Abstract
The influence of soil fungi on soil organic carbon (OC) from surface residue was tested in outdoor plots in southern Ontario, Canada, 2004. Fungal hyphal length, soil aggregation, OC and light and heavy fractions of organic matter were compared with factors of plant growth (with or without oat [Avena sativa]) and surface residue (no residue, oat straw (low C:N) or corn (Zea mays) stalks (high C:N)) in a factorial arrangement. Significant increases were observed in soil OC from the oat plants, and from corn stalks compared to straw residue, in the growing season with very moist, high OC, sandy soil. In treatments with corn stalk residue, fungal hyphal length was increased with interaction from the oat plants and residue and was positively correlated with the heavy fraction organic matter along with soil OC. Fungal hyphae, plant roots and high C:N residue were all factors in soil OC increases.
Introduction
The amount of crop residue left on the surface of the soil is considered as the most important variable in determining the potential to increase soil organic matter and thus organic carbon (OC) content (Clapp et al. Citation2000; Whilhelm et al. Citation2004). The practice of ‘no-till’ agriculture, which generates abundant surface residue, has been increasing since 1990 and is currently around 50% of cropping acreage on the Canadian Prairies (Statistics Canada Citation2006). There is a controversy over soil OC increases; studies in Western Canada have shown significant increases over time with no-till (Van den Bygaart et al. Citation2003), while studies in more humid conditions in Eastern Canada report varied results over the long term (Angers et al. Citation1997b).
In long-term field experiments that tested the effects of tillage, residue and fertilization on continuous corn (Zea mays) cropping, Clapp et al. (Citation2000) found that OC increased with no-till, provided residue was returned to the surface. With various crops, residue treatments and soil types in field sites in Eastern Canada, soil OC increased in the surface 10 cm after 11 years of no-till, as compared to conventional tillage, although no significant changes occurred when OC was measured to 60 cm depth (Angers et al. Citation1997b). With no-till, the response of OC to carbon input is mainly in the surface 15 cm (Kern and Johnson Citation1993), whereas tillage can increase soil OC below 15 cm (Allmaras et al. Citation2004).
No-till agriculture allows greater fungal colonization, as fungi are the dominant decomposers of litter in aerobic conditions at the soil surface (Hu et al. Citation1995). Holland and Coleman (Citation1987) found that a greater portion of 14C was retained in the soil when straw was surface applied rather than incorporated into the soil in a litterbag study in the field. Fungi also have slower decomposition time compared to bacteria (Parton et al. Citation1987) because of the 60% chitin in the cell walls (Langley and Hungate Citation2003) and the protein glomalin (Wright and Upadhyaya Citation1998). It has also been shown that slow turnover time maximizes the stability of microaggregates (<250 µm) (Tisdall and Oades Citation1982) and the transfer of macroaggregates (>250 µm) into stable OC (Besnard et al. Citation1996; Six et al. Citation2004).
Improved stability of OC and aggregates from increased fungal activity may explain increased conservation of OC in no-till (Six et al. Citation2006) as links have been established between fungal abundance and aggregation. The protein glomalin is thought to contribute to soil OC (Rillig and Allen Citation1999) from increased resistance of soils to decomposition (Rillig and Mummey Citation2006), and through increased soil aggregation (Rillig et al. Citation2002). In tall-grass prairie, Jastrow et al. (Citation1998) found that mycorrhizal root colonization had the largest single effect on soil aggregation, followed by root length and microbial biomass. Reports on the interaction of mycorrhiza and decomposer fungi in field conditions and the influence of surface crop residue on growth of mycorrhizal mycelia are needed to elucidate the role of fungi in soil dynamics that can influence OC retention.
The light fraction (LF) of organic matter is recently arrived, partly decomposed plant material, and its OC content (OCLF) has been reported as representative of the amount and effect of active decomposition of residue (Janzen et al. Citation1992). The remaining heavy fraction (HF) of organic matter is composed of organic matter adsorbed to mineral surfaces within aggregates (Christensen Citation1992). The C:N ratio is thought to control the speed of decomposition where the amount of nitrogen available limits microbial growth. Conti et al. (Citation1997) reported an increased decomposition rate with maize stubble residue added to soil with low C:N and OC levels, although no growing crop was included in the analysis. While speed of decomposition may be increased with LF, the retention of soil OC is variable. Studies where the decomposition rate varies inversely to soil OC content suggest recalcitrant residue associated with the heavy fraction organic matter, may result in greater increases in OC (Conti et al. Citation1997; Thomsen et al. Citation2008).
Oat (Avena sativa) was sown in outdoor plots with a factorial arrangement of plant (presence/absence) and surface residue (none/oat straw/corn stalks) treatments to test the relative effects of oat plants to residue, and comparative residue C:N of residue on fungal hyphal length, soil aggregation, soil OC and density fractions of organic matter. The hypotheses were: (1) plants and residue would both increase fungal hyphal length and their effects would be additive, (2) increased hyphal length would correspond to increases in soil aggregation, moisture and OC, and (3) with a lower C:N ratio, straw would increase hyphal length, OCLF and soil OC compared to corn residue.
Methods
Study plots
This study is the 2nd season of a three-year study using outdoor plots in the southern Ontario municipality of Clarington (44°03' N, 78°32' W) (Manns et al. Citation2007). A wooden frame was divided into 24 sections, each 60 cm square, and was filled with a 12 cm depth of soil by volume. The bottom of the plots had a perforated plastic lining to prevent upward movement of water and weed growth. The soil originated from the surface (0–10 cm) of a nearby garden where compost had been surface applied over 10 years without tilling on a base classified as ‘Brighton Sand’ (Manns et al. Citation2007). The particle size distribution of the soil was 89% sand (fine and very fine), 11% silt,<1% clay, with pH of 7.5 (electrode measurement in 2:1 soil:deionized water) and bulk density 1.15 g cm−3. Each year, the total volume of soil needed to fill the frame was mixed first to reduce variance in soil conditions and provide comparable conditions for each study. Initial OC averaged 69.0±6.0 g kg−1 (mean±SEM) as detailed for OC sampling (see Soil testing).
Treatments
The six treatments were presence (OT) or absence (NP) of oat plants in factorial with no residue (NR), straw residue (ST) or corn stalk residue (CS), each replicated four times and randomly assigned to the 24 sections of the frame. Oat straw from a neighboring farm and corn stalks that had stood the winter in a nearby field were mulched coarsely in a chipper-shredder resulting in segments with random length up to 10 cm and diameter of 0.3–0.6 cm for straw and 0.3–1.5 cm for corn stalks. Mulch was placed on the soil surface of designated sections, lined with a plastic netting of 2 cm mesh that allowed the mulch to be easily lifted up and weighed at harvest. The initial weight of straw and corn mulch was 533–555 and 733–750 g m−2, respectively, representing a 3–4 cm depth. This rate of addition represents 6.0 t ha−1 for straw and 8.2 t ha−1 for corn residue and could accumulate on the surface after two seasons of harvest with NT in southern Ontario from observation in local fields. The mulch rate assumes a decomposition rate of 50% per year that is slightly less than the rate of 13C incorporation found in soil aggregates in controlled incubation experiments following wheat straw decomposition (Angers et al. Citation1997a).
