korea) at 0.05% for 24 h. These seeds were grown in seedling boxes for 45 days. The paddy field was divided in many plots (3.3 m2 in area). Each plot was covered well by the plastic board for anti-penetration from other plots. One seedling of each rice cultivar was carefully planted by hand in each plot on 26 May 2006. Two weeks after rice growing, 40 day-old barnyardgrass plants taken from the seedling boxes were inter-planted in five rows across the rice rows (30×15 cm). No herbicide was applied. The pesticides were applied following the conventional method of rice cultivation in Korea. The plots, planted with barnyardgrass seedlings only, were used as the control treatment. Measurement was determined 67 days after planting. Barnyardgrass plants from each row were harvested and the highest extended leaf was recorded as plant height (cm). Other natural weeds growing in each experimental plot were controlled by hand. The biomass of weeds naturally growing in other experimental plots in each rice cultivar was also examined by hand picking in the area of 50×50 cm2. For dry weight, barnyardgrass seedlings were kept in oven-dried conditions at 60°C for five days and weighed. In addition, the 1000 grain weight and rice seedling height of the screened rice cultivars were also calculated. The inhibition percentage between treatments and the control was calculated using the following equation:
The inhibition magnitudes against barnyardgrass growth including the shoot length (SL), dry weight of sampled barnyardgrass were averaged as average inhibition (AI).
Statistical analysis
The laboratory bioassay was conducted with six replications and repeated three times, and greenhouse and field experiments were carried out in a completely randomized design with three replications. Statistical analysis was performed using analysis of variance (SAS Institute Citation1997) to analyze treatment differences. The means were separated on the basis of the least significant differences (LSD) at the 0.05 probability level. Correlation coefficient between laboratory-field and greenhouse-field was calculated against 18 rice cultivars, of which the inhibition against shoot length and dry weight of barnyardgrass was presented as the allelopathic factors.
Results
Laboratory screening
The inhibitory effect of barnyardgrass germination (G), shoot length (SL), root length (RL), dry weight (DW) and the average inhibition (AI) of 73 rice cultivars are shown in . Bao Dam cultivars showed the greatest inhibition (30.0%) on G of barnyardgrass, followed by Nhi Uu (16.0%) and 13 rice cultivars showed over 15.0% inhibition. Two cultivars Y1 and U17 showed the highest inhibition for SL (33.3%) and RL (35.9%), respectively. Nep Thom and Lua Huong exhibited the strongest suppression on RL of barnyardgrass (40.0% and 43.6%). For DW, 12 cultivars showed arrange inhibition from 12.0–21.0%, whereas, eight cultivars showed slight stimulation. HT-1 was the highest weed enhancement by over 20.0% (). For AI, two cultivars including Nhi Uu and Y-1 exhibited the highest suppression (around 23.0%), of which the inhibition against root elongation of barnyardgrass was 29.0–34.0%. The following cultivars stunted growth of barnyardgrass by 18.0–22.0% included Bao Thai, Bao Dam, Khang Dan, Khau Van, Nep Thom, Moc Tuyen, Lua Hai Dong, Lua Can, Lua Huong, and U17. Of which, barnyardgrass roots were the most influenced such as Lua Huong, Lua Can and Lua Hai Dong showed 43.6%, 35.9% and 34.8% of inhibition, respectively. The cultivars which caused the lowest inhibition (0.7–5.5%) were DT 36, T-10, Nep 69, Nep Thom-LS, and BTST cultivars ().
Table 1. The inhibition effects of 73 rice cultivars against barnyardgrass in laboratory screening.
In general, all screened rice cultivars inhibited germination of barnyardgrass, which varied in a range of 1.7–30.0%, except for Phuc Tien, which showed no effect (0.0%). For SL of barnyardgrass, it was slightly promoted by CH207, HDB-1, Nep-352, Nep Meo, Nep Muong Trang, Te 108, TM, and T-10 cultivars (0.2–13.7%). On the other hand, Nep My, Nhi Uu, Nep Con Gioi, and Q5 showed the highest suppression against barnyardgrass SL (). For RL, it was negligibly stimulated by two rice cultivars including DT-36 and Nep 69 (2.0–3.0%). However, 15 cultivars exhibited inhibition greater than 30.0%, of which Lua Huong and Nep Thom showed the strongest suppression (> 40.0%). In the case of DW, it was promoted by eight rice cultivars consisting of AC-4, HDB6, HT-1, Nep Quyt, Nep Con Gioi, T10, and VH5 (1.2–20.4%). The rice cultivars that reduced DW of barnyardgrass up to 20% were Nep Muong Trang, Nep Ha Giang, Lua Can, and Xe Liem Man Te ().