Several days following preparation of the plots, oats (A. sativa cv. Ida) were seeded (6 May 2004) by broadcasting seed over the mulch, or lightly raking the seeds into bare soil. Plots were kept weed-free by hand weeding. Oats were thinned to a normal field density of 333 plants m−2 (Welch Citation1995). Although dry weather delayed germination, supplemental watering was not needed, as higher than normal amounts of rain fell during the rest of the season.
Sampling
Soil samples were collected during creation of the plots in May before the mulch and seeds were applied. A composite sample of approximately 250 g was formed from four independent samples through a 10 cm depth for each of the 24 plots. Sampling was repeated monthly until harvest (9 August 2004). In plots with oat plants, the soil sample was taken close to the oat roots without disturbing the plants. Half of each sample was promptly stored at 4°C and the other half frozen at −20°C. At harvest the plants were cut 2 cm above the ground, dried 24 h at 80°C and weighed. The lifted mulch was washed to remove adhering soil and dried for 24 h at 80°C prior to weighing. Plant roots were loosened gently and extracted from the soil and the mass recorded following washing and air-drying. A small sample of fine roots was removed for mycorrhizal analysis.
Soil testing
Soil moisture was determined by loss of mass after oven drying at 105°C and expressed as a percentage of dry weight (Page Citation1982). The oven dried soil samples were passed through a 0.5 mm sieve and soil OC was determined from duplicate samples by loss on ignition at 375°C for 16 h in a muffle oven (Ball Citation1964). The percentage OC was calculated from the proportion of the mass loss after ignition over the dry mass prior to ignition divided by 1.8, the proportion of organic matter to OC (Page Citation1982) and subsequently expressed in g kg−1.
The aggregate size distribution was measured by shaking 5 g of air-dried soil through a nest of sieves, sizes 4, 2, 1, and 0.25 mm, for 15 min as described by Klute (Citation1982). Each size sieve mass was recorded and the percentage macroaggregates calculated as follows: [mass of aggregates > 0.25 mm/total dry wt of sample (5 g)]×100
The mean weight diameter index (MWD) was calculated from the aggregate size distribution as the sum of the mean diameter of each weight class×the percentage mass of each size class of air-dried aggregates for all size classes (Klute Citation1982). The 1–2 mm size aggregates were further analyzed for wet aggregate stability. Each sample absorbed water slowly over 10 min and was immersed in water on a 1 mm sieve with a 5 s cycle time in 20°C water for 5 min (Klute Citation1982). The water stability of aggregates (WSA) was expressed as a percentage of the mass after drying at 105°C for 24 h over the initial dry mass. A soil sample was tested for an effect of sand particles. Since the sand particles were less than 0.5 mm in size and not retained on the 1 mm sieve, no correction was made for primary mineral particles (Black Citation1965).
Fungal hyphal length and mycorrhizal colonization
A soil suspension was made by combining 1 g of thawed soil with 100 ml autoclaved water at high speed in a blender for 15 s. The soil solution with suspended organic matter was decanted off from sand grains that remained in the bottom of the beaker 10 s after stirring and stored at 4°C. Measurements were adjusted for the moisture percentage in the soil where fresh/frozen soil was used for testing.
Hyphal length is a measure of the density of fungal growth in the soil (Kabir et al. Citation1997) and was estimated from hyphae stained with lactophenol aniline blue in a soil suspension. A constant volume of soil mycelia is considered dead (West Citation1988), inactive or simply not growing (Walker Citation1975). Phenolic aniline blue effectively colored viable hyphae, but not ‘ghost’ hyphae, and estimated hyphae length of in vitro fungal cultures (West Citation1988). The distinctly blue stain adheres to β-glucan linkages in fungal cell walls of living hyphae (Paul and Clark Citation1982). The length of each piece of blue hyphae was estimated in 10 ml of suspension with three drops of stain in the glass Petri dish with 1.2 cm gridlines under 20× magnification with a stereoscopic microscope. For each treatment, the hyphal lengths were summed for triplicate samples and averaged to give the total length in cm g−1 soil dry wt. The length estimation of individual hyphae was confirmed by microscope photos.
Mycorrhizal colonization on oat roots was enumerated with root staining by ink/vinegar stain following an adaptation of the method of Vierheilig et al. (Citation1998) as detailed in Manns et al. (Citation2007). Evidence of hyphae, vesicles or arbuscules that occurred on each 1 cm piece on a single view of the microscope at 100× magnification was scored as a percentage occurrence of the total number of inspected pieces (approximately 40 per treatment) (Gryndler et al. Citation2002).
Density fractionation of organic matter
Light fraction organic matter is described as plant matter in the early stages of decomposition that is high in carbon, lipids and lignin (Lavelle and Spain Citation2001) and was found to be correlated to the microbial respiration rate in response to management inputs (Janzen et al. Citation1992). The heavy fraction (HF) is humic and mineral material or complexes that have a much higher density and thus can be separated from LF by specific gravity (Lavelle and Spain Citation2001).
A 5 g sample of field moist soil from each plot was wet-sieved to separate organic matter from sand. The organic matter was further separated into LF and HF with LUDOXTM HS-30 colloidal silica (Sigma Aldrich, Oakville, Ont., Canada), 1.2 g cm−3 specific gravity (van den Pol-van Dasselaar and Oenema Citation1999), following the method of Meijboom et al. (Citation1995). The LF and HF were calculated as the % dried mass of the fraction divided by the initial 5 g of whole soil adjusted for moisture content to dry weight. The LF and HF sediment were further tested for % OC content by weight as discussed previously for soil testing. The % OC in each fraction was expressed as OCLF and OCHF in g kg−1.
Residue organic carbon and nitrogen
The straw and corn residue were assessed for OC, and organic nitrogen at planting and harvest sampling. The harvest residue was placed in 0.5 mm netting to contain all material for washing, dried at 80°C for 24 h and ground in a Wiley mill prior to analysis. Residue OC and organic nitrogen were determined with a LECO FP428 autoanalyzer using the Dumas method for nitrogen (Carter Citation1993).
Soil OC mass balance
To account for the changes in soil OC, a mass balance was computed to compare the amount of OC potentially available from plant photosynthesis based on published carbon fixation rates of Paul and Kucey (Citation1981), to the actual changes in OC in the residues and soil in each treatment. To calculate the gross carbon from photosynthesis, the carbon fixation rate of 7.6 mg OC per g of shoot per hour (Paul and Kucey Citation1981) was applied to the fresh plant weight at harvest over an average time of 30 days to take into account reduced biomass during the early growing period and reduced photosynthesis during plant senescence.
The amount of carbon that could potentially have been accumulated in plant root growth, respiration and exudates was calculated from the allocation percentages of 19%, 28% and 0.5%, respectively (Paul and Kucey Citation1981). A shoot:root ratio of 2.5 was used to estimate root biomass, as this was the approximate ratio of the dried plant: root mass, and also the ratio observed for oats in field studies (Bolinder et al. Citation2007). The actual changes in OC from residue that could have contributed to soil OC were also calculated from the measured biomass and organic carbon and nitrogen content at harvest and planting (g dry wt). Fungal biomass was calculated from measured hyphal length over the season using 0.33 g cm−3 as a conversion factor that assumes 45% OC composition of fungi (Berg et al. Citation1998) and an average 14-day turnover time in the soil.
Statistics
Results of the 2×3 factorial treatments were examined with two-way ANOVA model (Statistica, Statsoft) to detect significant differences due to main effects of the plant (factor 1) – presence/absence and surface residue (factor 2) – no residue/oat straw/corn stalks. Significant differences among the three residue treatments were further tested with Fisher's Least Significant Difference (LSD) post-hoc test. Straw and corn residue results were combined for analysis as ‘residue’ where there were no significant differences among the individual treatments. Normality was ascertained with the Ryan-Joiner test. The Pearson product-moment method was used to assess correlation among the soil variables. Linear regression further analyzed the amount of variance explained by the dependence of one variable on another where biological associations were implied.
Results
Main effects
Surface residue had a significant effect on the % macroaggregates and soil OC at harvest sampling, while the oat plant effects on both variables were dependent on the plant growth stage (Two-way ANOVA, ). Average rainfall over the growing season (350 mm) resulted in abundant growth of the plants and fungal hyphae. Soil moisture is not detailed further in this account.
Table 1. Two-way model ANOVA (oats×residue) p values of main effects of the oat plant and residue on soil variables along with interaction term. Post-hoc comparison among residue treatment means by LSD test; No residue (NR), oat straw (ST) with low C:N, corn stalks (CS) with high C:N.
Fungal hyphal length
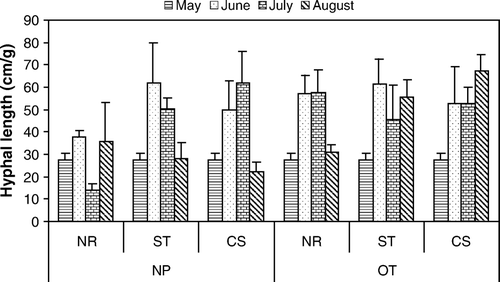
Fungal hyphal length averaged 27.6±2.7 cm g−1 soil in the soil control throughout the experimental period. In all other plots, hyphal length increased to a range of 40–60 cm g−1 soil through June and July. In August hyphal length then decreased to levels observed for bare soil in May, in all plots except with the combination of oat plants and residue where hyphal length was maintained or increased (). There was a significant effect of the oat plant (p=0.007) to maintain hyphal length only when accompanied with interaction (p=0.04) of the plant with both straw and corn stalk residue ().
Figure 1. Fungal hyphal length (cm g−1 soil) measured over time in plots with treatments of no plant (NP) or oat plants (OT) and with no residue (NR), straw residue (ST) of low C:N, or corn residue (CS) of high C:N from initial planting (May) to harvest (August) 2004. May figures were averaged across all treatments. Error bars represent SEM (n=4).
Soil aggregates
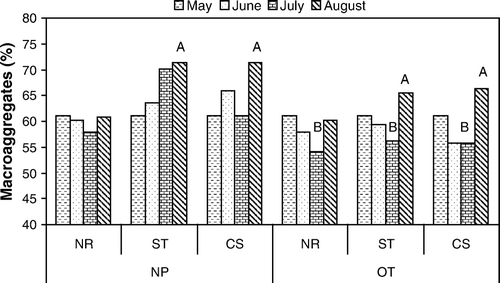
The oat plants significantly decreased aggregate size (p=0.02) in July (). The % macroaggregates was subsequently increased by both straw (p=0.03) and corn stalk (p=0.025) residue in August over the no residue control in plots with and without oat plants. There was a significant correlation of % macroaggregates to hyphal length only in treatments with both corn stalk residue and oat plants (r 2=0.99, p=0.006, n=4).
Figure 2. Percentage of soil macroaggregates measured over time in plot treatments with no plants (NP) or oats (OT) and with no residue (NR), straw residue (ST) with low C:N, or corn residue (CS) with high C:N from initial planting (May) to harvest (August) 2004. May figures were averaged across all treatments. Plots marked A are significantly greater (p=0.05) than other treatments at August sampling, and B are significantly less (p=0.05) than other treatments at July sampling (n=4) by two-way ANOVA.
The water stability of the aggregates (WSA) declined from May to June (t-test, p<0.001) from an average of 82.4±3.5% to 55.3±1.4%, which was attributed to the mixing of the soils in the creation of the plots. From June to August WSA increased to an average of 76.9±1.3% in all plots which approached the May value, but treatment affects were not significant (data not shown). The linear regression of %WSA = 0.14×hyphal length + 71.27 (r 2=0.24, p=0.015, n=24) represented the strongest relationship between two variables observed over all plots. The weighted distribution of the aggregate size classes (MWD), calculated from the August distribution of soil aggregates, was correlated to soil OC (r 2=0.41, p=0.023) and hyphal length (r 2=0.38, p=0.03) over all treatments with oat plants (n =12).
Organic carbon (OC)
Soil OC increased in all plots over the three-month growing season, with the greatest increase in the last four weeks (). Soil OC in plots with residue (with and without oat plants) was increased significantly with straw (p=0.04) and corn stalk (p=0.04) residues compared to no residue by LSD comparison of means at July sampling (). There was a significant positive effect of the oat plants (p=0.01) on soil OC at August sampling. In addition, plots with corn stalk residue had significantly greater soil OC than with straw residue (p=0.045) over all treatments, while the soil plots were intermediate in soil OC between straw and corn stalk residue. When the changes in soil OC from May to August with residue and oat plants were adjusted for the changes in the no residue plots, there was a significant reduction in soil OC with straw residue compared to no residue (p=0.01) and to corn stalk residue (p=0.02) with and without oat plants ().