Greenhouse screening
In greenhouse screening, the inhibition against SL and DW of barnyardgrass among the 73 rice cultivars is presented in . Khau Van cultivar exhibited the maximum average inhibition by 37.6%, of which SL and DW of barnyardgrass were reduced by 29.1% and 45.8%, respectively. Eleven rice cultivars showed suppression against barnyardgrass (20.0–30.0%), consisting of AC-4, AC-5, C70, CH-208, DSCH, DT-36, HDB-1, MT508-1, Nep 69, PC-5, and Vien Cham (). Seven rice cultivars exhibited negligible reduction (0.0–5.0%) – CL-9, DT-37, Moc Tuyen, Nep Quyt, Nep-352, LT2, and VH-1. However, 10 rice varieties promoted growth of barnyardgrass including Bao Dam, CIDAD 141, DHB-5, Gao Chien, Nep Con Gioi, Nep My, Nep Tau Co Rau, Te 108, VH-3, and 8FA 338, in which Nep Tau Co Rau exhibited the highest promotion (21.3%). It was observed that the screened rice cultivars showed greater inhibition against DW of barnyardgrass than the SL. There were 22 rice cultivars which reduced barnyardgrass DW > 20.0%, of which Khau Van was the maximum (45.8%), but only four rice cultivars exerted inhibition up to 20.0% against SL of barnyardgrass. Generally, for rice cultivars which enhanced the growth of barnyardgrass, both SL and DW were simultaneously promoted, except for Nep Quyt and 8FA 337 which had either SL or DW inhibited ().
Table 2. The inhibitory effects of 73 rice cultivars against barnyardgrass in greenhouse screening.
Field screening
The influence of rice against barnyardgrass growth, consisting of the height of barnyardgrass SL, DW of sampled barnyardgrass is shown in . The observation showed that all screened rice cultivars inhibited growth of barnyardgrass in the field, except for two cultivars including Nep Muong Trang and Xe Liem Man Te, which stimulated dry weight biomass of natural weeds (DWB) by 3.8% and 16.7%, respectively. The inhibitory magnitudes against, DWB, SL, and DW of barnyardgrass were 5.3–27.9%, -3.8% to 80.2%, 8.3–38.8%, and 27.8–74.2%, respectively. Among those growth categories, the DWB and DW had the most influence. Most of the rice cultivars observed with high tiller capacity and grain seed weight showed stronger suppression on growth of barnyardgrass, such as Phuc Tien, Nep Thom-LS, Nep Xiem, and Thoc Te L1931 cultivars which showed inhibition for DWB (60.6%), SL (38.8%), DW (74.2%) and AI (50.4%), respectively (). For AI, among varietal groups of the F, LNS, TS, H, TUS, and LUNS cultivars, the AI values varied by 26.4–33.7%, 25.8–50.4%, 32.4–42.6%, 40.1–46.3%, 22.3–26.2% and 6.7–28.7%, respectively ().
Table 3. The inhibitory effects of 18 rice cultivars against emergence of barnyardgrass in the field screening.
Comparison of allelopathic activity of rice cultivars among the screening methods and varietal groups
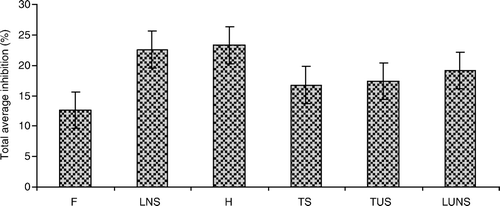
The inhibitory magnitudes of rice cultivars exerted in laboratory, greenhouse, and field screenings, and among the varietal groups were compared. The inhibitory percentage against SL and DW of barnyardgrass of the 18 cultivars selected for screening in the field exhibited in laboratory, greenhouse, and field, were averaged to compare their activities () and total average inhibition (). In general, H cultivars, and LNS cultivars showed the strongest inhibition in laboratory, greenhouse, and field screenings, as compared with other rice varietal groups. The growth reduction against barnyardgrass in fields caused by F cultivars was not significantly different from TUS, TS, and LUNS cultivars. However, in laboratory and greenhouse screenings, it was much lower than that of TUS, TS, and LUNS (). On the other hand, growth of barnyardgrass was much more suppressed in fields than in the laboratory and greenhouse. In the field, the AI (between SL and DW of barnyardgrass) ranged from 31.0–42.9%; however, it did not exceed 15% in the laboratory and greenhouse (). In terms of rice varietal groups in the laboratory, greenhouse and field, H cultivars showed the highest inhibition (23.3%) for AI, followed by the LNS cultivars by 22.6%, and F, TS, TUS, and LUNS cultivars ranged from 12.6–19.1%, respectively ().