Figure 3. Soil OC (g kg−1dry soil) comparison over time in plots with no plants (NP) or oats (OT) and with treatments of no residue (NR), straw residue (ST) with low C:N or corn residue (CS) with high C:N from initial planting (May) to harvest (August) 2004. May figures were averaged across all treatments. Error bars represent SEM (n=4).
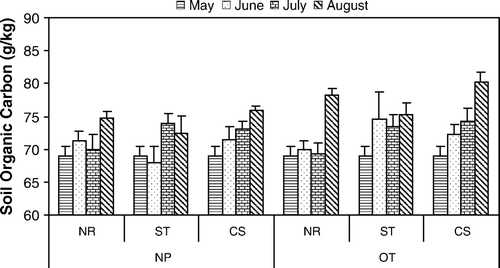
Table 2. Means and SEM in g m−2 of plant and root biomass, and estimated contributions to changes in soil OC from May to August 2004 in each treatment with oat plants with residue treatments; no residue (NR), straw (ST) of low C:N or corn stalk (CS) with high C:N.
Residue decomposition and carbon mass balance
The initial C:N ratios for straw and corn were 31 and 74, respectively. There was no difference in the total residue mass loss (g m−2) or residue OC loss (g m−2) between straw and corn stalk residue (). However, by percentage mass, corn stalks had less residue loss (p<0.001%), OC loss (p=0.001) and % nitrogen loss (p<0.001) than straw residue.
Table 3. Comparison of means±SEM of residue decomposition by loss of mass, OC and Nitrogen in % and mass (g m−2), with no plants (NP) or oat plants (OT) between straw (ST) with low C:N and corn (CS) with high C:N. Two-way model ANOVA p values give a comparison of variables between straw and corn residue (n=16).
Surface residue decomposition, plant roots, root exudates and fungal mycelia all contribute to increases in soil OC. The OC loss from residue averaged 123.5±9.9 g m−2 (), which could represent a gain of 60 g m−2 OC in the soil (< 10% of soil OC increase) after allowing for 50% loss of OC in microbial respiration (). The fresh root biomass of the oat plants at harvest was estimated to be from 600–800 g m−2. Root biomass OC could range up to 1000 g C m−2 based on the OC fixation input from harvested shoot weights in this study (). Calculated plant root biomass could account for 76% and 86% of the potential additions to soil OC for treatments, with and without residue, respectively. Root exudates based on the oat plant biomass would be less than 30 g m−2 (< 3%). Fungal biomass calculated from hyphal length in our study could not have contributed more than 7 g m−2 (< 1%) OC over the season.
Organic matter density fractions
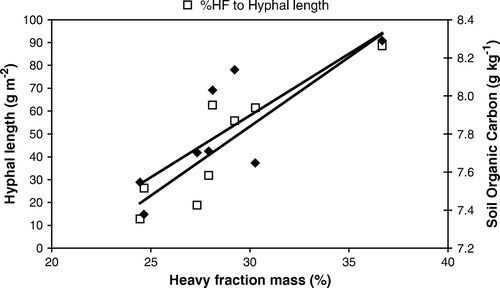
There were no significant differences between treatments in %LF and %HF or the OC in each fraction. The OCLF and OCHF averaged 13.4 and 45.0 g kg−1, respectively. There was little change in the average %HF between sampling times (31.1±4.0% and 29.6±3.1% in July and August, respectively). However, average %LF decreased (p<0.001) from 14.5±2.2% in July to 7.2±1.2% in August. In plots with oats in corn stalk residue (n=4), there was negative correlation between LF and HF (r 2=0.96, p=0.015). The %LF was negatively correlated to hyphal length (r 2=0.94, p=0.03) and % macroaggregates (r 2=0.89, p=0.05), while %HF was positively correlated to both variables. There were significant linear regressions of %HF on hyphal length (Hyphal length = 0.0648×%HF + 5.9518, r 2=0.78, p=0.004) and OC (OC = 0.0648×%HF + 5.9518, r 2=0.63, p=0.02) in all treatments with corn stalk residue (n=8) ().
Figure 4. Linear regression of heavy fraction organic matter, expressed as a % of total dry soil mass on hyphal length (g m−2) (?) y = 6.0583x - 128.42, r 2=0.7851, p=0.89 and soil organic carbon (g kg−1) (?) y = 0.0648x + 5.9518, r 2=0.6301, p=0.79 in all plots with corn stalk residue (with and without oat plants) at harvest (n=8).
Discussion
Significant effects
The study highlighted the different effects of surface residue and plant roots on major variables (hyphal length, WSA, aggregation and soil OC), and with plant growth stages. Surface residue significantly increased soil OC in July, while at harvest the dried oat plants had a greater positive effect on soil OC. There was a significant interaction term between residue and plant on fungal hyphal length at both sampling times suggesting that an interaction of mycorrhizal and saprophytic fungi may have contributed to the increases in soil OC. Variable effects of saprohytic fungi and arbuscular mycorrhiza interactions have been observed in previous studies (Fracchia et al. Citation1998), although changes were only related to germination, hyphal growth and community species composition of fungi. Interaction (Milleret et al. Citation2009) and additive (Andrade et al. Citation1998) effects of mycorrhiza and plant roots have been connected to the stability of aggregates but no published literature was found on links of fungal interaction to soil OC.
Hyphal length
The separate effects of saprophytic fungal hyphae and mycorrhizal hyphae were not varied directly in this study, but were separated by treatments, by their growth pattern over the season and by diameter observed in the previous study. The types of fungi were separated within plot treatments with no plant plus residue (saprophytic) and with oat plants plus no residue (mycorrhizal and saprophytic). Plots with oat plants plus straw and corn residue showed a sustained increase in fungal hyphal length from May to August, 2004, whereas plots with only residue or plants, experienced high fungal hyphal length in July that decreased to that of the base level of bare soil at harvest sampling.
The changes in hyphal length could also indicate a change in the type of fungi, with fast growing saprophytic fungi dominant in June and July, and slow growing mycorrhizal fungi dominant at August sampling. Saprophytic fungal growth is at a maximum with warm temperature, which corresponds to the highest fungal hyphal length recorded in July in this study. A high population of very small diameter hyphae (< 5 µm) was also found in July in our previous study for which oats were grown with and without straw surface residue in the same soil, resulting in ratios of hyphal fragments by diameter (< 5 µm:>5 µm) of 80:20 in July and 45:55 in August (Manns et al. Citation2007). Assuming large hyphae were mycorrhizal (Ayres and Boddy Citation1986; Schreiner and Bethlenfalvay Citation2003) an increase in mycorrhizal hyphae, which are more resistant to decomposition and which grow at a slow and steady rate over the season, would correspond to a peak of mycorrhizal growth in September previously observed in studies (Biederbeck and Campbell Citation1971; Kabir et al. Citation1997).