Figure 1. Comparison of inhibition against growth of barnyardgrass among rice varietal groups in laboratory, greenhouse, and field screenings. F, foreign; H, hybrids; TS, traditional sticky; TUS, traditional upland sticky; LNS, local non-sticky; LUNS, local upland non-sticky. The bars indicate mean±standard error.
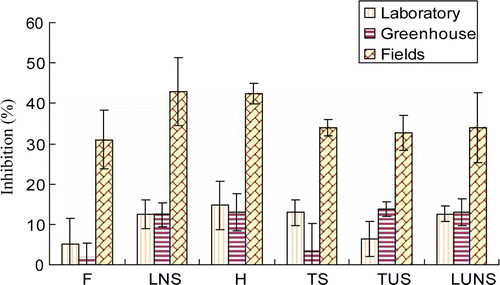
Figure 2. Correlation coefficient of rice against shoot length and dry weight of barnyardgrass in laboratory, greenhouse, and field screenings. NS, no significance
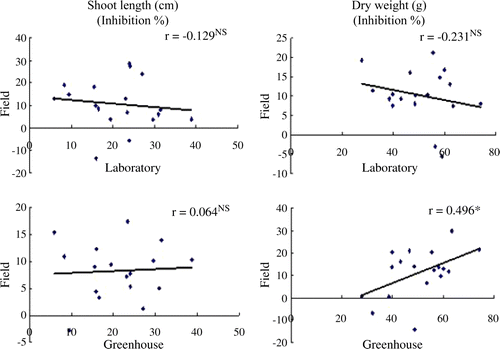
Figure 3. Total average inhibition of rice cultivars with different varietal groups against the growth of barnyardgrass in laboratory, greenhouse and field screenings. F, foreign; H, hybrids; TS, traditional sticky; TUS, traditional upland sticky; LNS, local non-sticky; LUNS, local upland non-sticky. The bars indicate mean±standard error.
The correlation coefficient between the allelopathic magnitude of rice cultivars (18 cultivars used in field screening) exhibited in laboratory-field, and greenhouse-field is shown in . The inhibition against SL and DW of barnyardgrass were presented as the rice allelopathic factors. For SL, the correlation coefficient showed no significant difference (NS) in both laboratory-field and greenhouse-field (r = -0.129 and 0.064, respectively). However, for DW, the intercepts for the lines showed significant difference between the greenhouse-field and more correlation (r = 0.496) than the laboratory (r = -0.213), and both were in greater magnitudes than the shoot length ().
Discussion
Barnyardgrass was selected in this study as the indicator plant because it is one of the most harmful paddy weeds growing abundantly in upland and lowland paddy fields in Vietnam. It causes rice yield reduction and is troublesome to farmers. Allelopathic activity of Vietnamese rice cultivars showed cultivar-dependence and varietal group-dependence against the growth of barnyardgrass in the laboratory, greenhouse and field experiments (Tables , and ). In laboratory screening, the inhibition of RL showed higher than that of SL of barnyardgrass. The greater inhibition of root may be due to their more intimate contact with the treated filter paper. Also, the obtained result agreed with the report of Olofsdotter and Navarez (Citation1996) that allelopathic rice cultivars significantly suppressed root rather than shoot growth of indicator plants. The inhibitory percentage against barnyardgrass of rice cultivars showed a lower value than the other previous reports (Ahn and Chung Citation2000; Chung et al. Citation2001; Lee et al. Citation2004). The main reasons may be due to using different screening methods. Some screening methods were developed with the use of either aqueous or chemical solvent extracts of rice leaves, stems, mixture rice residues and hulls (Fujii and Shibuya Citation1992; Ahn and Chung Citation2000; Lee et al. Citation2003; Jung et al. Citation2004; Chung et al. Citation2004). However, most of those screening methods have been based on the artificially prepared extracts of rice residues, which are then concentrated or diluted arbitrarily for assaying against the indicator plants. Therefore, authors examined the allelopathic effects of rice from its root exudates, which is a better choice to estimate actual allelopathy potential of rice than any use of organic solvents to extract rice residues.
The results showed, in general, rice cultivars exhibited higher weed suppression in field than that observed in laboratory and greenhouse screenings (Tables and, ), of which, the AI between SL and DW ranged from 31.0-42.9%, but was not over than 15.0% in both laboratory and greenhouse screenings (). This result was consistent with some previous research conducted in the controlled and natural conditions (Stowe Citation1979; Rice Citation1984; Leather and Einhellig Citation1988; Inderjit and Weston Citation2000; Olofsdotter Citation2001; Inderjit et al. Citation2005). Moreover, allelopathic activity of rice cultivars was reported to be significantly different by releasing phytotoxic substances at different times of the growing stages (Lee et al. Citation1999; Dilday et al. Citation2001; Jung et al. Citation2004; Kato-Noguchi et al. Citation2008). Also, in this study, the laboratory screening using the Petri dish germination test was applied for seven days, and the greenhouse and field for 50 and 126 days, respectively, to record the inhibitory effects. Therefore, the wide variation of allelopathy activity of rice cultivars was observed, which is similar to the report of Lee et al. (Citation1999), who indicated allelopathic activity of rice reached to the maximal levels at the heading stage. Similar cultivars and varietal groups of rice cultivars may show different ranges of allelopathic activity among laboratory bioassay, greenhouse and field screenings. This may depend on many factors such as screening applied methods, total time screening, and many other physiological factors in nature. However, the results of laboratory and greenhouse screening (controlled conditions) are not always applicable to the field because other components of plant competition play a more key role in the total interference between crops and weeds.
Most of the rice cultivars observed with high tiller capacity and grain seed weight showed stronger suppression of weeds than low tiller and grain seed cultivars in this study (), such as Phuc Tien, Nep Thom-LS, Nep Xiem, and Thoc Te L1931. It was similar to a report by Garrity et al. (Citation1992), who suggested that the characteristics of allelopathic potential cultivars is in direct proportion with high yield, strong tillers and sufficient height and leaf areas. Our findings showed that almost all Vietnamese rice cultivars exerted allelopathic potential against growth of barnyardgrass and exhibited a great variation of the allelopathic potential among rice cultivars and rice varietal groups. Of which H group cultivars showed the highest inhibitory effects, followed by LNSC, LUNSC, TUSC and the lowest was F cultivar, respectively ( and ). The results agreed with the report of Lin et al. (Citation2000). Thousands of worldwide rice cultivars have been evaluated for their allelopathic potential by either screening in laboratory, greenhouse and field or isolating allelochemical from rice residues and root exudates (Fujii Citation1992; Dilday et al. Citation1994, Citation2000; Olofsdotter et al. Citation1997; Hassan et al. Citation1998; Kato-Noguchi et al. Citation2002; Chung et al. Citation2003; Lee et al. Citation2004; Berendji et al. Citation2008). They reported that allelopathic activity is variety dependent and origin dependent, which supports our findings in this study. However, there is controversy in rice allelopathy research about the role of allelopathy in bioassay and natural settings, especially the paucity of evidence of allelochemical interactions, because competition and allelopathy factors cannot be separated under field conditions (Olofsdotter et al. Citation1999; Khanh et al. Citation2007).
According to the results from , the slope of the regression line was significantly different, which showed the relationship between field and greenhouse performance. The strength of inhibition against barnyardgrass recorded in greenhouse screening was more correlated to the results obtained in field than the laboratory screening, despite the fact that both greenhouse and laboratory screening may not reflect correctly the weed suppressing ability of rice exhibited in fields. Greenhouse screening results were more correlated to field screening. Contrarily, laboratory screening showed less correlation to field results than the greenhouse, but it may be useful to estimate weed suppression in the initial examination of rice allelopathy. Rice cultivars with potent weed suppressing ability can be re-examined in the greenhouse to evaluate the weed suppression in conditions that are close to natural settings, which include competitive and interferential factors and finally need to be reconfirmed in the field. SL is one of the parameters which is used to measure the influence of allelopathy; however, DW may be more useful and accurate to reflect the actual allelopathic effects by showing reduction/promotion against a plant biomass.