Our observed interaction of oat plants and residue on fungal hyphal length suggests there is greater total hyphal length when mycorrhizal and saprophytic fungi co-exist in a complex environment. Assuming that 50% of hyphae were from mycorrhizas in August, as in the previous season (Manns et al. Citation2007), the significantly higher hyphal length, which only occurred with interaction of residue and oat plant, may represent a synergy of mycorrhizal and saprophytic fungi or beneficial effects of surface residue on mycorrhizal growth. Current literature using isolated microorganisms in controlled studies indicates mycorrhizal and decomposer fungal interactions can be either positive or negative, depending on the species (Dighton et al. Citation1987).
Klironomos and Kendrick (Citation1995) found increased mycorrhizal fungal hyphae in the presence of saprophytic decomposers of surface litter in simulated forest microcosms resulting from reduced predation by soil microarthropods on mycorrhizal hyphae. Milleret et al. (Citation2009) found that earthworms reduced fungal biomass and noted that grazing on fungal hyphae can either stimulate growth or reduce fungal biomass. A recent greenhouse study by Ortiz-Ceballos et al. (Citation2007) reported that mulch decreased arbuscular mycorrhizal fungi, unless worms were also present. Mycorrhizal fungal hyphae (Boddy et al. Citation1989) and plant roots (Dommergues and Krupa Citation1978) act as sinks for ammonium that is released from saprophytic fungi after decomposition of the residue. Improved nutrient cycling in the soil food web from surface residue could also explain the increased growth of mycorrhizal and saprophytic fungi in concert.
Mycorrhizal colonization
Mycorrhizal fungal colonization on the oat roots averaged 88%, but no significant treatment effects were evident. The colonization of mycorrhizal fungi on oat plant roots may not correspond directly to hyphal length (Schenck Citation1982) since mycelial development varies with species and soil conditions (Bethlenfalvay and Linderman Citation1992). The calculated OC that could be retained in the observed length of fungal hyphae was only 1% of the increase in soil OC, so mycorrhizal hyphae could only be an indirect factor in increasing soil OC.
Mycorrhizal hyphae have been found to contain a protein glomalin which is water-insoluble and heat stable (Rillig et al. Citation2002) and contains a high percentage OC (Rillig and Allen Citation1999). Rillig and Mummey (Citation2006) maintained mycorrhizas influence root and shoot growth directly and soil aggregates indirectly from altered soil water and rhizodeposition. Rillig et al. (Citation2002) found an indirect effect of hyphae on glomalin and root length that explained significant amounts of variation in WSA, while the direct effect of hyphae on WSA was minimal.
Glomalin can be linked to soil OC indirectly from its association with increased aggregation (Rillig et al. Citation2002) and through slow decomposition of fungal hyphae (Rillig et al. Citation2003). A large greenhouse study comparing five mycorrhizal species and nine plant species with 10 replications found significant interaction of plant and fungal species on increasing 1–2 mm diameter water stable aggregates and a positive correlation of hyphal length with %WSA (Piotrowski et al. Citation2004). The plant roots had high density, although glomalin-related soil protein was not measurable because of high background levels in the soil. The interaction effects of mycorrhiza with plant roots may arise from effects on root physiology such as reduced root weight, specific root length and fineness (Rillig et al. Citation2002) or modification of root exudates (Fracchia et al. Citation1998).
Soil aggregation
Fungal hyphal length significantly correlated to the % macroaggregates only in the treatment of oat plants with corn stalk residue with limited sample size (n=4). However, the MWD expressed a positive correlation of aggregate size to hyphal length and OC over all plant treatments that is in agreement with previous reports that hyphal length increased OC in association with increased aggregate size (Hu et al. Citation1995; Beare et al. Citation1997; Angers et al. Citation1997b). Fungi have been associated with increased soil aggregation in grasslands (Tisdall and Oades Citation1979) and with winter rye (Lolium perenne) planted into sorghum residue in NT (Beare et al. Citation1997) for which there is a high abundance of mycorrhizal fungi.
Oats have a lower amount of mycorrhizal association compared to grasses, winter rye or corn, which may have contributed to the lack of correlation in the other treatments between hyphal length and% soil macroaggregates in this study compared to reported literature. Bossuyt et al. (Citation2001) found that saprophytic fungal biomass from wheat straw decomposition was essential to development of macroaggregates in 14 days in a microcosm study. The authors also suggested that only a threshold amount of fungal hyphae was necessary for aggregate formation and this may relate to the absence of a correlation in our high organic matter soil.
The significant correlation of fungal hyphal length to WSA over all plots indicates that fungal hyphae were a significant factor in the resistance of aggregates to decomposition at harvest sampling. This supports the work of current authors on a strong relationship between mycorrhizal hyphal length and WSA. Van der Heijden et al. (Citation2006) tested varied species of mycorrhizal inoculant and seedlings with sterile high quality soil in microcosms and found mycorrhizal colonization increased soil aggregation and %WSA. Andrade et al. (1998) ascertained a close relationship of fungal hyphal length to %WSA in controlled microcosm experiments growing Sorghum bicolor for 10 weeks with 43 µm mesh dividing hyphae from roots of S. bicolor. The relationship to WSA was highest with roots plus mycorrhiza, followed by roots alone, mycorrhiza alone, and the soil control in that order (Andrade et al. 1998). Piotrowski et al. (Citation2004) also found a positive correlation of %WSA to hyphal length and root biomass researching the difference in five arbuscular mycorrhizal species and nine plant species in a greenhouse study over 11 months. In a microcosm experiment, Milleret et al. (Citation2009) found no effect on water stability of aggregates individually from individual effects of mycorrhiza Glomus intraradices and roots of Allium porrum, but WSA increased significantly when there was significant interaction of mycorrhiza and roots.
The decrease in the percentage of macroaggregates in this study from the oat plants in July, followed by an increase in aggregate size from residue in August, is consistent with the effect of high exudation rates early in the growing season, which diminish as the plant dries (Whilhelm et al. Citation2004). The action of an abundance of roots limits soil aggregation as the disturbance and bacterial action stimulate decomposition (Jastrow et al. Citation1998). At plant senescence, root exudation stops and is replaced by saprophytic fungal decomposers of root material (Wamberg et al. Citation2003) that would contribute to the increases in hyphal length and OC from the oat plants.