In conclusion, our findings showed a wide variation of allelopathic activity based on cultivar-dependence and varietal group-dependence. The results might be useful for further developing rice cultivars with better and acceptable allelopathic traits as well as utilizing rice allelopathy for weed control in this country. Further investigation is needed to analyze allelochemical substances in the varietal variation.
Acknowledgements
The authors would like to acknowledge the Japan Science for the Promotion and Society (JSPS) for providing a Postdoctoral Fellowship (ID No: P08095).
References
- Ahn , JK and Chung , IM . 2000 . Allelopathic potential of rice hulls on germination and seedling growth of barnyarngrass . Agron J. , 92 : 1162 – 1167 .
- Berendji , S , Asghari , JB and Matin , AA . 2008 . Allelopathic potential of rice (Oryza sativa) varieties on seedling growth of barnyardgrass (Echinochloa crus-galli) . J Plant Inters. , 3 : 175 – 180 .
- Chau , DPM , Kieu , TT and Chin , DV . 2008 . Allelopathic effects of Vietnamese rice varieties . Allelo J. , 22 : 409 – 412 .
- Chin , DV . 2001 . Biological and management of barnyardgrass, red sprangletop and weedy rice . Weed Biol Manag. , 1 : 37 – 41 .
- Chou , CH . 1999 . Role of allelopathy in plant biodiversity and sustainable agriculture . Crit Rev Plant Sci. , 18 : 609 – 636 .
- Chung , IM , Ahn , JK and Yun , SJ . 2001 . Assessment of allelopathic potential of barnyardgrass (Echinochloa crus-galli) on rice (Oryza sativa L.) cultivars . Crop Prot. , 20 : 921 – 928 .
- Chung , IM , Kim , KH , Ahn , JK , Lee , SB , Kim , SH and Hahn , SJ. 2003 . Comparison of allelopathic potential of rice leaves, straw and hull extracts on barnyardgrass . Agron J. , 95 : 1063 – 1070 .
- Chung , IM , Xuan , TD , Hahn , SJ , Khanh , TD and Ahn , JK . 2004 . “ Methods to screen allelopathic rice varieties against weeds ” . In Research methods in plant science: Allelopathy: Plant Protection , Edited by: Singh , S and Walia , RK . Vol. II , 292 Jodhpur, , India : Scientific Publisher .
- Dilday , RH , Nastasi , P and Smith , RJ . 1989 . Allelopathy observation in rice (Oryza sativa L.) to ducksalad (Heteranthera limosa) . Arkansas Acad Sci. , 43 : 21 – 11 .
- Dilday , RH , Lin , E and Yan , WG . 1994 . Identification of allelopathy in the USDA-ARS germplasm collection . Aust J Exp Agric. , 34 : 907 – 910 .
- Dilday RH , Mattice JD , Moldenhauer KA . 2000 . An overview of rice allelopathy in the USA . In : Kim HU , Shin DH . Proceedings of International Workshop in Rice Allelopathy, Kyungpook National University . Taegu , Korea . p. 15 – 26 .
- Dilday RH , Nastasi P , Lin J , Smith RJ . 2001 . Allelopathic activity in rice (Oryza sativa L.) against ducksalad (Heteranthera limosa (Sw) . In: Hanson JD , Shaffer MJ , Bell DA , Code CV . Proceedings of Sustainable Agriculture for the Great Plains , 19–20 January 1989, Belsville, MD . Springfield, MD : USDA, ARS . p. 193 – 201 .
- Fujii Y. 1992 . The allelopathic effect of some rice varieties , Proceedings of the International Symposium on Biological Control Integrated Management of Paddy and Aquatic Weeds in Asia. National Agricultural Research Center , Tsukuba , Japan . p. 1 – 6 .
- Fujii , Y and Shibuya , T . 1992 . Establishment of a new bioassay specific to allelopathy . Survey of allelopathic plant by the Plant Box Method. Weed Res (Jp). , (Suppl.) 36 : 152 – 153 .
- Garrity , DP , Movillon , M and Moody , K . 1992 . Differential weed suppression ability in upland rice cultivars . Agron J. , 84 : 586 – 591 .