Soil OC
The increase in soil OC from the oat plants may be related to high root mass stimulated by the high rainfall. Plants roots have been shown to increase OC in controlled studies. In a pot study under growth chamber conditions, Gale and Cambardella (Citation2000) found that a greater amount of 14C was retained in the soil from oat roots (42%) compared to oat leaves left to decompose on the surface (16%). Those authors suggested that a portion of root OC is rapidly released into the soil after plant senescence, followed by increases in stabilized, protected, slow decomposition forms (Gale and Cambardella Citation2000). The authors observed rapid initial decomposition with 80% of coarse roots decomposed within 90 days. Increases in SOC across a chronosequence of prairie restoration had the closest association to very fine roots (Jastrow et al. Citation1998), possibly from mycorrhizal associations. Therefore, it would be possible for a large volume of very fine roots from the oat plant growth to turnover during the 90-day growing season and be measurable in the soil OC by harvest. This could explain the plant effect on soil OC in our very moist conditions, where soil OC increased primarily between July and August sampling.
From our OC mass balance calculations, plant root biomass accounted for the dominant portion of OC additions, with a small difference accounted for by residue (< 10%), root exudates (< 3%) and fungal hyphae (< 1%). Thus, plant roots and residue could explain the OC increases in oat plant plots after adjustment for changes in the soil controls. However, the increases in soil OC without oat plants of up to 1000 g m−2 must represent another factor that is not accounted for in our study. It is possible that soil algae were capable of fixing up to 127.4 g m−2 OC in conditions with high levels of phosphorous, organic matter (Lavelle and Spain Citation2001) and soil moisture that occurred in the four weeks preceding harvest.
Residue C:N
Plots with corn stalk residue had higher soil OC compared to plots with straw residue despite a higher C:N than straw residue and no difference in the amount of OC (by mass) released from both residues to the soil. Corn stalk residue also has a higher lignin content than straw that could be stabilized directly into slowly decomposing organic matter pools (Carter and Stewart Citation1996) represented by HF in this study. Our results are in contrast to Conti et al. (Citation1997) who reported that lower C:N in corn stubble from fertilization resulted in an increased decomposition rate, microbial activity and OCLF, without change in humic acids or total C and N. Their study concluded that higher OCLF also mineralized more rapidly, and did not contribute to the synthesis of stable forms such as humic acids, that may involve lignin or phenolic polymers (Conti et al. Citation1997). In our study, the positive correlation of HF to hyphal length and OC in the case of corn residue was not found with straw residue suggesting the recalcitrance of the residue to decomposition was more important than the amount of residue recently decomposed as LF in maintaining aggregation and OC in the sandy soil and wet conditions.
Density fractions
Heavy fraction represents slow decomposition into more stable forms rather than the LF commonly associated with plant root exudates or residue decomposition that are more labile. The contrasting trend observed between %LF (negative) and %HF (positive) correlations to hyphal length and macroaggregates in plots with oat plants plus corn stalk residue, indicates that the transfer of fungal hyphae, corn residue and/or oat roots into macroaggregates and soil OC was more closely associated with HF than LF. This agrees with Grandy and Robertson (Citation2007) who found heavy fraction (>1.6 g cm−3) had the highest OC accumulation in the largest aggregate size class when examining soil from the top 0–5 cm in varied ecosystems. Increases in soil OC in agricultural systems were highest with perennial alfalfa, followed by no-till (55% and 43%) over 12 years, with average OC accumulation in the average range of 30 g C m−2 g−1 for the mid-western United States, and HF accounting for 82% of increased OC with no-till (Grandy and Robertson Citation2007). In contrast, other reports found increases in %LF from mulching (Hassink Citation1995; Bending et al. Citation2000), and correlation to high organic matter (Six et al. Citation2004). Our study may vary from published data due to the density separation at 1.2 g cm−3.
The use of high organic matter sandy soil, conducive to decomposer and mycorrhizal fungal growth, and high rainfall to drive root growth and decomposition provided ultimate conditions, along with low variance, to obtain measurable increases in soil OC. The monthly changes verify that the soil OC slowly increased to a peak at the end of the oat season, where root decomposition was at a maximum. The increases in soil OC support the previous season of this plot study, where only with surface applied residue, soil OC increased in one season (Manns et al. Citation2007).
In a recent review of literature, Six et al. (Citation2006) maintained that conservation of OC in no-till was due to fungal mediated aggregate stability with increased resistance to decomposition. This theory is supported by our study results with corn residue, and we suggest that the interaction of plant roots and mycorrhiza was enhanced by surface residue resulting in increased stability of aggregates and soil OC. Understanding the role of fungi in soil OC and determining a sustainable amount of surface residue for no-till agriculture are important factors for addressing climate change.
Conclusions
In contrast to field scale studies that show soil OC only increases in the long-term with surface residue, this study found significantly greater soil OC with corn stalk residue compared to straw residue surface applied over the growing season of the oat crop. The correlation of %HF with hyphal length and soil OC with corn residue suggested slower decomposition of mycorrhizal hyphae, corn residue and oat roots were factors in the increased soil OC. Soil OC increased with corn stalk residue compared to straw residue, although corn stalks have a higher C:N than straw, and the amount of OC decomposed or measured in the LF of soil was similar in both treatments. Soil aggregation was increased from both straw and corn stalk residue after seed set of the oat plants but the oat plants were more important than residue for the increase in soil OC at plant senescence. The increased fungal hyphal length with interaction of the oat plants and surface residue could be explained from prolonged growth of saprophytic and mycorrhizal hyphae from the plant and residue environment together. Our results indicate plant roots combined with high C:N residue on the soil surface results in the greatest support for soil OC in the effort to reduce atmospheric CO2 through agricultural management.
Acknowledgements
The support of the Symons Trust Fund for Canadian Studies at Trent University is gratefully acknowledged for making this study possible. The assistance of Professor Bev Kay, Land Resource Science, University of Guelph, on the study design and analysis and manuscript editing was very much appreciated.
References
- Allmaras , RR , Linden , DR and Clapp , CE. 2004 . Corn-residue transformations into root and soil carbon as related to nitrogen, tillage and stover management . Soil Sci Soc Am J. , 68 : 1366 – 1375 .
- Andrade , G , Mihara , KL , Linderman , RG and Bethlenfalvay , GH. 1998 . Soil aggregation status and rhizobacteria in the mycorrhizosphere . Plant Soil , 2002 : 89 – 96 .
- Angers , DA , Recous , S and Aita , C. 1997a . Fate of carbon and nitrogen in water-stable aggregates during decomposition of CN labeled wheat straw . Eur J Soil Sci. , 48 : 295 – 300 .
- Angers , DA , Bolinder , MA , Carter , MR , Gregorich , EG , Drury , CF , Liang , BC , Voroney , RP , Simard , RR , Donald , RG , Beyaert , R and Martel , J. 1997b . Impact of tillage practices on organic carbon and nitrogen storage in cool, humid soils of Eastern Canada . Soil Tillage Res. , 41 : 191 – 201 .