- Hassan , SM , Aidy , IR , Bastawisi , AO and Draz , AE . 1998 . “ Weed management in rice using allelopathic varieties in Egypt ” . In Proceedings of the Workshop on Allelopathy in Rice. 25–27 November 1996 , Edited by: Olofsdotter , M . 27 – 37 . Makati City, Manila, , The Philippines : International Rice Research Institute .
- Inderjit and Weston , LA . 2000 . Are laboratory bioassays for allelopathy suitable for prediction of field responses? . J Chem Ecol. , 26 : 2111 – 2118 .
- Inderjit , Weston , LA and Duke , SO . 2005 . Challenges, achievements and opportunities in allelopathy research . J Plant Interact. , 1 : 69 – 81 .
- Jung , WS , Kim , KH , Ahn , JK , Hahn , SJ and Chung , IM. 2004 . Allelopathic potential of rice (Oryza sativa L.) residues against Echinochloa crus-galli . Crop Prot. , 23 : 211 – 218 .
- Kato-Noguchi , H , Ino , T , Sata , N and Yamamura , S . 2002 . Isolation and identification of a potent allelopathic substance in rice root exudates . Physiol Planta. , 115 : 401 – 405 .
- Kato-Noguchi , H , Ota , K and Ino , T . 2008 . Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities . Allelo J. , 22 : 321 – 328 .
- Khanh , TD , Xuan , TD , Chin , DV , Chung , IM , Elzaawely , AA and Tawata , S . 2006 . Current status of biological control of paddy weeds in Vietnam . Weed Biol Manage. , 6 : 1 – 9 .
- Khanh , TD , Xuan , TD and Chung , IM . 2007 . Rice allelopathy and the possibility for weed management . Ann App Biol. , 153 : 325 – 339 .
- Leather , GR and Einhellig , FA . 1988 . Bioassay of naturally occurring allelochemicals for phytotoxicty . J Chem Ecol. , 14 : 1821 – 1828 .
- Lee , CW , Yoneyama , K , Takeuchi , Y and Konnai , M . 1999 . Momilactone A and B in rice straw harvested at different growth stage . Biosci Biotechnol Biochem. , 63 : 1318 – 1320 .
- Lee , SB , Kim , KH , Hahn , SJ and Chung , IM . 2003 . Evaluation of screening methods to determine the allelopathic potential of rice varieties against Echinochloa crus-galli . Beauv. var. Oryzicola Ohwi. Allelo J. , 12 : 37 – 52 .
- Lee , SB , Ku , YC , Kim , KH , Hahn , SJ and Chung , I.M . 2004 . Allelopathic potential of rice germplasm against barnyardgrass . Allelo J. , 13 : 17 – 28 .
- Lin , W , Kim , KU , Liang , K and Guo , Y . 2000 . “ Hybrid rice with allelopathy ” . In Proceedings of the International Workshop in Rice Allelopathy. 17–19 August 2000 , Edited by: Kim , KU and Shin , DH . 49 – 56 . Kyungpook National University, Taegu, Korea .
- Navarez D , Olofsdotter M . 1996 . Seeding technique for screening allelopathy rice (Oryza sativa L.) . In: Brown H . Proceedings of 1996 2nd International Weed Control Congress , Denmark , Copenhagen . p. 1285 – 1290 .
- Olofsdotter M , Navarez D . 1996 . Allelopathic rice for Echinochloa crus-galli control . Proceedings of the 2nd International Weed Control Congress , Copenhagen , Denmark . p. 1175 – 1181 .
- Olofsdotter M , Navarez D , Rebulanan M . 1997 . Rice allelopathy – where are we and how far can we get? In: The 1997 Brighton Crop Protection Conference, Brighton . p. 99 – 104 .
- Olofsdotter , M , Navarez , D , Rebulanan , M and Streibig , JC . 1999 . Weed-suppressing rice cultivars – does allelopathy play a role? . Weed Res. , 39 : 441 – 474 .
- Olofsdotter , M . 2001 . Rice – a step toward use of allelopathy . Agron J. , 93 : 3 – 8 .
- Rice , EL . 1984 . Allelopathy. Physiological ecology , Orlando, FL : Academic Press .
- SAS Institute . 1997 . SAS/STAT User's Guide, 162 Ed . SAS Institute , Cary, NC .
- Stowe , LG . 1979 . Allelopathy and its influence on the distribution of plants in an Illinois old field . J Ecol. , 67 : 1065 – 1085 .