- Ayres P , Boddy L. 1986 . Water, fungi and plants, Symposium of the British Mycological Society 1985 . In : Water relations of mycorrhizal fungi and their host plants . Cambridge : Cambridge University Press . pp 287 – 303 .
- Ball , DF. 1964 . Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcaerous soils . J Soil Sci. , 15 : 84 – 92 .
- Beare , M , Hu , S , Coleman , D and Hendrix , P. 1997 . Influences of mycelial fungi on soil aggregation and organic matter storage in conventional and no-tillage soils . Appl Soil Ecol. , 5 : 211 – 219 .
- Bending , GD , Putland , C and Rayns , F. 2000 . Changes in microbial community metabolism and labile organic matter fractions as early indicators of the impact of management on soil biological quality . Biol Fertil Soils. , 31 : 78 – 84 .
- Berg , M , Kniese , J and Verhoef , H. 1998 . Dynamics and stratification of bacteria and fungi in organic layers of a Scots pine forest soil . Biol Fertil Soils. , 26 : 313 – 322 .
- Besnard , E , Chenu , C , Balesdent , J , Puget , P and Arrouays , D. 1996 . Fate of particulate organic matter in soil aggregates during cultivation . Eur J Soil Sci. , 47 : 495 – 503 .
- Bethlenfalvay GJ , Linderman RG. 1992 . Mycorrhizae in sustainable agriculture . In : Vesicular-arbuscular mycorrhizae and cultural stresses. Madison , WI : American Society of Agronomy, Soil Science Society of America, Crop Science Society of America Inc . p 71 – 100 .
- Biederbeck , V and Campbell , C. 1971 . Influence of simulated fall and spring conditions on the soil system I. Effect on soil microflora . Soil Sci Soc Am J. , 35 : 474 – 479 .
- Black CA . 1965 . Methods of soil analysis, Pt. 1 . Size distribution of aggregates . Madison, WI : American Society of Agronomy . p 499 – 510 .
- Boddy L , Marchant R , Read DJ. 1989 . Nitrogen, phosphorous and sulfur utilization by fungi . In : The nitrogen nutrition of mycorrhiza fungi and their host plants . Cambridge : Cambridge University Press . p 181 – 204 .
- Bolinder , MA , Janzen , HH , Gregorich , EG , Angers , DA and VandenBygaart , AJ. 2007 . An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada . Agr Ecosys Environ. , 118 : 29 – 42 .
- Bossuyt , H , Denef , K , Six , J , Frey , SD , Merckx , R and Paustian , K. 2001 . Influence of microbial populations and residue quality on aggregate stability . Appl Soil Ecol. , 16 : 195 – 208 .
- Carter MR . 1993 . Soil sampling and methods of analysis . In : Total nitrogen. Boca Raton , FL : Lewis Publishers, CRC Press . p 201 – 211 .
- Carter MR , Stewart BA. 1996 . Structure and organic matter storage in agricultural soils . Boca Raton , FL : CRC Press, Lewis Publishers .
- Christensen , BT. 1992 . Physical fractionation of soil and organic matter in primary particle size and density separates . Adv Soil Sci , 20 : 1 – 90 .
- Clapp , CE , Allmaras , RR , Layese , MF , Linden , DR and Dowdy , RH. 2000 . Soil organic carbon and 13C abundance as related to tillage, crop residue and continuous corn management in Minnesota . Soil Tillage Res. , 55 : 27 – 142 .
- Conti , M , Arrigo , N and Marelli , H. 1997 . Relationship of soil carbon light fraction, microbial activity, humic acid production and nitrogen fertilization in the decaying process of corn stubble . Biol Fertil Soils. , 25 : 75 – 78 .
- Dighton , J , Thomas , E and Latter , P. 1987 . Interactions between tree roots, mycorrhizas, saprotrophic fungus and the decomposition of organic substrates in a microcosm . Biol Fertil Soils. , 4 : 145 – 150 .
- Dommergues Y , Krupa S. 1978 . Interactions between non-pathogenic soil microorganisms . In : The rhizosphere . Amsterdam : Elsevier . p 243 – 264 .
- Fracchia , S , Mujica , MT , Gracia-Romera , I , Gracia-Garrido , JM , Martin , J , Ocampo , JA and Godeas , A. 1998 . Interactions between Glomus mosseae and arbuscular mycorrhizal sporocarp-associated saprophytic fungi . Plant Soil. , 200 : 131 – 137 .
- Gale , WJ and Cambardella , CA. 2000 . Carbon dynamics of surface residue- and root-derived organic matter under simulated No-till . Soil Sci Soc Am J. , 64 : 190 – 195 .
- Grandy , AS and Robertson , GP. 2007 . Land-use intensity effects on soil organic carbon accumulation rates and mechanisms . Ecosystems , 10 : 58 – 73 .
- Gryndler , M , Vosatka , M , Hrselova , H , Chvatalova , I and Jansa , J. 2002 . Interaction between arbuscular mycorrhizal fungi and cellulose in growth substrate . Appl Soil Ecol. , 19 : 279 – 288 .
- Hassink , J. 1995 . Density fractions of soil macroorganic matter and microbial biomass as predictors of C and N mineralization . Soil Biol Biochem. , 27 : 1099 – 1108 .
- Holland , EA and Coleman , DC. 1987 . Litter placement effects on microbial and organic matter dynamics in an agroecosystem . Ecology , 68 : 425 – 433 .
- Hu , S , Coleman , DC , Beare , MH and Hendrix , PF. 1995 . Soil carbohydrates in aggrading and degrading agroecosystems: Influences of fungi and aggregates . Agri Ecosys Environ. , 54 : 77 – 88 .
- Janzen , HH , Campbell , CA , Brandt , SA , Lafond , GP and Townley-Smith , L. 1992 . Light-fraction organic matter in soils from long-term crop rotations . Soil Sci Soc Am J. , 56 : 1799 – 1806 .
- Jastrow , JD , Miller , RM and Lussenhop , J. 1998 . Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie . Soil Biol Biochem , 30 : 905 – 916 .
- Kabir , Z , O'Halloran , IP , Fyles , JW and Hamel , C. 1997 . Seasonal changes of arbuscular mycorrhizal fungi as affected by tillage practices and fertilization: Hyphal density and mycorrhizal root colonization . Plant Soil. , 192 : 285 – 293 .
- Kern , JS and Johnson , MG. 1993 . Conservation tillage impacts on national soil and atmospheric carbon levels . Soil Sci Soc Am J. , 57 : 200 – 210 .
- Klironomos , JN and Kendrick , WB. 1995 . Stimulative effects of arthropods on endomycorrhizas of sugar maple in the presence of decaying litter . Funct Ecol. , 9 : 528 – 536 .
- Klute A . 1982 . Methods of soil analysis, Part 1. Agronomy series # 9, 2nd ed . In : Aggregate stability and size distribution . Madison, WI : American Society of Agronomy, Soil Science Society of America . p 425 – 442 .
- Langley , JA and Hungate , BA. 2003 . Mycorrhizal controls on belowground litter quality . Ecology , 84 : 2302 – 2312 .
- Lavelle P , Spain AV. 2001 . Soil ecology . Dordrecht : Kluwer Academic Publishers .
- Manns , HR , Maxwell , CD and Emery , RJN. 2007 . The effect of ground cover or initial organic carbon on soil fungi, aggregation, moisture and organic carbon in one season with oat (Avena sativa) plots . Soil Tillage Res. , 96 : 83 – 94 .
- Meijboom , FW , Hassink , J and van Noordwijk , M. 1995 . Density fractionation of soil macroorganic matter using Silica suspension . Soil Biol Biochem. , 27 : 1109 – 1111 .
- Milleret , R , Le Bayon , RC and Gobat , JM. 2009 . Root, mycorrhiza and earthworm interactions: Their effects on soil structuring processes, plant and soil nutrient concentration and plant biomass . Plant Soil. , 316 : 1 – 12 .
- Ortiz-Ceballos , AI , Pena-Cabriales , JJ , Fragoso , C and Brown , GG. 2007 . Mycorrhizal colonization and nitrogen uptake by maize: Combined effect of tropical earthworms and velvetbean mulch . Biol Fertil Soils. , 44 : 181 – 186 .
- Page A 1982 . Methods of soil analysis. Part 2. Agronomy series # 9, 2nd ed . In: Total carbon, organic carbon and organic matter. Madison , >WI : American Society of Agronomy, Soil Science Society of America . p 539 – 579 .
- Parton , WJ , Schimel , DS , Cole , CV and Ojima , DS. 1987 . Analysis of factors controlling soil organic matter levels in Great Plains grasslands . Soil Sci Soc Am J. , 51 : 1173 – 1179 .
- Paul , EA and Clark , FE. 1982 . Soil microbiology and biochemistry , San Diego : Academic Press .
- Paul , EA and Kucey , RMN. 1981 . Carbon flow in plant microbial associations . Science , 213 : 473 – 474 .
- Piotrowski , JS , Denich , T , Klironomos , JN , Graham , JM and Rillig , MC. 2004 . The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species . New Phytol. , 164 : 365 – 373 .
- Rillig , MC and Allen , MF. 1999 . What is the role of arbuscular mycorrhizal fungi in plant-to-ecosystem responses to elevated atmospheric CO2? . Mycorrhiza , 9 : 1 – 8 .
- Rillig , MC and Mummey , DL. 2006 . Mycorrhizas and soil structure . New Phytol. , 171 : 41 – 53 .
- Rillig , M , Wright , S and Eviner , V. 2002 . The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species . Plant Soil. , 238 : 325 – 333 .
- Rillig , MC , Ramsey , PW , Morris , S and Paul , EA. 2003 . Glomalin, an arbuscular-mycorrhizal fungal soil protein, responds to land-use change . Plant Soil. , 253 : 293 – 299 .
- Schenck , N. 1982 . Methods and principles of mycorrhizal research , St Paul, MN : APS Press .
- Schreiner , RP and Bethlenfalvay , GJ. 2003 . Crop residue and colembola interact to determine the growth of mycorrhizal pea plants . Biol Fertil Soils. , 39 : 1 – 8 .
- Six , J , Bossuyt , H , Degryze , S and Denef , K. 2004 . A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics . Soil Tillage Res. , 79 : 7 – 31 .
- Six , J , Frey , SD , Thiet , RK and Batten , KM. 2006 . Bacterial and fungal contributions to carbon sequestration in agroecosystems . Soil Sci Soc Am J. , 70 : 555 – 569 .
- Statistics Canada 2006 . Census of agriculture . Ottawa : Government of Canada .
- Tisdall , JM and Oades , JM. 1979 . Stabilization of soil aggregates by the root systems of ryegrass . Austral J Soil Res. , 17 : 429 – 441 .
- Tisdall , JM and Oades , JM. 1982 . Organic matter and water-stable aggregates in soils . J Soil Sci. , 33 : 141 – 163 .
- Thomsen , IK , Petersen , BM , Bruun , S , Jensen , LS and Christensen , BT. 2008 . Estimating soil C loss potentials from the C to N ratio . Soil Biol Biochem. , 40 : 849 – 852 .
- van den Bygaart , AJ , Gregorich , EG and Angers , DA. 2003 . Influence of agricultural management on soil organic carbon: A compendium and assessment of Canadian studies . Can J Soil Sci. , 83 : 363 – 390 .
- van den Pol-van Dasselaar , A and Oenema , O. 1999 . Methane production and carbon mineralization of size and density fractions of peat soils . Soil Biol Biochem. , 31 : 877 – 886 .
- Van der Heijden , MGA , Streitwolf-Engel , R , Riedle , R , Siegrist , S , Neudecker , A , Ineichen , K , Boller , T , Wiemken , A and Sanders , IR. 2006 . The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland . New Phytol. , 172 : 739 – 752 .
- Vierheilig , H , Coughlan , AP , Wyss , U and Piche , Y. 1998 . Ink and vinegar: A simple staining technique for Arbuscular-Mycorrhizal Fungi . Appl Environ Microbiol. , 64 : 5004 – 5007 .
- Walker N. 1975 . Soil microbiology . In : Soil fungi . New York : J Wiley and Sons . p 165 – 180 .
- Wamberg , C , Christensen , S , Jakobsen , I , Muller , AK and Sorensen , SJ. 2003 . The mycorrhizal fungus (Glomus intraradices) affects microbial activity in the rhizosphere of pea plants (Pisum sativum) . Soil Biol Biochem. , 35 : 1349 – 1357 .
- Welch , RW. 1995 . The oat crop , London : Chapman and Hall .
- West , AW. 1988 . Specimen preparation, stain type, and extraction and observation procedures as factors in the estimation of soil mycelial lengths and volumes by light microscopy . Biol Fert Soils. , 7 : 88 – 94 .
- Whilhelm , WW , Johnson , JMF , Hatfield , JL , Voorhees , WB and Linden , DR. 2004 . Crop and soil productivity response to corn residue removal: A literature review . Agron J. , 96 : 1 – 17 .
- Wright , SF and Upadhyaya , A. 1998 . A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi . Plant Soil. , 198 : 97 – 107 .